Abstract
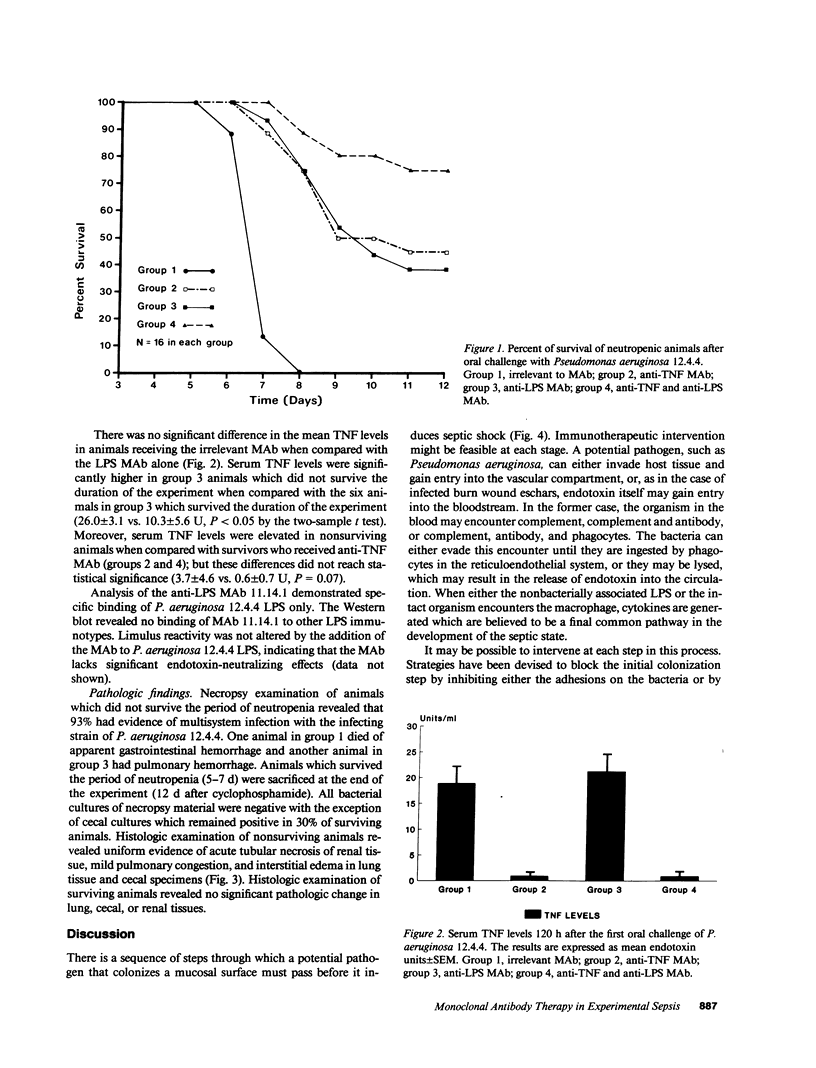
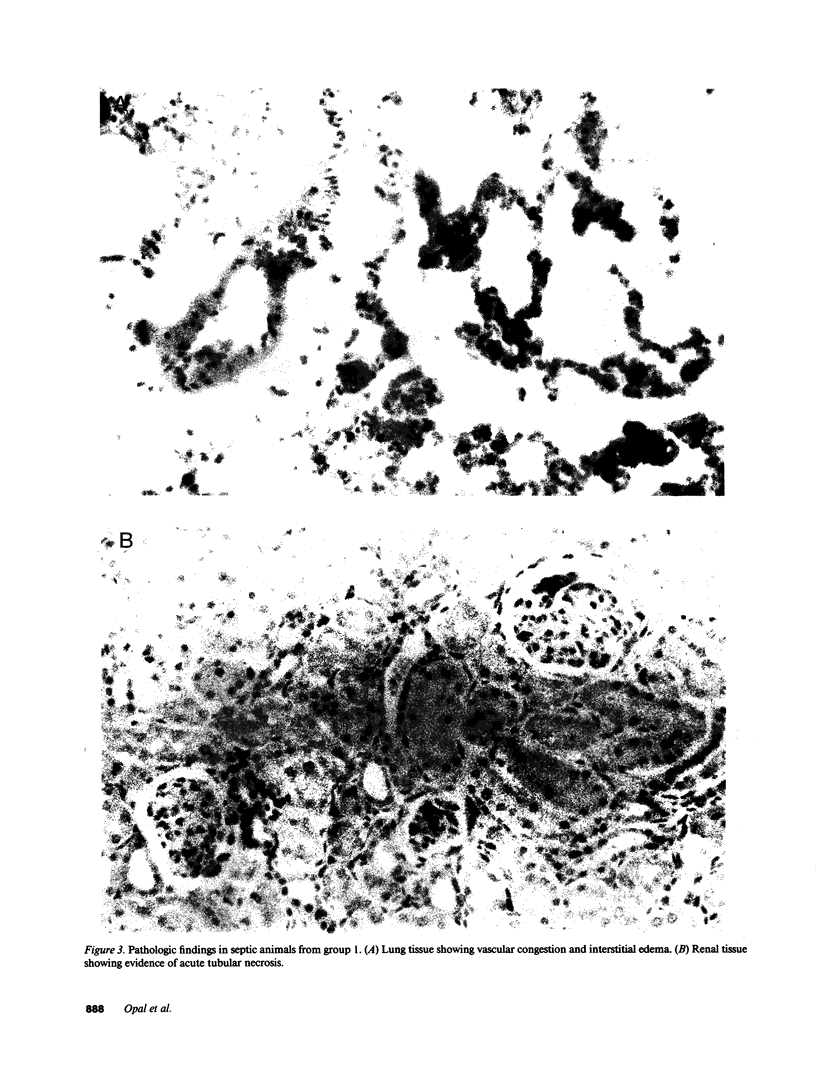
Monoclonal antibodies (MAb) directed against bacterial lipopolysaccharide (LPS) and tumor necrosis factor-alpha (TNF) provide partial protection in experimental models of septic shock. To determine if additional benefit accrues from a combination of anti-TNF and anti-LPS MAb in the treatment of septic shock, a neutropenic rat model was developed to study active infection with Pseudomonas aeruginosa 12.4.4. Animals were treated intravenously with an irrelevant MAb (group 1); anti-TNF MAb (group 2); MAb directed against P. aeruginosa 12.4.4 LPS (group 3); or a combination of anti-TNF and anti-LPS MAb (group 4). None of the control animals in group 1 survived the 7-d period of neutropenia (0/16). In contrast, the survival rate was 44% in group 2 (P less than 0.02); 37% in group 3 (P less than 0.05); and 75% in group 4 (P less than 0.0002). The combination of monoclonal antibodies provided greater protection than either MAb given alone (P less than 0.05). Serum TNF levels during infection were significantly greater in groups 1 and 3 (20.1 +/- 3.3 U, mean +/- SE) than in groups 2 and 4 (0.9 +/- 0.8 U, P less than 0.0001). These results indicate that a combination of monoclonal antibodies to LPS and TNF have additive benefit in experimental Pseudomonas aeruginosa sepsis. This immunotherapeutic approach may be of potential utility in the management of serious, gram-negative bacterial infection in neutropenic patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin: more than a tumor necrosis factor. N Engl J Med. 1987 Feb 12;316(7):379–385. doi: 10.1056/NEJM198702123160705. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Cannon J. G., Tompkins R. G., Gelfand J. A., Michie H. R., Stanford G. G., van der Meer J. W., Endres S., Lonnemann G., Corsetti J., Chernow B. Circulating interleukin-1 and tumor necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990 Jan;161(1):79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- Chia J. K., Pollack M., Guelde G., Koles N. L., Miller M., Evans M. E. Lipopolysaccharide (LPS)-reactive monoclonal antibodies fail to inhibit LPS-induced tumor necrosis factor secretion by mouse-derived macrophages. J Infect Dis. 1989 May;159(5):872–880. doi: 10.1093/infdis/159.5.872. [DOI] [PubMed] [Google Scholar]
- Collins H. H., Cross A. S., Dobek A., Opal S. M., McClain J. B., Sadoff J. C. Oral ciprofloxacin and a monoclonal antibody to lipopolysaccharide protect leukopenic rats from lethal infection with Pseudomonas aeruginosa. J Infect Dis. 1989 Jun;159(6):1073–1082. doi: 10.1093/infdis/159.6.1073. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Kelly N., Bernton E., Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med. 1989 Jun 1;169(6):2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley A. R., Cohen J., Buurman W., Owen R., Hanson G., Lumley J., Aulakh J. M., Bodmer M., Riddell A., Stephens S. Monoclonal antibody to TNF in severe septic shock. Lancet. 1990 May 26;335(8700):1275–1277. doi: 10.1016/0140-6736(90)91337-a. [DOI] [PubMed] [Google Scholar]
- Girardin E., Grau G. E., Dayer J. M., Roux-Lombard P., Lambert P. H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988 Aug 18;319(7):397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- Hesse D. G., Tracey K. J., Fong Y., Manogue K. R., Palladino M. A., Jr, Cerami A., Shires G. T., Lowry S. F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988 Feb;166(2):147–153. [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Männel D. N., Northoff H., Bauss F., Falk W. Tumor necrosis factor: a cytokine involved in toxic effects of endotoxin. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S602–S606. doi: 10.1093/clinids/9.supplement_5.s602. [DOI] [PubMed] [Google Scholar]
- Opal S. M., Cross A. S., Kelly N. M., Sadoff J. C., Bodmer M. W., Palardy J. E., Victor G. H. Efficacy of a monoclonal antibody directed against tumor necrosis factor in protecting neutropenic rats from lethal infection with Pseudomonas aeruginosa. J Infect Dis. 1990 Jun;161(6):1148–1152. doi: 10.1093/infdis/161.6.1148. [DOI] [PubMed] [Google Scholar]
- Sadoff J. C., Wright D. C., Futrovsky S., Sidberry H., Collins H., Kaufmann B. Characterization of mouse monoclonal antibodies directed against Pseudomonas aeruginosa lipopolysaccharides. Antibiot Chemother (1971) 1985;36:134–146. doi: 10.1159/000410478. [DOI] [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Shenep J. L., Flynn P. M., Barrett F. F., Stidham G. L., Westenkirchner D. F. Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J Infect Dis. 1988 Mar;157(3):565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- Silva A. T., Appelmelk B. J., Buurman W. A., Bayston K. F., Cohen J. Monoclonal antibody to endotoxin core protects mice from Escherichia coli sepsis by a mechanism independent of tumor necrosis factor and interleukin-6. J Infect Dis. 1990 Aug;162(2):454–459. doi: 10.1093/infdis/162.2.454. [DOI] [PubMed] [Google Scholar]
- Silva A. T., Bayston K. F., Cohen J. Prophylactic and therapeutic effects of a monoclonal antibody to tumor necrosis factor-alpha in experimental gram-negative shock. J Infect Dis. 1990 Aug;162(2):421–427. doi: 10.1093/infdis/162.2.421. [DOI] [PubMed] [Google Scholar]
- Teng N. N., Kaplan H. S., Hebert J. M., Moore C., Douglas H., Wunderlich A., Braude A. I. Protection against gram-negative bacteremia and endotoxemia with human monoclonal IgM antibodies. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1790–1794. doi: 10.1073/pnas.82.6.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Fahey T. J., 3rd, Albert J. D., Fong Y., Hesse D., Beutler B., Manogue K. R., Calvano S., Wei H. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., Fisher C. J., Jr, Sprung C. L., Straube R. C., Sadoff J. C., Foulke G. E., Wortel C. H., Fink M. P., Dellinger R. P., Teng N. N. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin. A randomized, double-blind, placebo-controlled trial. The HA-1A Sepsis Study Group. N Engl J Med. 1991 Feb 14;324(7):429–436. doi: 10.1056/NEJM199102143240701. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J., McCutchan J. A., Fierer J., Glauser M. P., Sadoff J. C., Douglas H., Braude A. I. Treatment of gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982 Nov 11;307(20):1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- Ziegler E. J. Protective antibody to endotoxin core: the emperor's new clothes? J Infect Dis. 1988 Aug;158(2):286–290. doi: 10.1093/infdis/158.2.286. [DOI] [PubMed] [Google Scholar]
- van Dijk W. C., Verbrugh H. A., van Erne-van der Tol M. E., Peters R., Verhoef J. Escherichia coli antibodies in opsonisation and protection against infection. J Med Microbiol. 1981 Nov;14(4):381–389. doi: 10.1099/00222615-14-4-381. [DOI] [PubMed] [Google Scholar]