Abstract
The outcome of hepatitis C virus (HCV) Infection on patient and gratt survival after orthotopic liver transplantation (OLT) has been controversial. An earlier experience with a higher dose of tacrolimus (≥0.1 mg/kg/d intravenously and ≥0.2 mg/kg/d orally) was associated with a worse clinical outcome in patients infected with HCV. The clinical outcome of 183 liver transplant recipients with end-stage liver disease (ESLD) secondary to HCV infection (HCV group) was compared with a contemporary cohort of 556 patients with HCV infection who underwent transplantation for nonviral, nonmalignant ESLD (control group). All patients were prospectively screened for anti-HCV antibodies and HCV RNA by reverse-transcriptase polymerase chain reaction. All OLT patients were receiving low-dose tacrolimus immunosuppression. Cumulative patient survival rates for the HCV group were 80% after 1 year and 75% after 3 years compared with rates of 84% and 78%, respectively, in the control group (P = .452). Primary graft survival rates at the same time intervals for the HCV group and the control group were 72% and 77.5% at 1 year and 67% and 72% at 3 years, respectively (P = .144). The incidence of re-transplantation (re-OLT) in the HCV group and the control group was 12.6% and 10.4%, respectively (P = .42). Chronic HCV infection as an indication for OLT with a lower dose of tacrolimus immunosuppression (≤ 0.05 mg/kg/d intravenously and ≤0.1 mg/kg/d orally) is associated with a similar patient and graft survival as those without HCV infection.
Patients with end-stage liver disease (ESLD) associated with hepatitis C virus (HCV) infection account for approximately 25% of those awaiting orthotopic liver transplantation (OLT) in the United States. With improvements in diagnostic techniques to detect HCV, it has been observed that recurrent HCV infection after OLT is almost universa1.1,2 Feray et al3 showed a high degree of molecular homology between pretransplantation and post-transplantation viral isolates, confirming that the same strain of HCV is responsible for recurrent viral infection. Furthermore, Chazouilleres et al4 have shown that the levels of serum HCV RNA increased significantly after OLT, and this is probably caused by the presence of immunosuppression in the postoperative period.
The recurrence of hepatitis B virus (HBV) infection among transplant recipients has been associated with decreased patient and graft survival5; these results have recently improved with long-term prophylactic strategies,6,7 and novel antiviral agents may continue to improve the outcome of these patients. By contrast, the outcome of patients who have undergone OLT for ESLD associated with HCV infection has not been well defined. Recent reports have shown that long-term graft and patient survival are similar between patients with and without HCV infection,8-10 although these findings may change when patients are followed up for more than 5 years after OLT.
We have examined, in a retrospective cohort study, the clinical outcome of HCV infection defined by the incidence of re-transplantation (re-OLT), incidence and timing of acute cellular rejection, graft and patient survival, causes of graft failure, and death among patients infected with HCV who underwent OLT for ESLD using a low-dose tacrolimus immunosuppressant.
Materials and Methods
Between August 1991 and December 1995, 1041 adult patients (aged ≥18 years) underwent primary OLT at the University of Pittsburgh Medical Center (Pittsburgh, PA) and received tacrolimus and steroids as immunosuppression treatment after transplantation. The initial dose of tacrolimus was 0.05 mg/kg/d or less intravenously and 0.1 mg/kg/d or less orally. All OLT patients were prospectively screened for HCV infection.
HCV Diagnostic Testing
Serology
Plasma samples obtained before June 1992 were assayed for HCV antibodies using a first-generation (C100-3) enzyme immunoassay (EIA) (EIA-1; Abbot Laboratories, Abbot Park, IL). From June 1992 to December 1992, specimens were processed by using both a second-generation EIA (C100-3, HC-31, and HC-34: Abbot Laboratories) and a second-generation recombinant immunoblot assay (RIBA II; Chiron Corporation, Emeryville, CA).
Reverse-transcription polymerase chain reaction
Since January 1993, detection of HCV RNA in plasma was performed by reverse-transcription polymerase chain reaction using liquid hybridization detection.1,11
Pathology
All explanted livers from patients with a positive serological screen for HCV were routinely examined, and a diagnosis consistent with HCV-associated cirrhosis was verified histopathologically.
Patients
Of the 1041 OLTs performed during the study period, 183 (18%) were performed for HCV-associated ESLD. This group included 46 patients with a history of alcohol abuse confirmed by psychiatric evaluation; these patients fulfilled a period of 6 months of sobriety before transplantation. The HCV group was compared with a contemporary cohort of 556 patients (control group) who underwent transplantation for ESLD that was not associated with either hepatobiliary malignancy or viral causes, including HCV infection.
Donor and recipient characteristics are listed in Table 1. The two groups were similar with respect to donor age and sex, cold ischemia time, and United Network for Organ Sharing (UNOS) status. There was a predominance of men and younger patients in the HCV group compared with the control group.
Table 1.
Donor and Recipient Characteristics
| HCV Group (n = 183) | Control Group (n = 556) | P | |
|---|---|---|---|
| Donor age (yr) | 37 ± 16 | 38 ± 17 | .325 |
| Donor sex M/F (%) | 59/41 | 63/37 | .292 |
| Ischemia time (hr) | 14 ± 4 | 14 ± 5 | .673 |
| Recipient age (yr) | 50 ± 9 | 52 ± 12 | .013 |
| Recipient sex M/F (%) | 74/26 | 56/44 | .0001 |
| UNOS 1+2 status (%) | 73 | 75 | .558 |
| Mean follow-up (mo) | 41 ± 14 | 39 ± 15 | .258 |
The histopathologic diagnosis of acute rejection required the presence of a predominantly mononuclear, but mixed portal and/or perivenular inflammatory infiltrate, with inflammatory infiltration and damage of a majority of the bile ducts and subendothelial/perivenular inflammation of the portal vein branches and/or terminal hepatic venules. Chronic rejection was identified by the presence of biliary epithelial cell atrophy/pyknosis in a majority of bile ducts, with or without varying degrees of ductopenia, in a patient in whom biliary tract strictures have been excluded. These criteria were more stringently used in liver allografts with HCV infection.
Statistical Analysis
Continuous variables are presented as the mean ± standard deviation, and categorical variables as percentages.
The standard two-sample t test was used to test differences between group means, whereas differences in proportions were tested by Pearson's chi-squared test or by Fisher's exact test, if expected frequencies were less than 5.
Patient survival was calculated from the time of OLT until death, and primary graft survival from the time of OLT until the first re-OLT or death with primary graft. Survival curves were generated using the Kaplan-Meier (product-limit) method12 and were compared by the log-rank (Mantel-Cox) test.13 The cumulative risk for the first re-OLT was computed using the Kaplan-Meier method. Risk estimates were calculated as 1 – s(t) where s(t) = the cumulative probability of being re-OLT free at time t. The same methodology was used to compute the cumulative risk for acute rejection. There were 24 patients, however, who were excluded because they either died or underwent re-OLT within 2 days of OLT. Five patients belonged to the HCV group and 19 to the control group; 8 patients died of primary graft failure and 16 required re-OLT because of primary graft failure (9 patients) and technical problems (7 patients). Cox's proportional hazards model14 was used to compute the relative risk (RR) for failure of the primary graft, RR for mortality for the HCV group, and 95% confidence intervals (CIs). Multivariate Cox regression was used to adjust the RR for UNOS status at the time of OLT, recipient sex, and year of OLT. Cox's model was also used to adjust the RR for donor sex, recipient and donor age, and cold ischemia time. For analysis of overall mortality, time to re-OLT was incorporated into the multivariate model as a time-dependent covariant.
Results
Incidence of Re-OLT and Causes of Graft Failure
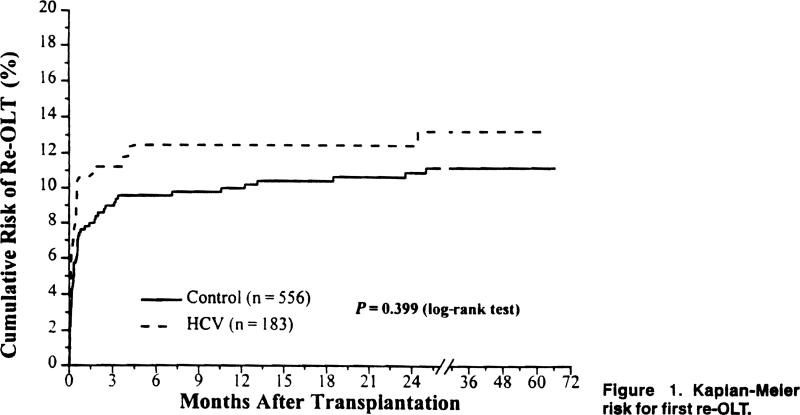
The overall incidence of re-OLT was similar in both groups. Twenty-three patients (12.5%) in the HCV group required re-OLT versus 58 patients (10.4%) in the control group (P = .422). The cumulative risk for re-OLT over time for both groups is shown in Figure 1.
Figure 1.
Kaplan-Meier risk for first re-OLT.
The indications for first re-OLT in the HCV and control groups are shown in Table 2. The most common indication for both groups was primary nonfunction (7.2%), followed by technical complications (2.4%). The incidence of acute and chronic rejection was similar in both study groups:
Table 2.
Indications and Incidence for First Re-OLT
| Indications | HCV Group (%) | Control Group (%) | P |
|---|---|---|---|
| PNF | 16 (8.7) | 38 (6.8) | |
| Technical | 5 (2.7) | 12 (2.2) | |
| ACR | 0 | 1 (0.2) | |
| Chronic R | 2 (1.1) | 5 (0.9) | |
| Others | 0 | 2 (0.4) | |
| Total | 23/183 (12.6) | 58/556 (10.4) | .422 |
Abbreviations: R, rejection; PNF, primary nonfunction.
Incidence and Timing of Acute Cellular and Chronic Rejection
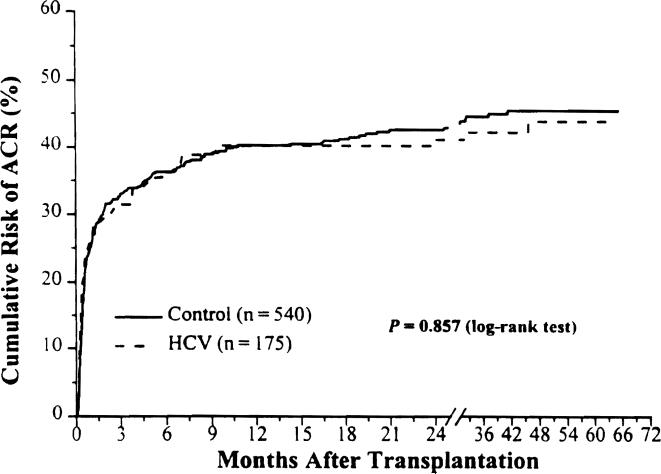
The cumulative risk for acute cellular rejection (ACR) over time for both groups is shown in Figure 2. There was no difference in the incidence and timing of ACR in both study groups (Table 3). The overall incidence of ACR was 36.6% in the HCV group compared with 39.6% in the control group. The median time to the first rejection episode and the incidence of multiple episodes of ACR were also similar in both groups (Table 3).
Figure 2.
Kaplan-Meier risk for acute rejection.
Table 3.
Incidence and Timing of ACR
| HCV Group (n = 175)* | Control Group (n = 540)* | P | |
|---|---|---|---|
| Patients with ≥1 episode ACR (%) | 64 (36.6) | 214 (39.6) | .471 |
| Cumulative risk for ACR at 1 year | 39.5 ± 3.9 | 40 ± 2.2 | .857 |
| Median time (d) to first episode (range) | 13 (2-1390) | 18(2-1237) | .088 |
| Multiple episodes (%) | |||
| 2 | 9 (14.1) | 48 (22.4) | |
| 3 | 4 (6.3) | 11 (5.1) | |
| 4 | 1 (1.6) | 2 (0.9) | |
| 5 | 1 (1.6) | 0 |
Patients who died or underwent re-OlT within 2 days post-OlT were excluded.
The cumulative incidence of chronic rejection at the end of the first year was 4.6% in the control group and 6.3% in the HCV group. At the end of the fifth year, the cumulative incidence was 9.6% and 13.2%, respectively. These differences were not statistically Significant.
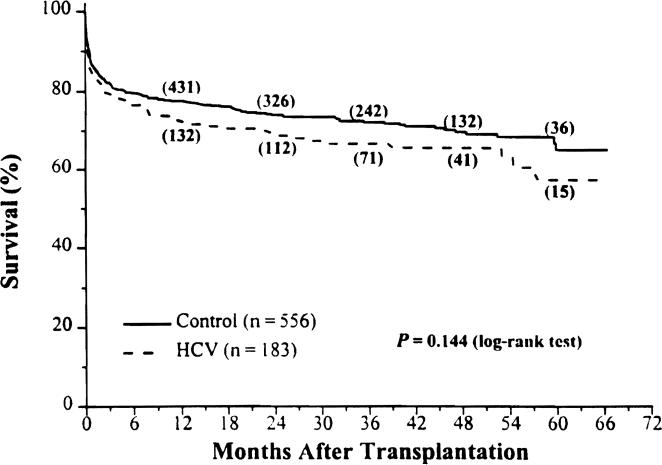
The Kaplan-Meier primary graft survival for the HCV and control groups were 72% and 78%. 67% and 72%, and 57% and 65% at 1, 3, and 5 years after OLT, respectively (Fig. 3; P = .144).
Figure 3.
Kaplan-Meier primary graft survival from August 18, 1991, to December 31, 1995. The numbers in parentheses are the number of patients remaining at risk.
Patient Survival
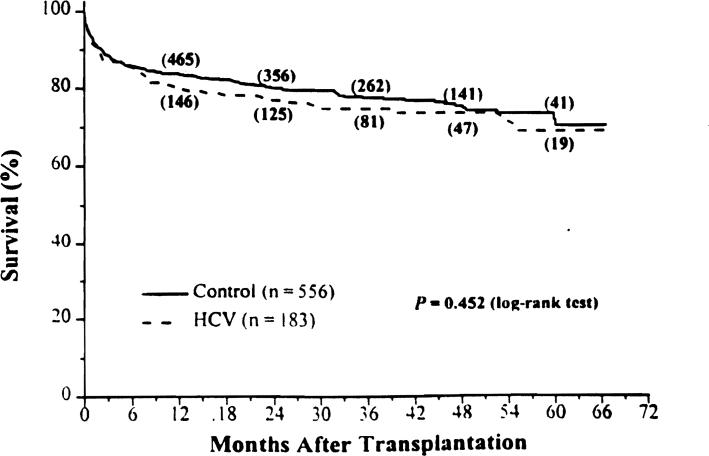
The Kaplan-Meier survival rates for the HCV and control groups were 80% and 84%. 75% and 78%, and 69% and 70% at 1, 3, and 5 years after OLT, respectively (Fig. 4; P = .452).
Figure 4.
Kaplan-Meier patient survival from August 18, 1991, to December 31, 1995. The numbers in parentheses are the number of patients remaining at risk.
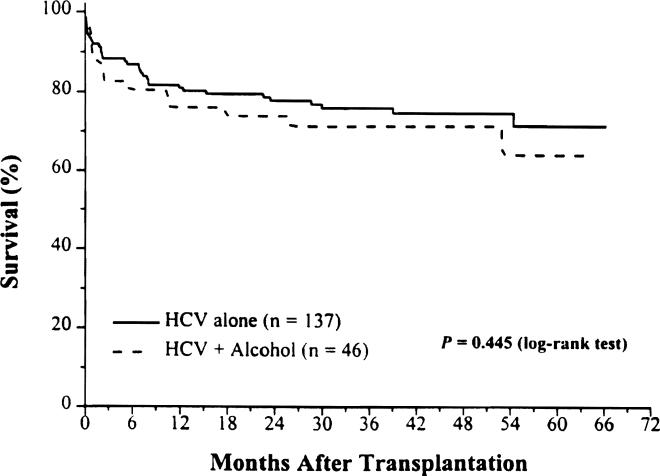
Forty-six of 183 patients in the study group also had a history of alcohol abuse, and they were diagnosed to have ESLD caused by both HCV infection and alcohol. When we compared patient survival rates between these two subsets of patients, HCV alone (n = 137) versus HCV plus alcohol (n = 46). there was no significant difference (adjusted RR = 1.13; 95% CI. 0.59 to 2.14). Survival rates were 81% and 76%, 78% and 74%, and 72% and 64% at 1, 3, and 5 years after OLT among the groups with HCV alone and HCV plus alcohol, respectively (Fig. 5; P = .445).
Figure 5.
Kaplan-Meier patient survival from August 18, 1991, to December 31, 1995.
Causes of Patient Death
The overall mortality was similar in both groups. Forty-eight patients in the HCV group (26%) and 128 patients in the control group (23%) died during the period of observation. Infection caused by bacteria and fungus were the leading causes of death in both groups (Table 4). Other causes of death included cardiac failure, multisystem organ failure, and post-transplant lymphoproliferative disorders and were Similarly distributed in both groups.
Table 4.
Primary Causes of Death After OLT
| Causes | HCV Group (%) | Control Group (%) |
|---|---|---|
| Bacterial sepsis | 20 (42) | 48 (38) |
| Fungal infections | 6 (12) | 6 (5) |
| Cardiac failure | 4 (8) | 14 (11) |
| Multisystem failure | 11 (23) | 19 (15) |
| PTLD | 1 (2) | 2 (2) |
| Intraoperative | 1 (2) | 7 (5) |
| Others/unknown | 5 (10) | 32 (25) |
| Total | 48 (100) | 128 (100) |
Abbreviation: PTLD, post-transplant lymphoproliferative disorders.
Multivariate Analyses
The comparison of the HCV group with the control group with respect to patient and graft survival was adjusted for selected baseline characteristics (see Methods) using Cox's proportional hazards model. The adjusted RR for mortality for HCV patients was 1.11 (95% CI. 0.78 to 1.60) and the RR for primary graft failure was 1.28 (95% CI. 0.93 to 1.75).
Discussion
OLT for ESLD caused by HCV infection was associated with a similar patient and graft survival when compared with those patients without HCV infection. The observed patient and graft survival rates among HCV-infected patients were acceptable despite almost universal recurrent infection: therefore, these patients should be considered for OLT as a therapeutic option.
Our earlier experience with HCV-infected patients with a higher dose of tacrolimus (≥0.1 mg/kg/d intravenously and ≥0.2 mg/kg/d orally) was associated with a worse clinical outcome when compared with a control group consisting of patients with nonviral, nonmalignant disease.15 This observation raised concerns that tacrolimus-based immunosuppression might have a deleterious impact on HCV-infected patients. However, under the current dosing of tacrolimus (≤0.05 mg/kg/d intravenously and ≤0.1 mg/kg/d orally), the observed patient and graft survival were similar to those achieved in the control group, as reported by other studies.8-10
It was not surprising that approximately 25% of the patients with chronic HCV in this study also had a history of heavy alcohol intake. This association has been established previously and these patients were more likely to have a severe histopathologic picture than HCV alone.16,17 This has been attributed to a synergistic effect between HCV and alcohol. More recently, Oshita et al18 have reported that alcoholic patients infected with HCV have increased serum HCV RNA levels, and this was interpreted as reflecting an impaired cellular immunity associated with alcohol intake. In our series, the outcome after OLT of HCV-infected patients with a history of alcoholism was similar to those with HCV infection alone, which would indicate that the negative effect of this combination may disappear after OLT. This could be caused by the lack of additional hepatic injury from alcohol in a population that is more likely to be abstinent after transplantation.19 Because of the retrospective nature of this study, the data on alcohol recidivism was not complete; therefore, an analysis on the impact of recidivism in the natural history of HCV infection after liver transplantation could not be optimally performed. It would be worthwhile to investigate this issue further among patients with HCV who continue or resume alcohol use and to determine whether this synergistic effect continues to be observed after OLT.
None of our transplant recipients infected with HCV presented, after OLT, an accelerated clinical course characterized by cholestasis and hepatic failure. This clinical course has been reported among immunosuppressed patients. It was initially reported in a heart transplant recipient who had been infected with HCV.20 Schluger et al21 have recently reported this unique clinical course in 10 of 135 patients who underwent transplantation for HCV disease. The failed grafts showed either cirrhosis or confluent hepatic necrosis. Eight of these pauents required re-OLT.
Fifty-eight patients in the HCV group received interferon alfa therapy at some point in their clinical course. None of the patients presented a sustained virological or biochemical response. Twelve patients had a transient biochemical response that returned to pretreatment level as soon as the medication was stopped. There was no discernible effect on the end points analyzed in this subset of patients.
Bacterial sepsis and fungal infection were more prevalent in the HCV group as a cause of death after OLT, although it did not reach statistical significance. Singh et al22 have reported an increased prevalence of serious infections in liver transplant recipients with recurrent HCV. This higher incidence of infection has been attributed to a depressed cell-mediated immunity among patients infected with HCV. This viral infection may have an immunosuppressant effect in itself, as has been described with cytomegalovirus.23 Epstein Barr virus,24 and HBV,25 although it has not been established so far.
Other findings, as well as those reponed by other groups, showed a similar patient and graft survival in a rather short observation period (≤5 years). We anticipate that, as with immunocompetent patients, HCV infection may slowly evolve toward ESLD in OLT patients, and a worse outcome might become evident with a longer follow-up period. The significant challenge that we face is to identify risk factors for disease progression and to define prophylactic and therapeutic strategies to prevent and treat recurrent HCV in this patient population.
References
- 1.Mateo R, Demetris A, Dico E, Frye C, Wang LF, el-Sakhawi Y, et al. Early detection of the novo hepatitis C infection in post–liver transplant patients by reverse-transcriptase polymerase chain reaction. Surgery. 1993;114:442–448. [PubMed] [Google Scholar]
- 2.Wright TL, Donegan E, Hsu HH, Ferrell L, Lake JR, Kim M, et al. Recurrent and acquired hepatitis C viral infection in liver transplant recipients. Gastroenterology. 1992;103:317–322. doi: 10.1016/0016-5085(92)91129-r. [DOI] [PubMed] [Google Scholar]
- 3.Feray C, Samuel D, Thiers V, Gigou M, Pichon F, Bismuth A, et al. Reinfection of liver graft by hepatitis C Virus after liver transplantation. J Clin Invest. 1992;89:1361–1365. doi: 10.1172/JCI115723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chazouilleres O, Kim M, Combs C, Ferrell L, Bacchetti P, Roberts J, et al. Quantitation of hepatitis C virus RNA in liver transplant recipients. Gastroenterology. 1994;106:994–999. doi: 10.1016/0016-5085(94)90759-5. [DOI] [PubMed] [Google Scholar]
- 5.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991;13:619–626. [PMC free article] [PubMed] [Google Scholar]
- 6.Samuel D, Bismuth A, Mathieu D, Arulnaden JL, Reynes M, Benhamou JP, et al. Passive immunoprophylaxls after liver transplantation in HBsAg-positive patients. Lancet. 1991;337:813–815. doi: 10.1016/0140-6736(91)92515-4. [DOI] [PubMed] [Google Scholar]
- 7.Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, et al. Liver transplantation in European patients with hepatitis B surface antigen. New Engl J Med. 1993;329:1842–1847. doi: 10.1056/NEJM199312163292503. [DOI] [PubMed] [Google Scholar]
- 8.Gane EJ, Portmann BC, Naoumov NV, Smith HM, Underhill JA, Donaldson PT, et al. Long-term outcome of hepatitis C infection after liver transplantation. New Engl J Med. 1996;334:815–820. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 9.Boker KHW, Dalley G, Bahr MJ, Maschell H, Tillmann HL, Trutwein C, et al. Long-term outcome of hepatitiS C virus infection after liver transplantation. Hepatology. 1997;25:203–210. doi: 10.1002/hep.510250137. [DOI] [PubMed] [Google Scholar]
- 10.Feray C, Gigoou M, Samuel DS, Paradis V, Wilber J, David MF, et al. The course of hepatitis C virus infection after liver transplantation. Hepatology. 1994;20:1137–1143. doi: 10.1002/hep.1840200506. [DOI] [PubMed] [Google Scholar]
- 11.Mateo R, Faruki H, Cooper D, Demetris AJ, Ehrlich G. Detection of hepatitis C virus RNA in liver and plasma by reverse-transcriptase PCR using liquid hybridization. In: Ehrlich G, editor. PCR-based diagnostics in infectious diseases. Blackwell Scientific; London: 1993. pp. 124–132. [Google Scholar]
- 12.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Mantel PN. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 15.Casavilla A, Mateo R, Rakela J, Irish W, Demetris AJ, Starzl TE, et al. Impact of hepatitis C viral infection on survival following primary liver transplantation under FK 506 (Prograft™) [abstract]. Hepatology. 1994;20:133A. [Google Scholar]
- 16.Pares A, Barrera JM, Caballeria J, Ercilla G, Bruguera M, Caballeria L, et al. Hepatitis C virus antibodies in chronic alcoholic patients: Association with severity of liver injury. Hepatology. 1990;12:1295–1299. doi: 10.1002/hep.1840120608. [DOI] [PubMed] [Google Scholar]
- 17.Rosman AS, Paronetto F, Galvin K, Williams RJ, Lieber CS. Hepatitis C virus antibody in alcoholic patients. Association with the presence of portal and/or lobular hepatitis. Arch Intern Med. 1993;153:965–969. [PubMed] [Google Scholar]
- 18.Oshita M, Hayashi N, Kashara A, Hagiwara H, Mita E, Naito M, et al. Increased serum hepatitis C virus RNA levels among alcoholic patients with chronic hepatitis C. Hepatology. 1994;20:1115–1120. [PubMed] [Google Scholar]
- 19.Starzl TE, Van Thiel D, Tzakis AG, Iwatsuki S, Todo S, Marsh JW, et al. Orthotopic liver transplantation for alcoholic cirrhosis. JAMA. 1988;260:2542–2544. [PMC free article] [PubMed] [Google Scholar]
- 20.Lim HL, Lau GK, Davis GL, Dolson DJ, Lau JYN. Cholestatic hepatitis leading to hepatitis failure in a patient with organ-transmitted hepatitis C virus infection. Gastroenterology. 1994;106:248–253. doi: 10.1016/s0016-5085(94)95829-7. [DOI] [PubMed] [Google Scholar]
- 21.Schluger LK, Sheiner PA, Thung SN, Lau JY, Min A, Wolf DC, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology. 1996;23:971–976. doi: 10.1002/hep.510230505. [DOI] [PubMed] [Google Scholar]
- 22.Singh N, Gayowski T, Wagener MM, Marino IR. Increased infections. I. Liver transplant recipients with recurrent hepatitis C virus. Transplantation. 1996;61:402–406. doi: 10.1097/00007890-199602150-00014. [DOI] [PubMed] [Google Scholar]
- 23.Ho M. Advances in understanding cytomegalovirus infection after transplantation. Transplant Proc. 1994;26:7–11. [PubMed] [Google Scholar]
- 24.Haider S, Coutinho M de L, Emond RT, Sutton RN. Tuberculin anergy and infectious mononucleosis. Lancet. 1973;2:74. doi: 10.1016/s0140-6736(73)93265-0. [DOI] [PubMed] [Google Scholar]
- 25.Nouri-Aria KT, Arnold J, Davison F, Portmann BC, Meager A, Morris AG, et al. Hepatic interferon alfa gene transcripts and products in liver specimens from acute and chronic hepatitis B virus infection. Hepatology. 1991;13:1029–1034. [PubMed] [Google Scholar]