Abstract
INTRODUCTION:
The functional evaluation has become increasingly important in the understanding and management of patients with interstitial lung diseases. The cardiopulmonary exercise test and the six‐minute walk test (6MWT), through their isolated variables, have been used to do this evaluation, with some limitations.
OBJECTIVES:
We proposed a new composite index (desaturation distance ratio using continuous peripheral oxygen saturation (SpO2) and the distance walked as a more reliable tool for doing a functional evaluation of these patients.
METHODS:
6MWT was performed by interstitial lung diseases patients and controls. Analyzed parameters were walked distance and desaturation area (DAO2), obtained by taking the difference between maximal SpO2 possible (100%) and patient's SpO2 every 2 seconds. desaturation distance ratio was calculated using the ratio between DAO2 and distance walked.
RESULTS:
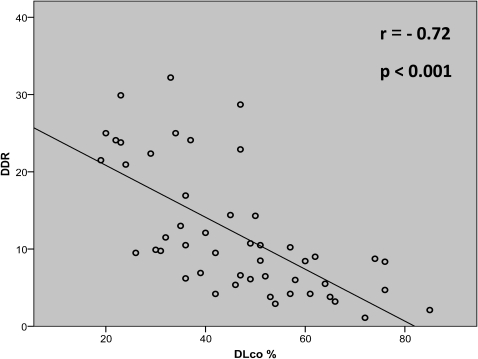
Forty‐nine interstitial lung diseases patients and 11 control subjects completed the protocol. The mean (SD) age was 60 (12) years and 65 (9) years, respectively (p:NS). Data obtained from 6MWT showed a significant statistical difference between interstitial lung diseases patients and controls: mean walked distance (430 and 602 meters, respectively); SpO2 minimal maintained at least 10 seconds ‐ SpO2 min (85% and 94%, respectively), and median desaturation distance ratio (10 and 2.5, respectively). A correlation analysis, considering interstitial lung diseases patients, revealed the best correlation between desaturation distance ratio and DLco (r = ‐ 0.72; p<0.001), being the correlation between SpO2 min and DLco of 0.61 (p<0.001) and among walked distance and DLco of 0.58 (p<0.05).
CONCLUSION:
Desaturation distance ratio is a promising concept and a more reliable physiologic tool to assess pulmonary diseases characterized by involvement of the alveolar‐capillary membrane, such as interstitial lung diseases.
Keywords: The six‐minute walk test, Pulmonary function tests, Interstitial lung diseases
INTRODUCTION
The functional capacity evaluation has become increasingly important in understanding and managing patients with chronic lung diseases.1-3 The cardiopulmonary exercise test (CPET) using a cycle ergometer or treadmill protocols have been used in patients with restrictive and obstructive lung diseases, and they provide a good correlation between survival and functional capacity among individuals in these groups.1,2,4-6
In patients with interstitial lung diseases (ILD), especially those with diffuse fibrotic lung diseases, these protocols typically show a fall in peripheral oxygen saturation (SpO2) during exercise.7,8 However, some patients may be unfamiliar with this type of assessment, it may not be possible to extrapolate their daily life activities from its results, it may have limited reproducibility in patients with idiopathic pulmonary fibrosis (IPF), and it requires specific equipment with a high cost.1,5,9 Recently, researchers observed that some patients in rehabilitation programs did not have oxygen desaturation during CPET but had such an event during walking tests.6
The six‐minute walk test (6MWT) is a simple, efficient, and low‐cost tool used to evaluate the performance of individuals during submaximal exercise, and it can be safely applied in patients with heart and/or advanced lung disease.1,10-12
Despite the controversy regarding the use of walked distance or oxygen desaturation for better correlation with pulmonary function or survival data, recent studies have demonstrated the efficacy of using the 6MWT to evaluate the effectiveness of therapy in patients with interstitial lung diseases, pulmonary rehabilitation follow‐up, marking criteria for transplantation and indication for supplementary oxygen, concluding that it offers a good correlation with the functional capacity and survival of these patients.1-4,9,10,13
Desaturation during 6MWT is associated with higher mortality and has a good correlation with variables of pulmonary function, such as forced vital capacity (FVC), diffusion of carbon monoxide (DLco), total lung capacity (TLC), and SpO2 at rest in patients with interstitial lung diseases.4,5,13 Nevertheless, some researchers have concluded that the distance covered in 6MWT has a stronger correlation with severity and survival of patients with IPF than SpO2 alone.10,14 Eaton and colleagues showed that the covered distance in 6MWT is highly reproducible and has a better prognostic value than SpO2 due to its large variability and because of the fact that it may not be a reliable parameter in fibrotic idiopathic interstitial pneumonia.9
Lettieri and colleagues proposed an index that includes both parameters: walked distance and degree of desaturation during 6MWT.1 Using the product of the lowest SpO2 identified by the distance in meters (distance ‐ saturation product – DSP), they developed a composite index with higher sensitivity and specificity to determine 12‐month survival in IPF patients compared to pulmonary function data or isolated variables of 6MWT. Despite the fact that this index includes these two parameters, it is limited to a specific minimum value of SpO2 during the 6MWT, which does not reflect the entire oxygen saturation during the test, so it may underestimate the functional condition of the patient.1
The purpose of this study was to create a new composite index based on the 6MWT, using oximetry (continuous peripheral oxygen saturation registered every 2 seconds during the test) and the distance walked, in order to develop a more reliable tool for functional assessment of patients with interstitial lung diseases.
MATERIALS AND METHODS
Study design
We developed a transversal study and invited patients to participate during their regular visits in our Interstitial Pulmonary Outpatient Division. If the patient filled in all inclusion criteria noted below (evaluated through medical records review and physical examination during the visit) he or she was invited to sign the free informed consent form approved by the hospital ethics committee.
All patients included in the study needed to be able to maintain peripheral resting saturation in room air (SpO2) ≥ 88% and present no clinical signs of exacerbation.
Subjects
Fifty‐one patients were assessed during their visits to the Interstitial Pulmonary Outpatient Division of the University of Sao Paulo Medical School. Twenty‐five of them had been diagnosed as having idiopathic pulmonary fibrosis (IPF), 6 patients had chronic hypersensitivity pneumonitis (CHP), 5 patients had centrilobular fibrosis secondary to gastroesophageal reflux disease (GERD), and 15 patients received the diagnosis of lymphangioleiomyomatosis (LAM).15,16
Two of the IPF patients were not able to sustain room air SpO2 ≥ 88% at rest. Therefore, 49 patients (23 IPF patients) were recruited to be a part of this study.
The diagnosis criteria for IPF was based on American Thoracic Society / European Respiratory Society (ATS/ERS) guidelines. The CHP diagnosis was established in individuals exposed to a known offending antigen who exhibited a relationship between symptoms and exposure, and there were compatible pulmonary tomographic findings, and/or the diagnosis was proven by a biopsy that excluded other causes.17-19
The definition of GERD‐related centrilobular fibrosis is the combination of GERD‐related symptoms (heartburn, regurgitation, and dysphagia), compatible tomographic features, clinical improvement after specific anti‐reflux therapy, and/or proven by a biopsy that excluded other causes. Patients who had biopsy presented a pattern compatible to centrilobular fibrosis (CLF).15,20,21
Lymphangioleiomyomatosis diagnosis was established by the presence of pulmonary cystic lesions, combined or not with angiomyolipomas and with typical features found in a lung biopsy, such as smooth muscle cell positive anti‐actin antibody and positive antibody human melanoma black (HMB45) in an immunohistochemistry study.22
A control group was made up of 11 healthy volunteers with no history of tobacco use.
Measurements
Demographic data were recorded before the pulmonary function tests were performed.
The six‐minute walk test (6MWT) was carried out by a trained technician, according to ATS guidelines, and it was symptom‐limited. Analyzed parameters were the walked distance (in meters), SpO2 minimum maintained at least 10 seconds, and difference between basal saturation (at rest) and the minimal one achieved in the test (SpO2 basal – SpO2 min).12,23
We created a new index named the desaturation ‐ distance ratio (DDR) based on a physiologic and a holistic interpretation of 6MWT, considering that desaturation and walked distance are equally important variables for pulmonary functional assessment.
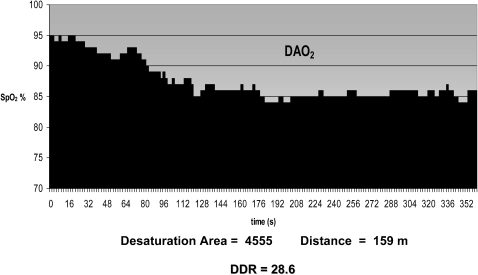
During the 6MWT that lasted 360 seconds, the patient used the pulse oxymeter holter Nonin WristOx® 3100 (Plymouth, MN, USA) to record SpO2 and heart frequency every 2 seconds. Data obtained during the 6MWT were entered into a computer program, nVISION® software (Plymouth, MN, USA) and then exported to an EXCEL worksheet. Then, each recorded SpO2 was subtracted from 100% (maximal SpO2) and plotted on a graph that showed the desaturation area (DAO2) (gray area, Figure 1). Following that, the DDR index was obtained using the ratio between the desaturation area and the distance walked (see an example in Figure 1). Patients who presented the worst performance during 6MWT, with a low distance walked and extensive periods of desaturation, presented a high DDR index value.
A complete pulmonary function test (PFT) was performed at the Laboratory of Pulmonary Physiology, Hospital das Clinicas, within a period no longer than three days after 6MWT, using a whole‐body plethysmograph of the Elite Series Medgraphics ® (Saint Paul, MN, USA). The values measured were forced vital capacity (FVC), forced expiratory volume in one second (FEV1), FEV1/FVC ratio, inspiratory capacity (IC), total lung capacity (TLC), residual volume (RV), and carbon monoxide diffusion capacity (DLco). PFT was held to levels that met the recommendations of the reference values used for spirometry established by Pereira and colleagues. For lung volumes and DLco, the reference values were those recommended by Neder and associates.24,25,26
The assessment of dyspnea was performed using the Borg score, obtained by using a visual analogue scale, which evaluates the intensity of dyspnea by quantifying the effort during the exercise, using a range that varies from 0 to 10 points, where 0 is the absence of symptoms and 10 the worst feeling of dyspnea or fatigue.20
Statistical analysis
Parametric variables were compared using Student's t test, and the Pearson coefficient was used to establish correlations. The Mann‐Whitney and Spearman coefficients were used for non‐parametric variables. All data were two‐tailed, and p values of less than 0.05 were assumed to represent statistical significance. We used the software package SPSS 15.0 (SPSS, Inc., Chicago, IL, USA) to perform statistical analysis.
RESULTS
Forty‐nine ILD patients and 11 control subjects completed the protocol. The mean (SD) age was 60 (12) years in the first group and 65 (9) years in the second one. Nineteen ILD patients (39%) were ex‐smokers. PFT analysis revealed restrictive pattern in ILD patients, with a moderate reduction in DLco (46% of predicted). Control patients presented normal pulmonary functional parameters (Table 1).
Table 1.
Demographic and functional parameters in ILD patients and controls.
| ILD | ||||||
| CONTROLS (N = 11) | TOTAL (N = 49)♦ | IPF (N = 23) | LAM (N = 15) | CHP (N = 6) | CLF (5) | |
| Age years (SD) | 65 (9) | 60 (12)* | 68 (9) | 47 (7) | 63 (11) | 58 (11) |
| Sex (M / F) | 9 / 2 | 24 / 25 | 17/6 | 0/15 | 2/4 | 4/1 |
| Ex‐ smokers | 0 / 11 | 19 / 49 | 15/23 | 3/15 | 4/6 | 1/5 |
| FVC % (SD) | 103 (11) | 76 (16)† | 72 (24) | 89 (13) | 70 (22) | 74 (18) |
| FEV1 % (SD) | 106 (11) | 75 (18)† | 79 (26) | 71 (24) | 76 (21) | 81 (19) |
| TLC % (SD) | 98 (11) | 80 (19)† | 74 (26) | 98 (13) | 72 (17) | 73 (19) |
| DLco % (SD) | 103 (21) | 46 (16)† | 39 (16) | 55 (18) | 46 (12) | 56 (12) |
ILD – interstitial lung disease; IPF – interstitial pulmonary fibrosis; LAM – lymphangioleiomyomatosis; CHP – chronic hypersensitivity pneumonitis; CLF – centrilobular fibrosis; SD ‐ Standard deviation; FVC % ‐ predicted forced vital capacity; FEV1 % ‐ predicted forced expiratory volume in the first second; TLC % ‐ predicted total lung capacity; DLco % ‐ predicted diffusion capacity for carbon monoxide
t test between controls and all 49 ILD patients
p value not significant †p < 0,05
Data obtained from 6MWT showed significant statistical differences between ILD patients and controls, with emphasis on the mean walked distance (430 and 602 meters, respectively) and the median difference between SpO2 basal and SpO2 minimal (sustained for at least 10 s) during the test, resulting in a 10‐point drop in the first group and only 3 points in the control group. The median DDR was higher among ILD patients (10) than in controls (2.5), due to the high desaturation and reduced walk distance observed in those individuals (p<0.05) (Table 2).
Table 2.
Six‐minute walk test parameters in ILD patients and controls.
| ILD | ||||||
| CONTROLS (N = 11) | TOTAL (N = 49)♦ | IPF (N = 23) | LAM (N = 15) | CHP (N = 6) | CLF (5) | |
| Distance (SD) | 602 m (95) | 430 m (122)† | 373 m (114) | 480m (116) | 484 m (117) | 474 m (87) |
| SpO2 basal % (SD) | 96.5 (2) | 95 (2)* | 94 (3) | 95 (3) | 95 (2) | 96 (1) |
| SpO2 min % (SD) | 94 (2) | 85 (8)† | 82 (7) | 87 (10) | 86 (5) | 86 (4) |
| SpO2 basal ‐ min (IQ) | 3 (2 – 4) | 10 (6 ‐ 13)† | 10 (9 ‐ 15) | 5 (2 ‐ 11) | 9 (6 ‐ 11) | 10 (6 ‐ 12) |
| DDR (IQ) | 2.5 (2 – 4.5) | 10 (6 ‐ 17)† | 11 (9 ‐ 23) | 6 (4 ‐ 12) | 8 (6 ‐ 10) | 8 (6 ‐ 8) |
SD ‐ Standard deviation; IQ – interquartile; Distance in meters; DDR ‐ desaturation distance ratio; SpO2 basal % ‐ peripheral oxygen saturation at rest; SpO2 min % ‐ minimal sustained SpO2 during ≥ 10 seconds
Comparison between controls and all 49 ILD patients
p value not significant †p < 0,05
Correlation analysis revealed significant and strong correlation between DDR and predicted DLco (Figure 2). The correlations between walked distance, SpO2 min, and the difference (SpO2 basal – SpO2 min) demonstrated a lower correspondence with DLco (Table 3). There was no correlation between DDR or other 6MWT variables with lung volumes (TLC and RV) (data not shown).
Table 3.
Correlations between six‐minute walk test parameters and functional variables in ILD patients.
| DD Ratio† | Distance* | SpO2 min %* | SpO2 basal ‐ min† | |
| DLco % | r = ‐ 0.72 | r = 0.58 | r = 0.61 | r = ‐ 0.67 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| FVC % | r = ‐ 0.46 | r = 0.33 | r = 0.43 | r = ‐ 0.50 |
| p = 0.001 | p = 0.02 | p = 0.002 | p < 0.001 | |
| FEV1 % | r = ‐ 0.52 | r = 0.25 | r = 0.59 | r = ‐ 0.60 |
| p < 0.001 | p = 0.08 | p < 0.001 | p < 0.001 |
Distance in meters; DD Ratio ‐ desaturation distance ratio; SpO2 min % ‐ minimal sustained peripheral oxygen saturation during ≥ 10 seconds; SpO2 basal % ‐ peripheral oxygen saturation at rest
Pearson †Spearman
DISCUSSION
Many researchers have employed functional evaluations using submaximal testing of patients with respiratory diseases, such as chronic obstructive pulmonary disease (COPD), pulmonary hypertension (PH), and interstitial lung disease (ILD). In particular, the 6MWT has been widely used.
Interstitial lung diseases, especially idiopathic pulmonary fibrosis (IPF), are associated with high mortality and a progressive decrease in exercise capacity.17 There is growing interest in diagnostic and prognostic methods in this population in order to improve understanding and management and provide better quality of life for these patients.
Protocols using a treadmill or cycle ergometer have been employed to assess the functional capacity of the population with ILD, but these tests may be unfamiliar to patients and require sophisticated and expensive equipment.1,5,9 The 6MWT is a simple tool with a low cost, and it is easily applied in functional evaluation of patients with ILD, but there is a disagreement in the literature about which is the best 6MWT parameter (walked distance or decrease in SpO2) to assess functional capacity, severity, or mortality in this population.2,4,9,10,13
In this study, we created a composite index ‐ DDR index ‐ that includes the two main variables obtained in 6MWT, the distance walked and the decrease in SpO2 evaluated at regular intervals during the 360‐second test. We hypothesized that the incorporation of these two main variables in a unique index may help researchers evaluate one of the main features of ILD, a disturbance in gas exchange that worsens during exercise.26,27 In this population, exercise leads to an increase in the alveolar‐arterial O2 gradient concomitant with a decrease in peripheral partial oxygen pressure (PaO2) and peripheral oxygen saturation (SpO2), secondary to multiple changes, especially in ventilation / perfusion mismatch and a diffusion limitation in the alveolar capillary membrane.26,27
According to Eaton and colleagues, walked distance showed less variability and better correlation with DLco and VO2 max than SpO2. 9 However, in a study done by Lama et al, patients who presented a decrease in SpO2 during a 6MWT, equal or below 88%, exhibited significantly lower values of FVC and DLco, and the decrease in saturation (SpO2 basal – lowest saturation) was a significant predictor of mortality, showing that for each percentage decrease in saturation, mortality increased 23%.4
The divergence in these results may be due to the fact that distance is highly dependent on patient efforts. Limited efforts may lead to a smaller distance, preventing the development of hypoxia. However, hypoxia can promote dyspnea and reduce the distance covered. A combination of these two parameters may minimize the impact of this effort factor.
We demonstrated that DDR had a strong and significant correlation with DLco but a weaker correlation with FVC and FEV1 (Table 3). Full oxymetry, measured every 2 seconds, may more accurately reflect the diffusion capacity of the alveolar – capillary membrane, which is reduced in patients with ILD due to impaired gas exchange (reflected by DLco).1,4,10,13
The strong correlation of DDR with DLco but not with FVC or with other lung volumes may be explained by the following factors. In the initial phases of ILD, decline in DLco may precede abnormalities in lung volume.17 Many patients in our sample were ex‐smokers with superimposed COPD, and, in this context, combined pulmonary fibrosis and emphysema can lead to preserved or mildly reduced lung volumes despite the significant decrease in DLCO.28,29 In patients with LAM, there is also airway flow obstruction caused by compression of the airways by smooth cell proliferation or loss of lung elastic coil, leading to an increase in lung volumes.30
We concluded that the combination of the desaturation area and distance walked in a six‐minute walk test (DDR index) is a promising concept and a more reliable physiologic tool to assess pulmonary diseases characterized by involvement of the alveolar‐capillary membrane, such as ILD, and other pulmonary disorders like pulmonary emphysema. Further studies are necessary to determine the role of DDR in predicting mortality, disease progression, and response to treatment in this subset of patients.
ACKNOWLEDGMENT
Alfredo N.C. Santana
Figure 1.
Desaturation ‐ Distance Ratio (DDR): calculated using the ratio between DAO2 [gray area ‐ obtained by subtraction between each recorded SpO2 at every 2 seconds from 100% (maximal SpO2 )] and the distance walked.1 ‐ Desaturation ‐ Distance Ratio (DDR): calculated using the ratio between DAO2 [gray area ‐ obtained by subtraction between each recorded SpO2 at every 2 seconds from 100% (maximal SpO2 )] and the distance walked.
Abbreviations: DAO2: desaturation area; SpO2: peripheral oxygen saturation
Figure 2.
Correlation between DLco% and DDR in ILD patients.2 ‐ Correlation between DLco% and DDR in ILD patients.
REFERENCES
- 1.Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF. The distance‐saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med. 2006;100:1734–41. doi: 10.1016/j.rmed.2006.02.004. 10.1016/j.rmed.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 2.Carter R, Holiday DB, Nwasuruba C, Stocks J, Grothues C, Tiep B. 6‐Minute walk work for assessment of functional capacity in patients with COPD. Chest. 2003;123:1408–15. doi: 10.1378/chest.123.5.1408. 10.1378/chest.123.5.1408 [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Gascon M, Sanchez A, Gallego B, Celli BR. Inspiratory capacity, dynamic hyperinflation, breathlessness, and exercise performance during the 6‐minute‐walk test in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2001;163:1395–9. doi: 10.1164/ajrccm.163.6.2003172. [DOI] [PubMed] [Google Scholar]
- 4.Lama VN, Flaherty KR, Toews GB, Colby TV, Travis WD, Long Q, et al. Prognostic Value of Desaturation during a 6‐minute walk test in Idiopathic Interstitial Pneumonia. Am J Respir Crit Care Med. 2003;168:1084–90. doi: 10.1164/rccm.200302-219OC. 10.1164/rccm.200302‐219OC [DOI] [PubMed] [Google Scholar]
- 5.Chetta A, Aiello M, Foresi A, Marangio E, D'Ippolito R, Castagnaro A, et al. Relationship between outcome measures of six‐minute walk test and baseline lung function in patients with interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:170–5. [PubMed] [Google Scholar]
- 6.Poulain M, Durand F, Palomba B, Ceugniet F, Desplan J, Varray A, et al. 6‐minute walk test is more sensitive than maximal incremental cycle testing for detecting oxygen Desaturation in patients with COPD. Chest. 2003;123:1401–7. doi: 10.1378/chest.123.5.1401. 10.1378/chest.123.5.1401 [DOI] [PubMed] [Google Scholar]
- 7.Spiro SG, Dowdeswell IRG, Clarck TJH. An analysis of submaximal exercise responses in patients with sarcoidosis and fibrosing alveolitis. Br J Dis Chest. 1981;75:169–80. doi: 10.1016/0007-0971(81)90050-4. 10.1016/0007‐0971(81)90050‐4 [DOI] [PubMed] [Google Scholar]
- 8.Agustí AG, Roca J, Gea J, Wagner PD, Xaubet A, Rodriguez‐Roisin R. Mechanisms of gas‐exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;143:219–25. doi: 10.1164/ajrccm/143.2.219. [DOI] [PubMed] [Google Scholar]
- 9.Eaton T, Young P, Milne D, Wells AU. Six‐minute walk, maximal exercise tests: Reproducibility in Fibrotic Interstitial Pneumonia. Am J Respir Crit Care Med. 2005;171:1150–7. doi: 10.1164/rccm.200405-578OC. 10.1164/rccm.200405‐578OC [DOI] [PubMed] [Google Scholar]
- 10.Caminati A, Bianchi A, Cassandro R, Mirenda MR, Harari S. Walking distance on 6‐MWT is a prognostic factor in idiopathic pulmonary fibrosis. Respir Med. 2009;103:117–23. doi: 10.1016/j.rmed.2008.07.022. 10.1016/j.rmed.2008.07.022 [DOI] [PubMed] [Google Scholar]
- 11.Chuang ML, Lin IF, Wasserman K. The body weight‐walking distance product as related to lung function, anaerobic threshold and peak VO2 in COPD patients. Respir Med. 2001;95:618–26. doi: 10.1053/rmed.2001.1115. 10.1053/rmed.2001.1115 [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society statement. Guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 13.Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, et al. Idiopathic pulmonary Fibrosis: prognostic value of changes in physiology and Six‐minute‐walk test. Am J Respir Crit Care Med. 2006;174:803–9. doi: 10.1164/rccm.200604-488OC. 10.1164/rccm.200604‐488OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lederer DJ, Arcasoy SM, Wilt JS, D'Ovidio F, Sonett JR, Kawut SM. Six‐minute‐walk distance predicts waiting list survival in Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2006;174:659–64. doi: 10.1164/rccm.200604-520OC. 10.1164/rccm.200604‐520OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho ME, Kairalla RA, Capelozzi VL, Deheinzelin D, do Nascimento Saldiva PH, de Carvalho CR. Centrilobular fibrosis: a novel histological pattern of idiopathic interstitial pneumonia. Pathol Res Pract. 2002;198:577–83. doi: 10.1078/0344-0338-00305. 10.1078/0344‐0338‐00305 [DOI] [PubMed] [Google Scholar]
- 16.Medeiros‐Junior P, Carvalho CRR. Linfangioleiomiomatose pulmonar. J Bras Pneumol. 2004;30:66–7. [Google Scholar]
- 17.American Thoracic Society. Idiopathic Pulmonary Fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS) and European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 18.Bourke SJ, Dalphin JC, Boyd G, McSharry C, Baldwin CI, Calvert JE. Hypersensitivity pneumonitis: current concepts. Eur Respir J Suppl. 2001;32:81s–92s. [PubMed] [Google Scholar]
- 19.Lacasse Y, Selman M, Costabel U, Dalphin JC, Ando M, Morell F, et al. HP Study Group. Clinical diagnosis of hypersensitivity pneumonitis. Am J Respir Crit Care Med. 2003;168:952–8. doi: 10.1164/rccm.200301-137OC. 10.1164/rccm.200301‐137OC [DOI] [PubMed] [Google Scholar]
- 20.Gaude GS. Pulmonary manifestations of gastroesophageal reflux disease. Ann Thorac Med. 2009;4:115–23. doi: 10.4103/1817-1737.53347. 10.4103/1817‐1737.53347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurski RR, da Rosa AR, do Valle E, de Borba MA, Valiati AA. Extraesophageal manifestations of gastroesophageal reflux disease. J Bras Pneumol. 2006;32:150–60. doi: 10.1590/s1806-37132006000200011. [DOI] [PubMed] [Google Scholar]
- 22.Johnson SR. Lymphangioleiomyomatosis. Eur Respir J. 2006;27:1056–65. doi: 10.1183/09031936.06.00113303. [DOI] [PubMed] [Google Scholar]
- 23.Paciocco G, Martinez FJ, Bossone E, Pielsticker E, Gillespie B, Rubenfire M. Oxigen desaturation on the six‐minute walk test and mortality in untreated primary pulmonary hypertension. Eur Respir J. 2001;17:647–52. doi: 10.1183/09031936.01.17406470. 10.1183/09031936.01.17406470 [DOI] [PubMed] [Google Scholar]
- 24.Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33:397–406. doi: 10.1590/s1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- 25.Neder JA, Andreoni S, Castelo‐Filho A, Nery LE. Reference values for lung function tests. I. Static volumes. Braz J Med Biol Res. 1999;32:703–17. doi: 10.1590/s0100-879x1999000600006. [DOI] [PubMed] [Google Scholar]
- 26.Neder JA, Andreoni S, Peres C, Nery LE. Reference values for lung function tests. III. Carbon monoxide diffusing capacity (transfor factor) Braz J Med Biol Res. 1999;32:729–37. doi: 10.1590/s0100-879x1999000600008. [DOI] [PubMed] [Google Scholar]
- 27.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exercise. 1982;14:377–81. [PubMed] [Google Scholar]
- 28.Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie‐Leblond I, et al. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26:586–93. doi: 10.1183/09031936.05.00021005. 10.1183/09031936.05.00021005 [DOI] [PubMed] [Google Scholar]
- 29.Rogliani P, Mura M, Mattia P, Ferlosio A, Farinelli G, Mariotta S, et al. HRCT and histopathological evaluation of fibrosis and tissue destruction in IPF associated with pulmonary emphysema. Respir Med. 2008;102:1753–61. doi: 10.1016/j.rmed.2008.07.010. 10.1016/j.rmed.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 30.Chu SC, Horiba K, Usuki J, Avila NA, Chen CC, Travis WD, et al. Comprehensive evaluation of 35 patients with lymphangioleiomyomatosis. Chest. 1999;115:1041–52. doi: 10.1378/chest.115.4.1041. 10.1378/chest.115.4.1041 [DOI] [PubMed] [Google Scholar]