Abstract
Between February 26, 1981, and July 30, 1987, 36 patients underwent orthotopic liver transplantation for primary sclerosing cholangitis associated with ulcerative colitis. Three of the 36 recipients died within 3 mo because of graft nonfunction or surgical complications. The other 33 (92%) lived for at least 1 yr. Two of the 33 died after 12 and 14 mo, respectively, of recurrent cholangiocarcinoma that was not diagnosed before transplantation. Four other patients died of recurrent liver failure (three cases) or immunoblastic sarcoma (one case) after 14, 21, 36 and 44 mo. Twenty-seven (75%) of the patients are still alive 23 to 81 mo after transplantation. Two patients have been diagnosed as having colorectal cancer 11 and 21 mo respectively, after transplantation, for an overall incidence of 5.6% (2 of 36) and a corrected incidence of 6.5% (2 of 31) if the three early deaths and two later deaths caused by cholangio-carcinomas are excluded. It is not known whether colorectal malignancies were present but undetected at the time of transplantation or whether they developed afterward. It is clear that patients who undergo liver transplantation for primary sclerosing cholangitis associated with ulcerative colitis should have careful follow-up of the colon, including colonoscopy and multiple biopsies of the colorectal mucosa. Whether proctocolectomy should be considered prophylactically after liver transplantation is an unresolved issue.
Primary sclerosing cholangitis (PSC) is an uncommon liver disease characterized by chronic inflammation and scarring of the intrahepatic or extrahepatic biliary tree, which leads to the development of secondary biliary cirrhosis (1-3). Orthotopic liver transplantation (OLT) is the only curative treatment (4). PSC is often associated with ulcerative colitis (UC) (1-3), which is known to carry an increased risk of colorectal cancer, especially when UC duration exceeds 10 yr (5-9). We report two patients who were diagnosed as having colorectal cancer after OLT for PSC associated with UC, and we review our experience with OLT for PSC accompanied by UC.
PATIENTS AND METHODS
Between February 26, 1981, and July 30, 1987, 580 adult primary OLTs were performed at the University Health Center of Pittsburgh. The indication for transplantation in 74 (12.8%) of these patients was PSC. UC also was diagnosed in 43 (58.1%) of the 74 recipients. Concomitant Crohn's disease was present in 6 (8.1%) of the 74 patients. Patients were considered to have primary sclerosing cholangitis when there were typical cholangiographical findings of the disease, pathological confirmation of the disease from the total hep-atectomy specimen or the absence of a history or pathological evidence consistent with secondary biliary cirrhosis from other causes (4).
Of the 43 patients who had PSC associated with UC, 7 were excluded from the study because of previous right colectomy for a Duke's class B carcinoma (one patient), total colectomy for UC (three patients) and proctocolectomy for UC (three patients). The ages of the 36 recipients without previous colorectal surgery ranged from 22 to 63 yr (mean = 41 yr); 26 (72.2%) were men. UC duration before OLT was 3 to 30 yr (mean = 14 yr). PSC had been diagnosed 2 to 14 yr (mean = 6 yr) before transplantation. UC preceded PSC in all but two patients.
Three patients who died within 3 mo of transplantation from graft nonfunction or technical complications were excluded because meaningful colon studies were precluded. Two patients with previously undiagnosed cholangiocarcinomas in their native livers were also excluded. Transplantation was done successfully in these patients, but they died of tumor recurrence 12 and 14 mo later. Excluding these five cases, 31 (86%) of the 36 recipients were eligible for long-term study. Four of the 36 died of recurrent liver failure (three patients) or immunoblastic sarcoma (one patient) after 14, 21, 36 and 44 mo. Twenty-seven (75%) of the 36 are still alive after 23 to 81 mo. The mean follow-up in the 31 patients admitted into the study group, including the four who died later, was 45 mo (Table 1).
Table 1.
Ulcerative colitis in 31 patients who developed colorectal cancer after liver transplantation for sclerosing cholangitis
| Duration of UC (onset to OLT in yr) |
Follow-upa after OLT (in mo) |
No. patients with cancer/total |
|---|---|---|
| <10 | 38 ± 14 (21-58) |
0/10 (0%) |
| 10-20 | 46 ± 21 (14-79) |
1/14 (7.1%) |
| >20 | 45 ± 9 (32-54) |
1/7 (14.3%) |
| total | 43 ± 17 | 2/31 (6.5%) |
UC = ulcerative colitis; OLT = orthotopic liver transplantation.
Mean ± S.D. and range ().
Surgical techniques used during OLT were not influenced by the UC. During the time of case accrual, donor livers were preserved with Euro-Collins solution. Immunosuppression was done with cyclosporine and azathioprine, to which OKT3 monoclonal antibody and/or azathioprine was added in a few cases (10).
RESULTS
Two (5.6%) of the 36 patients with PSC and UC were diagnosed as having colorectal cancer 11 and 21 mo after OLT, respectively. Without the five patients who were excluded because of early death or recurrent cholangiocarcinoma, the corrected incidence of colorectal cancer was 2 (6.5%) of 31. The two patients with colorectal cancer both had histories of UC for 17 and 21 yr, respectively. There were no colorectal cancers in patients whose UC had been present for less than 10 yr (Table 1).
Case Reports
Case 1
A 48-yr-old white man with a 21-yr history of UC and PSC of 4 yr underwent OLT on August 19, 1986. Colonoscopy in November 1985 showed no evidence of ulceration, stricture or neoplasm in the colon or rectum. Multiple biopsy samples did not show malignancy. The course after transplantation was complicated by acute cellular rejection, which required OKT3 monoclonal antilymphocyte globulin treatment, and by the transmission of human immunodeficiency virus from the donor organ (11).
In February 1988 the patient noted constipation and incomplete defecation. Manual examination in May 1988 revealed a rectal mass, and a barium-enema roentgenogram demonstrated circumferential stricture of the rectum. He underwent total proctocolectomy with end ileostomy on May 10, 1988. There was no evidence at surgery of regional or distant metastases. Macroscopically, a 6-cm concentric stricture involved the rectum from the dentate line upward 2 cm. Microscopically, sections from the stricture site demonstrated infiltrating, poorly differentiated adenocarcinoma with a marked lymphocytic inflammatory cell infiltrate. Cancer cells extended into the perirectal adipose tissue as well as nearby lymph nodes (Duke's class C). The patient's postoperative course was uneventful, and he has no evidence of recurrence 14 mo later.
Case 2
A 44-yr-old white woman with a 17-yr history of UC and PSC of 6 yr underwent uneventful OLT on July 28, 1986. Pretransplant colonoscopy on April 24, 1986, was normal except for colonic mucosal edema. Multiple biopsy samples did not show malignancy. The patient's course after transplantation was unremarkable except for an episode of easily reversible acute cellular rejection.
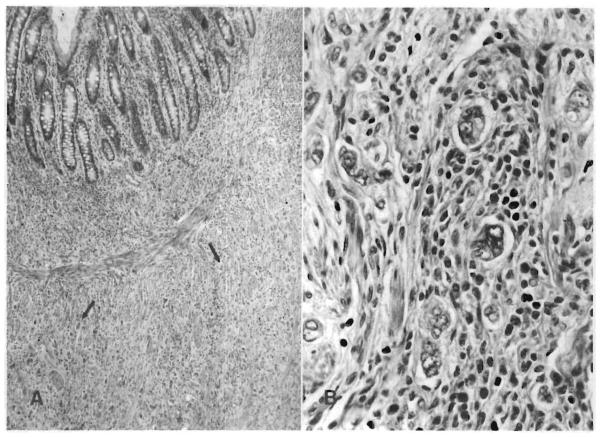
On July 1, 1987, she underwent colonoscopy for bloody diarrhea of 2 mo duration. Multiple colonic biopsy samples obtained at that time revealed severe dysplasia of the entire colon, for which total proctocolectomy with ileostomy was performed 11 mo after transplant. The surgical specimen contained an indurated ulcer measuring 3.0 × 2.5 cm in the midsection of the transverse colon. The lesion was a well-differentiated adenocarcinoma with a prominent inflammatory cell infiltrate that extended through the colonic wall (Duke's class B) (Fig. 1). The patient remains well without evidence of recurrence 24 mo after proctocolectomy.
Fig. 1.
(A) Section from proximal portion of tumor in Patient 2. Well-differentiated tubular adenocarcinoma extends into submucosa (arrows) (H & E, × 25). (B) High-power view of (A). Note prominent inflammatory cell infiltrate, predominant by lymphocytes and plasma cells (H & E, × 100).
DISCUSSION
Risk factors for the development of colorectal carcinoma in patients with UC include prolonged duration or early onset of UC and extensive involvement along with severe dysplasia of the colonic mucosa (5-9). The incidence of colorectal cancer after 18 to 25 yr of UC has been reported to be from 1.4% to 34% (7-9). Our two patients are the first examples, to our knowledge, of this complication after OLT. Whether transplantation contributed to or accelerated the malignant process is open to speculation.
It has been well documented that immunosuppression after transplantation leads to the development of certain forms of cancer (12, 13). Kidney transplant recipients treated with azathioprine and prednisone have a higher than normal incidence of lymphoma, Kaposi's sarcoma and a variety of epithelial tumors, probably including colorectal carcinoma (12, 13). However, under immunosuppression with cyclosporine, incidence of carcinomas of the colon, rectum, lung, prostate and female breast has not increased much—if at all (14)—although the risk of lymphoma and sarcoma remains high.
In our two patients, pretransplant colorectal examinations, including colonoscopy and biopsies, revealed no evidence of neoplasm or dysplasia. These studies were done 9 and 3 mo before transplantation. Unfortunately, they were not repeated. Because the intervals between OLT and the development of colorectal cancer were only 11 and 21 mo in the two patients, respectively, it is tempting to speculate that malignant transformation was stimulated by immunosuppression or other conditions of transplantation. However, there is no proof of this, and it is possible that the lesions were missed in the preoperative evaluation or developed between the examinations and the transplantation. Cancers arising in the colon or rectum involved with UC tend to be flat and to exhibit infiltrating growth. These qualities make it difficult, as in case 2, to establish the early diagnosis of colorectal cancer with the use of colonoscopy alone (6). Our patients had UC for 17 and 21 yr, respectively, increasing the risk of malignant degeneration.
It is clear that the changes caused by UC should be regarded as precancerous. Early detection of colorectal cancer in patients who have undergone OLT for PSC associated with UC should be attempted using semiannual colonoscopy with multiple mucosal biopsies and double-contrast barium enema (15). Our patients were an object lesson in that failure to be assiduous led to a delay in diagnosis. Whether proctocolectomy should be considered prophylactically after liver transplantation is an unresolved issue.
Acknowledgments
Supported by research grants from the Veterans Administration and project grant DK 29961 from the National Institutes of Health, Bethesda, Maryland.
REFERENCES
- 1.Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200–206. [PubMed] [Google Scholar]
- 2.Thompson HH, Pitt HA, Tompkins RK, Longmire WP. Primary sclerosing cholangitis: a heterogenous disease. Ann Surg. 1982;196:127–136. doi: 10.1097/00000658-198208000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaRusso NF, Wiesner RH, Ludwig J, MacCarty RL. Current concepts: primary sclerosing cholangitis. N Engl J Med. 1984;310:899–903. doi: 10.1056/NEJM198404053101407. [DOI] [PubMed] [Google Scholar]
- 4.Marsh JW, Iwatsuki S, Makowka L, Esquivel CO, Gordon RD, Todo S, Tzakis A, et al. Orthotopic liver transplantation for primary sclerosing cholangitis. Ann Surg. 1988;207:21–25. doi: 10.1097/00000658-198801000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennard-Jones JE, Morson BC, Ritchie JK, Shove DC, Williams CB. Cancer in colitis: assessment of the individual risk by clinical and histological criteria. Gastroenterology. 1977;73:1280–1289. [PubMed] [Google Scholar]
- 6.Collins RH, Feldman M, Fordtran JS. Colon cancer, dysplasia, and surveillance in patients with ulcerative colitis: a critical review. N Engl J Med. 1987;316:1654–1658. doi: 10.1056/NEJM198706253162609. [DOI] [PubMed] [Google Scholar]
- 7.Hendriksen C, Kreiner S, Binder V. Long term prognosis in ulcerative colitis—based on results from a regional patient group from the county of Copenhagen. Gut. 1985;26:158–163. doi: 10.1136/gut.26.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzka I, Brody RS, Morris E, Katz S. Assessment of colorectal cancer risk in patients with ulcerative colitis: experience from a private practice. Gastroenterology. 1983;85:22–29. [PubMed] [Google Scholar]
- 9.Kewenter J, Ahlman H, Hulten L. Cancer risk in extensive ulcerative colitis. Ann Surg. 1978;188:824–828. doi: 10.1097/00000658-197812000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Iwatsuki S, Shaw BW, Jr, Gordon RD, Esquivel CO. Immunosuppression and other nonsurgical factors in the improved results of liver transplantation. Semin Liver Dis. 1985;5:334–343. doi: 10.1055/s-2008-1040630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ward JW, Schable C, Dickinson GM, Makowka L, Yanaga K, Caruana R, Chan H, et al. Acute human immunodeficiency virus infection: antigen defection and seroconversion in immunosuppressed patients. Transplantation. 1989;47:722–724. doi: 10.1097/00007890-198904000-00030. [DOI] [PubMed] [Google Scholar]
- 12.Starzl TE, Penn I, Putnam CW, Groth CG, Halgrimson CG. Iatrogenic alterations of immunologic surveillance in man and their influence on malignancy. Transplant Rev. 1971;7:112–145. doi: 10.1111/j.1600-065x.1971.tb00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penn I. Cancer is a complication of severe immunosuppression. Surg Gynecol Obstet. 1986;162:603–610. [PubMed] [Google Scholar]
- 14.Penn I. Cancers following cyclosporine therapy. Transplantation. 1987;43:32–35. doi: 10.1097/00007890-198701000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Lennard-Jones JE, Morson BC, Ritchie JK, Williams CB. Cancer surveillance in ulcerative colitis: experience over 15 years. Lancet. 1983;2:149–152. doi: 10.1016/s0140-6736(83)90129-0. [DOI] [PubMed] [Google Scholar]