Cyclosporine (CsA),* a cyclic undecapeptide of fungal origin, is a potent immunosuppressive drug used to prevent the rejection of transplanted kidneys, livers, and hearts in man. Elimination of the drug is primarily biliary; only about 6% of the dose appears in the urine.
The disposition and metabolism of CsA remains poorly understood. Absorption from the gastrointestinal tract is incomplete and variable, and it is possible that the drug undergoes enterohepatic recirculation. Venkataramanan et al. observed increases in CsA blood levels following T tube clamping in liver transplant patients (1), and Kahan and coworkers noted the appearance of the drug in the ileostomy drainage of a patient (2).
CsA blood levels of transplant patients are routinely monitored in our laboratory by radioimmunoassay (RIA) or high-performance liquid chromatography (HPLC) of whole blood. The RIA measures CsA and many of its metabolites, whereas HPLC specifically measures the parent compound. A modification of both methods for determination of CsA in bile samples has been developed in our laboratory, and it has proved to be reliable in terms of accuracy, precision, and sensitivity. The determination of CsA concentration in bile is of potential value in the research setting as a tool for CsA disposition studies.
Bile samples were collected from a T tube in the common bile duct of five patients who had recently undergone orthotopic liver transplantation. Blank bile for preparation of assay standards and controls was from a patient not receiving CsA. Samples were obtained at various times after a dose of CsA and were stored at −20°C. CsA was administered as an oral dose (800–1200 mg; n = 3) or as a combination of i.v. infusion and oral dose (100–140 mg i.v., 450–1200 mg p.o.; n = 2).
For RIA, CsA standards and controls were prepared using a stock solution of 2.5 mg CsA in 25 ml of HPLC-grade methanol. The flask was wrapped in foil and stored upright at −20 °C. Two sets of standards, 10, 20, 40, 69, and 100 μg/ml and 15, 40, 50, and 80 μg/wl, were prepared by pipetting the appropriate volumes of stock solution into glass flasks, evaporating the methanol under a stream of dry nitrogen, and adding 1.0 ml of blank bile. The flasks were covered with foil and stirred at room temperature for 1.5 hr. Each set of standards was diluted 50-fold to the final concentration with assay buffer A (0.05 M TRIZMA, pH 8.5), aliquoted into capped plastic tubes, and frozen until needed. Patient samples were diluted 50-fold with buffer A before analysis. One set of standards was used to obtain a standard curve and the other set to assess the accuracy of the measurement. The Sandoz RIA kit was used to measure CsA in samples according to the manufacturer's instructions.
HPLC bile standards and controls were prepared as above, but directly from the stock solution to yield the desired concentrations; no further dilution with buffer was performed. Patient samples were analyzed without dilution. HPLC determination of CsA was performed on a C18 reverse-phase Bondapack column, using a modification of the method of Sawchuk and Cartier (3). The column temperature was maintained at 70°C. Flow rate of the mobile phase, acetonitrile/methanol/distilled water (49/22/29 by volume), was 1.0 ml/min. Column effluent was monitored at 214 nm. Peak heights were measured electronically, and the ratio of CsA to the internal standard CsD was used to calculate drug concentration.
An index of CsA metabolite production was calculated using the concentrations obtained by RIA and HPLC by the following equation:
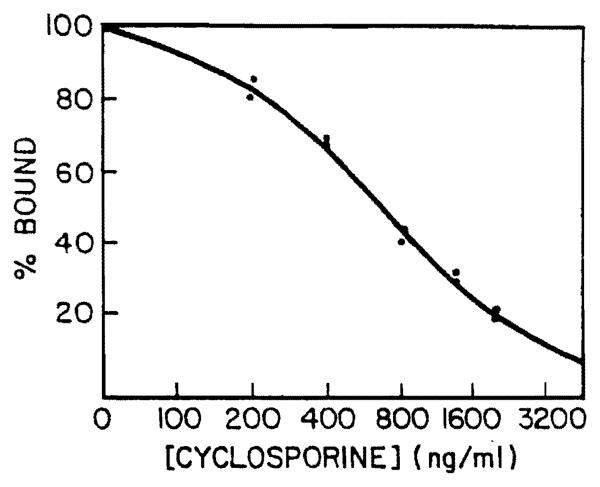
A typical linear/log RIA standard curve of CsA in bile is shown in Figure 1. The assay is linear between bile CsA concentrations of 0.2 and 2.0 μg/ml. Patient samples were assayed in duplicate, and the coefficient of variation for duplicates within the run was less than 10%. Precision of the assay in routine use was determined by analyzing 12 bile samples from five patients in duplicate daily for 10 days. The standard deviation of the difference of duplicate values was obtained for each set of determination, and from this data a coefficient of variation was calculated using standard statistical methods. Thus, the average between run variation was 11% with a range of 7%–14%. The highest variation was observed in a bile sample that contained pulpy debris. Four bile controls loaded with different concentrations of CsA were assayed in duplicate with each run of patient samples, and recovery of added CsA was 104±5.5% (Table 1).
Figure 1.
Linear/log RIA standard curve for determination of cyclosporine concentration in bile.
Table 1.
Recovery of CsA added to control bile
| CsA added (μg/ml) |
CsA measured (μg/ml) |
|
|---|---|---|
| RIA | HPLCa | |
| 0.3 | 0.29±0.048 | 0.29±0.019 |
| 0.8 | 0.85±0.079 | 0.81±0.075 |
| 1.0 | 1.1±0.15 | 0.96±0.082 |
| 1.6 | 1.7±0.13 | 1.7±0.13 |
n = 10 and 4, respectively, for RIA and HPLC for each concentration of CsA added to blank or control bile.
When CsA concentration was determined in bile using HPLC, the limit of sensitivity was 0.1 μg/ml, and recovery of CsA in bile controls at all levels of CsA averaged 95%. The coefficient of variation for bile standards assayed on consecutive days was approximately 10%. The volumes of patient bile samples received in the laboratory for assay were insufficient for repeat analyses by HPLC.
Ten bile samples from four patients were assayed by RIA and HPLC for CsA concentration, and the results are shown in Table 2. Considerably higher values were obtained using RIA because of the high level of CsA metabolites present in bile. The actual metabolite concentration may be much higher than these values indicate, since CsA metabolites only partially crossreact with the antibody used in this assay. In contrast, HPLC, which specifically measures the parent drug only, yielded values 8–48-fold lower than those obtained by RIA.
Table 2.
RIA and HPLC determinations of cyclosporine concentration in bile samples at various times after a dose
| Patient | Time after dose (hr) |
Cyclosporine (μg/ml) |
CMIb | |
|---|---|---|---|---|
| RIAa | HPLC | |||
| A | 4 | 97.7±12.8 | 6.69 | 0.93 |
| B | 1 | 36.8±5.50 | 2.64 | 0.93 |
| 4 | 47.9±7.95 | 4.63 | 0.90 | |
| 9 | 46.7±6.25 | 3.08 | 0.93 | |
| 11 | 39.9±5.10 | 2.55 | 0.94 | |
| 12 | 44.8±6.70 | 3.12 | 0.93 | |
| C | 5 | 29.6±3.85 | 0.714 | 0.98 |
| 7 | 28.5±3.50 | 0.708 | 0.98 | |
| 12 | 21.5±3.15 | 0.451 | 0.98 | |
| D | 2 | 15.6±3.15 | 1.85 | 0.88 |
Mean (n = 10) and standard deviation are shown for RIA results. Insufficient sample volume prevented more than one HPLC determination for each sample.
CMI-CsA metabolite index.
Table 2 also includes a CMI that appeared to be relatively constant in any given patient and fluctuated within a narrow range for all patients studied. The CMI was 0.98 for all three samples from patient C, even though the samples were collected at different times during the dosing interval. Similarly, patient B had an average CMI value of 0.93.
The introduction of CsA for immunosuppression has been recognized as one of the major breakthroughs in organ transplantation in recent years. A greater understanding of CsA disposition and metabolism in the human body may further enhance its usefulness. The question of relative amount of parent drug and metabolites at various dosages is an important one, especially if there is a saturation level above which the liver is unable to metabolize more drug in a single pass.
A previous publication from this laboratory presented data on CsA levels in human bile (1). The analytical methods have since been modified to further increase precision, sensitivity, and CsA recovery. The most significant improvement has been the use of dry CsA for preparation of the stock standard solution instead of the standard included in the Sandoz RIA kit, which had limited solubility in the blank bile such that CsA recovery from standards and controls was variable and unacceptably low. This, in turn, led to a variable underestimation of CsA concentration in bile. The present bile CsA method has a higu degree of precision, and recoveries are essentially 100%.
The present study shows that the levels of parent CsA in bile are quite low, and it is unlikely that bile CsA undergoes any significant enterohepatic recirculation. This is in general agreement with the results of Venkataramanan et al. (1), who found that less than 1% of the administered dose of CsA was excreted in bile. The question remains, however, whether CsA metabolites, which are present in bile in very high concentrations, are recycled to any significant extent. This is important because if any of these metabolites possess immunosuppressive properties, recycling may prolong the pharmacologic action of the drug. If, on the other hand, products of CsA metabolism are found to be toxic, their prolonged presence in the body could be harmful.
In this connection, the CMI could be a useful estimate of the level of CsA metabolites present in a sample. In blood, RIA determinations of CsA are two to three times higher than by HPLC (1), and the calculated CMI is found to be between 0.5 and 0.7. In contrast, the CMI for bile samples is considerably higher, around 0.93, consistent with the results of Venkataramanan and coworkers (1), from whose data a CMI value of approximately 0.97 may be calculated. This may be expected as the metabolism of CsA is primarily hepatic. Unexpected was the finding that the CMI values were virtually identical in a given patient, regardless of the time elapsed since the last dose of CsA (Table 2). These data imply that the hepatic breakdown of the drug is fairly constant throughout the dosing interval for the patients in this study.
This method can be of value in the research setting for studies involving the disposition and metabolism of CsA. The results of RIA and HPLC CsA determinations in bile can be compared to estimate the fraction of total dose being excreted through the bile, as well as the amount of parent drug that is leaving the body unmetabolized.
Acknowledgments
We thank Linda Markivich, Diane Phillips, and Toni Campbell for their excellent technical assistance.
Footnotes
Abbreviations used: CMI, cyclosporine metabolite index; CsA, cyclosporine; HPLC, high-performance liquid chromatopaphy; RIA, radioinimunoassay.
REFERENCES
- 1.Venkataramanan R, Starzl TE, Yang S, Burckart GJ, Ptachcinski RJ, Shaw BW, Iwatsuki S, Van Thiel DH, Sanghvi A, Seltman H. Biliary excretion of cyclosporine in liver transplant patients. Transplant Proc. in press. [PMC free article] [PubMed] [Google Scholar]
- 2.Kahan BD, Ried M, Newburger J. Pharmacokinetics of cyclosporine in human renal transplantation. Transplant Proc. 1983;15:446. [Google Scholar]
- 3.Sawchuck RJ, Cartier LL. Liquid chromatographic determination of cyclosporine A in blood and plasma. Clin Chem. 1981;27:1368. [PubMed] [Google Scholar]