Summary
Differential localization of calcium channel subtypes in divergent regions of individual neurons strongly suggests that calcium signaling and regulation could be compartmentalized. Region-specific expression of calcium extrusion transporters would serve also to partition calcium regulation within single cells. Little is known about selective localization of the calcium extrusion transporters, nor has compartmentalized calcium regulation within single neurons been studied in detail. Sensory neurons provide an experimentally tractable preparation to investigate this functional compartmentalization. We studied calcium regulation in the outer segment (OS) and inner segment/synaptic terminal (IS/ST) regions of rods and cones. We report these areas can function as separate compartments. Moreover, ionic, pharmacological, and immunolocalization results show that a Ca-ATPase, but not the Na+/K+, Ca2+ exchanger found in the OSs, extrudes calcium from the IS/ST region. The compartmentalization of calcium regulation in the photoreceptor outer and inner segments implies that transduction and synaptic signaling can be independently controlled. Similar separation of calcium-dependent functions is likely to apply in many types of neuron.
Introduction
Several different processes and mechanisms are known to regulate intracellular free calcium ([Ca2+]i) in neurons (reviewed by Carafoli, 1991 and Pozzan et al., 1994). [Ca2+]i may be controlled regionally within individual neurons (Lipscombe et al., 1988; Yuste et al., 1994; Kavalali et al., 1997); however, there is little data showing such compartmentalization or elucidating how calcium could be differentially regulated in specific regions within a cell via localized influx and extrusion mechanisms. Sensory cells provide an advantageous preparation to study the partitioning of calcium regulation because the sensory transduction and synaptic signaling compartments are well differentiated structurally. Furthermore, the roles of calcium are known to be very distinct in each region. Calcium regulation of transduction, which serves to control the gain (photoreceptors, reviewed by McNaughton, 1990; hair cells, Lenzi and Roberts, 1994; olfactory receptors, Kurahashi and Menini, 1997), differs from that in the output (synaptic) compartments (Rieke and Schwartz, 1996). In vertebrate photoreceptors, calcium enters the outer segments (OSs), the site of phototransduction, through cGMP-gated channels and is cleared from the cytosol via an Na+/K+, Ca2+ exchanger (reviewed by McNaughton, 1990; Korenbrot, 1995). The predominant influx pathway for Ca2+ entry into ISs is through L-type voltage-gated channels (Corey et al., 1984; Barnes and Hille, 1989; Rieke and Schwartz, 1996). However, virtually nothing is known about how calcium is extruded from the inner segments and synaptic terminals of rods and cones.
One primary goal of this present study was to elucidate how calcium is regulated and extruded from the ISs and synaptic terminals of photoreceptors. We tested to see if an Na+/K+, Ca2+ exchanger or a Ca-ATPase, the other principal type of calcium extrusion, played a role in calcium clearance. We found no evidence for an Na+/K+, Ca2+ exchanger but found pharmacological and immunocytochemical data supporting a principal role for a Ca-ATPase. These findings show conclusively that calcium influx and clearance differ between the outer segment and the inner segment/synaptic terminal regions and that there is a compartmentalization of [Ca2+]i in these sensory cells.
Results
Enzymatically isolated salamander retinal photoreceptors were plated onto coverslips and loaded with Fura 2–AM, a high affinity calcium indicator dye. We measured the time courses of spatially averaged changes of [Ca2+]i in rods and cones by integrating the ratiometric signal from regions of interest inscribed around the inner edges of the ISs and/or OSs in the field of view.
An Na+/Ca2+ Exchanger Extrudes Ca2+ from the Outer but Not from the Inner Segments
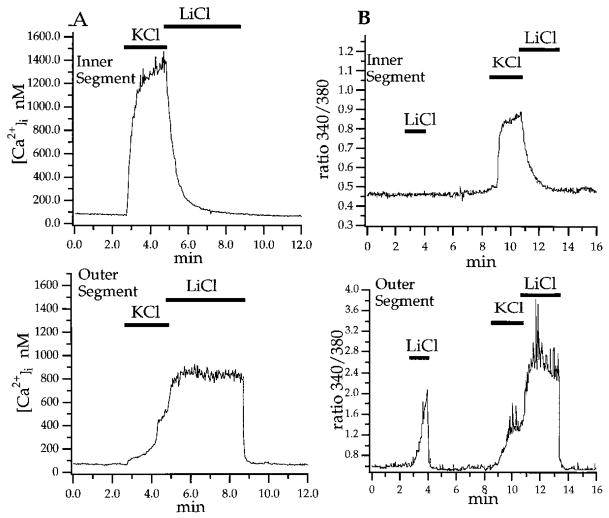
The ISs and OSs differed in how they responded to manipulations known to alter Na+/Ca2+ exchange. It has been demonstrated in earlier studies that Li+ and choline cannot substitute for Na+ in activation of Na+/Ca2+ exchange (Blaustein and Hodgkin, 1969; Yau and Nakatani, 1984). Also, high external potassium and low external sodium can inhibit the exchanger and cause it to switch into a “reverse mode,” i.e., to pump calcium into the cell as opposed to extruding it (the “forward mode”; Schnetkamp 1995). Figure 1A shows that [Ca2+]i rose rapidly in the IS and more slowly in the OS in response to KCl (90 mM, 2.1 min). Immediately following KCl, the rod was superfused with Li+ saline (in which all Na+ was replaced by Li+). In LiCl, outer segment [Ca2+]i remained elevated following KCl (Figure 1A), a result consistent with inhibition of the exchanger. In some cases, [Ca2+]i actually rose further upon LiCl substitution (Figure 1B), which suggests that the exchanger was reversed under these conditions in this particular rod. Upon restoration of normal extracellular Na+, the maintained high [Ca2+]i in the OSs returned to baseline exponentially, with a time constant of 3.0 s. The time constants for recovery of [Ca2+]i in OSs, upon switch to Na+-containing solution, averaged 3.9 ± 0.4 s. This value is similar to the time courses of Ca2+ extrusion measured in toad rod OSs (~2.5 s; Miller and Korenbrot, 1987), slower than the value reported for indo-1 dextran–loaded gecko rod OSs (~1.5 s; Gray-Keller and Detwiler, 1994), and between the two slower time constants reported for Ca2+ extrusion from OSs in Fura 2–loaded salamander retinas (~1.5 and 7.0 s, respectively; McCarthy et al., 1996).
Figure 1. Calcium Extrusion Is Regulated Independently in Photoreceptor Inner and Outer Segments.
Simultaneous measurements were made of the time courses of the spatially averaged [Ca2+]i in the IS and the OS of single rods.
(A) A switch from control saline (2 mM KCl) to high KCl superfusate (90 mM KCl) raised [Ca2+]i in both segments. Immediately following high KCl, the superfusate was switched to Li+ saline (0 mM NaCl, 2 mM KCl, and 97 mM LiCl). The IS [Ca2+]i returned to baseline with a time course that was fitted with a single exponential of t = 29.9 s (upper trace). The OS [Ca2+]i remained at a plateau in LiCl saline; it returned to baseline upon return to control saline with t = 3.0 s (lower trace).
(B) In another rod, control saline was replaced with Li+ saline. [Ca2+]i rose in the OS (lower trace) but remained unchanged in the IS. KCl elevated [Ca2+]i in both segments; again, the rise in the OS was slower. Upon switch to Li+, [Ca2+]i fell in the IS but rose further in the OS, an action we attribute to reversal of the Na+/K+, Ca2+ exchanger. Upon return to control saline, [Ca2+]i in the OS returned to baseline.
[Ca2+]i was calibrated for both of these cells using ionomycin. Actual [Ca2+]i was estimated using Kd = 224 nM.
In contrast to the OS, [Ca2+]i in the IS, upon switch to Li+, did not stay elevated but declined to baseline values with an exponential time constant of 29.9 s. The average time constant for calcium clearance from ISs in Na+-free Li+ saline was 34.2 ± 2.7 s (n = 4), comparable to recovery in Na+-containing saline (39.9 ± 4.0 s, n = 15). These data confirm that calcium extrusion in the OS is Na+ dependent and demonstrate that extrusion from the IS is Na+ independent.
To further investigate the involvement of Na+/K+, Ca2+ exchange, we compared changes in the resting levels of [Ca2+]i in OSs and ISs in the presence or absence of extracellular Na+. Substitution of [Na+]o with [Li+]o increased resting [Ca2+]i in rod OSs by 187 ± 62.6 nM (n = 5). [Na+]o substitution did not significantly change resting [Ca2+]i of most rod inner segments (30/34 rods). An example of this difference between ISs and OSs is shown in Figure 1B. Here, LiCl elevated [Ca2+]i in the OS but not the IS.
Compartmentalization of [Ca2+]i homeostasis was also observed in experiments in which OSs and ISs were depolarized in the presence of nifedipine, a dihydropyridine blocker of L-type voltage-gated calcium channels. We found that KCl-induced increases of [Ca2+]i in rod OSs were not prevented by high concentrations of nifedipine (40 μM, n = 3). Nifedipine (2–10 μM) did block the KCl-evoked [Ca2+]i increases in ISs of all rods (n = 11) and cones (n = 9) tested. These observations rule out the possibility that the increases in the OSs were caused by calcium influx into the ISs, via L-type calcium channels expressed in the ISs, and subsequent diffusion to the OSs. Taken together, these findings indicate that calcium rises in the OSs were most likely attributable to reversal of the Na+/K+, Ca2+ exchange in OSs, and this exchanger was not functioning to extrude calcium from ISs in control saline or after KCl-induced depolarizations.
Images of Fura 2–AM ratiometric signals were recorded to determine the spatiotemporal changes in [Ca2+]i in the photoreceptors. Figure 2 shows that in response to KCl, [Ca2+]i rose and fell fastest in the synaptic terminal, at the end of the long thin fiber emanating from the spherical inner segment. [Ca2+]i in the basal region of the IS rose next fastest and then [Ca2+]i rose in the apical IS region and the OS. Strikingly, these sequential images show how [Ca2+]i levels in the IS and OS of a single rod could move in opposite directions simultaneously. Switching to LiCl caused [Ca2+]i to drop in the synaptic terminal and IS but to rise even higher in the OS (Figure 2d). Upon return to control, [Ca2+]i dropped back to baseline in the OS (Figure 2e). By manipulation of Na+/K+, Ca2+ exchange, Figure 2 demonstrates again that [Ca2+]i can be regulated independently in the ISs and OSs. This figure, and many similar experiments not shown, also reveal that calcium changes occur faster in the synaptic terminal than in the IS, but calcium clearance from both regions is Na+ independent.
Figure 2. Spatiotemporal Dynamics of Calcium Changes in a Rod Photoreceptor.

Sequential images of [Ca2+]i changes were recorded from a Fura 2–loaded rod. Between (a) and (b), the rod was surperfused with high KCl. The images in (b) and (c) were captured 3 and 21 s after KCl application, respectively. The image in (d) was captured 15 s after KCl was replaced by LiCl. The image in (e) was captured 7 s after the return to control saline. These images show that KCl-evoked increases in [Ca2+]i occurred most rapidly in the synaptic terminal region and then in the basal region of the inner segment. In LiCl, the IS and synaptic terminal returned to baseline while [Ca2+]i in the OS rose, most notably at the tip. The pseudocolor scale representing the 256 gray levels of the 340/380 ratios is shown on the bottom; red indicates the largest changes. Scale bar, 10 μm.
Extrusion of Ca2+ from outer segments of cone photoreceptors also relies on Na+-dependent exchange (Nakatani and Yau, 1988). We were unable to measure [Ca2+]i signals in cone OSs because they were commonly lost during the isolation procedures (Maricq and Korenbrot, 1988). As in the rods, we found that the resting [Ca2+]i and the time course of [Ca2+]i recovery in cone ISs were not significantly changed by replacing [Na+]o with [Li+]o or [choline]o. On average, following short puffs of KCl in which [Ca2+]i rose up to ≤1 μM, the recovery time constants were 13.2 ± 1.4 s (n = 28) for cones exposed in Na+-free saline and 11.1 ± 0.6 s (n = 51) for cones measured in the control Na+-containing saline. For the rods stimulated with similar brief puffs of KCl, calcium transients recovered with time constants of 18.9 ± 1.0 s (n = 46) in Na+-free saline, not significantly different from 19.1 ± 0.9 s (n = 51), which was measured in the control Na+-containing solution. The time constants of recovery were consistently longer in rods than in cones (p < 0.0001).
In summary, these results indicate that separate mechanisms control calcium extrusion in rod ISs and synaptic terminals versus OSs: an Na+-dependent mechanism in the OS and an Na+-independent mechanism in the IS and synaptic terminals. Cone ISs also have an Na+-independent calcium extrusion mechanism.
Plasma Membrane Ca-ATPase Is Immunolocalized to Inner Segments
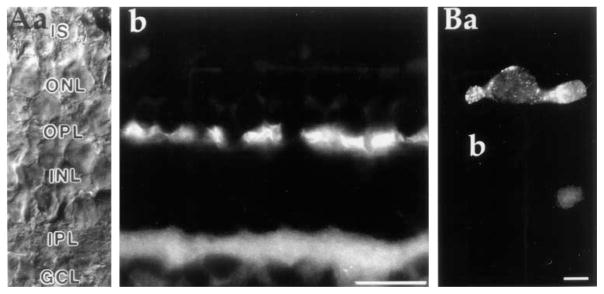
Haase et al. (1990) showed in the bovine retina that a monoclonal antibody against the Na+/K+, Ca2+ exchanger exclusively labeled rod outer segments. Their antibody did not recognize cone OSs, nor did it label ISs of either photoreceptor class. The lack of staining in inner segments suggests Ca2+ is extruded either via a different Na+/Ca2+ exchange protein or via a different type of calcium extrusion mechanism. Our results, demonstrated above (Figures 1 and 2), eliminate the first possibility. We thus tested for presence of a plasma membrane Ca-ATPase (PMCA) by labeling tissue sections and dissociated cells with an antibody that recognizes the “hinge domain” of all PMCA isoforms but does not label sarcoplasmic/endoplasmic reticulum Ca-ATPases (Adamo et al., 1992). Figure 3A shows heavy labeling of both plexiform layers in a vertical section of the salamander retina. In addition to strong labeling of the outer plexiform layer, where the synaptic terminals of rods and cones contact horizontal and bipolar dendrites, less intense but pronounced labeling was observed also in the basal regions of rod and cone inner segments. Figure 3Ba illustrates staining of an enzymatically isolated cone. Again, the most prominent staining was observed in the synaptic terminal with punctate staining in the inner segment and the ellipsoid. In control experiments, using only the secondary antibody, weak staining in the ellipsoid region was also observed, indicating that the ellipsoid staining was probably nonspecific (Figure 3Bb). PMCA antibodies displayed similar staining patterns in both rods and cones.
Figure 3. Antibodies to the Plasma Membrane Ca2+-ATPase Are Localized to the Inner Segments and Synaptic Terminals of Photoreceptors.
(A) Immunostaining of radially cut section of tiger salamander retina. Nomarski DIC image (a) and anti-PMCA immunofluorescence (b) of retinal section are shown. The strongest labeling occurred at regions of synaptic contacts in the outer and inner plexiform layers. Photoreceptor inner segments were also stained, albeit not as strongly. Scale bar, 40 μm.
(B) Example of an enzymatically dissociated cone labeled with a secondary antibody in the presence (a) and absence (b) of the primary PMCA antibody. The cells in (a) and (b) were plated and stained in parallel on concanavalin A–coated dishes. Scale bar, 10 μm.
Calcium Is Extruded from ISs via Plasma Membrane Ca-ATPase
The experiments below provide confirmatory evidence for PMCA function in photoreceptor ISs.
Lanthanum
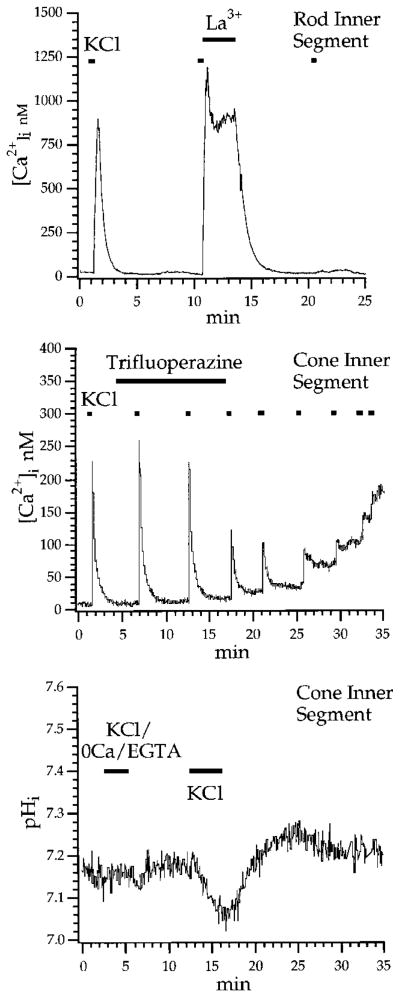
Extracellular La3+ has been used to block PMCA-dependent extrusion in several cell types (Milanick, 1990). This action is presumably mediated by increasing the steady-state level of the activated phosphoenzyme at its extra-cellular site (Herscher and Rega, 1996). Figure 4A shows that [La3+]o blocks Ca2+ extrusion in photoreceptor ISs. This rod was depolarized briefly by 1 s steps of KCl. [Ca2+]i increased rapidly to ~900 nM and decayed with a time constant of 23.7 s. Immediately following a second step of KCl, a switch to 1 mM La3+ saline effectively clamped [Ca2+]i to an elevated plateau, preventing its recovery to baseline. After La3+ washout, [Ca2+]i declined to baseline with a time constant of 50.7 s. The sustained [Ca2+]i plateau produced by La3+ was observed in 13 rods and 6 cones. The ability of La3+ to produce a plateau of high [Ca2+]i following depolarization-evoked increases in [Ca2+]i is opposite to its expected action as a blocker of these voltage-dependent calcium channels (Reichling and MacDermott, 1991). A parsimonious interpretation of these results is therefore that La3+ blocked the activity of the PMCA and thereby inhibited calcium clearance.
Figure 4. Calcium Is Extruded from Photoreceptor Inner Segments by a Ca-ATPase.
(A) The rod shown here was depolarized with sequential 1 s steps of 90 mM KCl. The cell was perfused with La3+-containing saline (1 mM) immediately following the second KCl step. After La3+, the superfusate was returned to control saline. That [Ca2+]i remained elevated during La3+ supports the idea that a Ca-ATPase is necessary for clearance of calcium following a calcium load.
(B) The cone shown here was repeatedly stimulated by brief puffs of KCl. Trifluoperazine (4 μM), a calmodulin antagonist, was added to the superfusate after the second KCl puff. Subsequent transient calcium increases to KCl declined, and the resting [Ca2+]i rose in a step-like manner after each puff.
(C) Effects of KCl on intracellular pH in a cone. The cell was loaded with 5 μM BCECF-AM and then stimulated with two sequential steps of KCl. The first step was applied in control superfusate containing 0 Ca/3 mM EGTA. There was no effect on baseline pH. A subsequent exposure to 90 mM KCl in control saline resulted in an acidification. pH changes were calibrated using nigericin.
Calmodulin Blockers
Calmodulin is an allosteric effector of PMCA that acts to extend its activation into the physiological range of [Ca2+]i (Carafoli, 1991). Accordingly, calmodulin antagonists decrease the rate of pumping by blocking the calmodulin-binding site and decreasing the pump affinity for Ca2+ (Gietzen et al., 1981). We found that superfusion of rod and cone ISs with the membrane-permeable calmodulin antagonists trifluoperazine (3–5 μM), calmidazolium (2–10 μM), and W-13 (1 μM) raised baseline [Ca2+]i and prolonged the recovery from calcium loads. In Figure 4B, brief puffs of KCl were periodically applied to a cone IS. Following exposure to 4 μM trifluoperazine, each puff of KCl resulted in a new, higher plateau resembling a staircase, suggesting that the cell was no longer capable of clearing calcium from its cytoplasm. This same action of trifluoperazine was observed in 7 rods and 4 cones. The staircase-like elevation following a series of depolarizing steps was similar to that seen in auditory hair cells of turtle following intracellular perfusion with 1 mM vanadate, an intracellular blocker of the PMCA (Tucker and Fettiplace, 1995). We have observed that exposure to the calmodulin antagonists calmidazolium at concentrations above 2 μM (n = 14/14 cones, 9/11 rods) and W-13 (n = 3 rods) (data not shown) often resulted in increases in resting [Ca2+]i, suggesting that PMCA may be tonically active in dissociated photoreceptors.
Intracellular pH
Ca-ATPase acts as an obligatory Ca2+/nH+ exchanger (where n = 1 or 2) in a variety of cell types (Niggli et al., 1982; Milanick, 1990; Trapp et al., 1996). This attribute of the pump predicts that exchanger-mediated Ca2+ efflux would be accompanied by an acidification due to an increase in the intracellular proton concentration. To test this idea, we loaded cells with the pH indicator dye BCECF-AM and evoked an increase in [Ca2+]i with KCl. Figure 4C shows the effects of depolarization on intra-cellular pH in a cone. First, the cell was depolarized for 2.4 min with 90 mM KCl in the absence of external Ca2+; no change in intracellular pH was detected. Next, a 3.2 min superfusion with high KCl in normal extracellular [Ca2+] acidified the cell by 0.13 pH units. The absence of acidification in 0 Ca2+ was seen in 6 of 6 cells. The acidification seen in normal calcium averaged −0.09 ± 0.01 pH units for 10 cones and ranged from −0.05 to −0.18 pH units. We found that the acidification was blocked by 10 μM nifedipine (n = 2; data not shown). This confirms that the calcium influx that led to the acidification was through L-type Ca2+ channels.
The KCl-induced acidification we observed did not result from Ca2+/H+ exchange between the cytoplasm and the intracellular stores. As evidence, we found that exposure to 5 μM cyclopiazonic acid, an inhibitor of the sarcoplasmic/endoplasmic Ca-ATPase, did not prevent the KCl-induced acidification (n = 2; data not shown). Additionally, no pH changes were observed when choline or Li+ was substituted for [Na+]o, indicating that the acidification was not produced by a reversal of the Na+/H+ exchanger (n = 6; data not shown). Finally, an analysis of the proton motive force indicates that the KCl-induced acidification was not caused by passive net influx of protons into the photoreceptor cytoplasm. At the control extracellular pH of 7.6 and resting membrane potential of −58 mV, the electrochemical equilibrium for [H+] would be at pHi = 6.60 (see Saarikoski et al., 1997). Depolarization to −10 mV, which would be expected from 90 mM KCl, would shift the equilibrium [H+]i to pH = 7.43. Thus any passive H+ flow would be outward and would alkalinize the cells, contrary to what we observed.
Discussion
Here, we report a compartmentalization of calcium regulation mechanisms in a sensory neuron. Not only are the calcium influx pathways different (McNaughton, 1990), but there is a polarized expression of calcium extrusion mechanisms in the different regions of the photoreceptors. By measuring calcium dynamics in vertebrate rods, we demonstrated that calcium is cleared from OSs through Na+/K+, Ca2+ exchange, whereas calcium clearance from ISs and synaptic terminals occurs via the plasma membrane Ca-ATPase. Furthermore, by means of independent manipulation of the two calcium extrusion processes, it has been possible to reveal that the IS and OS of single photoreceptors can function as independent compartments.
Mechanisms that Compartmentalize Calcium within Rods and Cones
The influx, extrusion, and restricted Ca2+ diffusion all contribute to its compartmentalization and differential regulation between OSs and ISs. Under our experimental conditions, as in bright light, the cGMP-gated channels in rod OSs are closed; therefore, Ca2+ would not be expected to enter through these channels. We show that by changing [K+]o and [Na+]o, the forward and reverse Ca2+ transport by the Na+/K+, Ca2+ exchanger in the rod OSs can be manipulated separately from the voltage-gated Ca2+ channels and PMCAs in the ISs and synaptic terminals. This result, concomitant with the fact that ISs but not OSs possess a variety of voltage-dependent conductances, predicts a functional uncoupling of [Ca2+]i between the sensory transduction and synaptic compartments of the cell. Such an uncoupling would be enhanced by the biophysical properties of Ca2+ diffusion. The range of Ca2+ actions in most physiological situations is highly restricted due to its small apparent diffusion coefficient in cytosol (D ≈ 10−7 cm2s−1 for Ca2+ versus D ≈ 10−5 cm2s−1 for Na+ and K+; Kushmerick and Podolsky, 1969), a consequence of calcium binding to immobile buffers and its sequestration into intracellular compartments (Fain and Schroder, 1990; Allbritton et al., 1992; Lagnado et al., 1992; see also McCarthy et al., 1996). In contrast, the unidirectional flow of Na+ that presumably carries the circulating current that enters the OSs and leaves the ISs in darkness (Hagins et al., 1970) will be relatively unhindered with respect to Ca2+ fluxes. Compartmentalization of Ca2+ is thus fundamentally different from that of free Na+ and K+.
The kinetic differences of calcium rise and clearance from synaptic terminals, basal regions, and apical regions of the ISs were pronounced. The rates of rise and fall of [Ca2+]i in the synaptic terminals were generally severalfold faster than in the basal and apical ISs. This may reflect the need for faster Ca2+ regulation required for synaptic function. We were unable to quantitate [Ca2+]i changes in synaptic terminals at the 100 ms time scale expected from the cone light response (Schnapf and Copenhagen, 1982), owing to the limitations in the temporal resolution (1 Hz) of the imaging system. The time constants for extrusion of Ca2+ from inner segments of rods and cones are similar to those reported for two other neurons specialized for tonic release, the bipolar cell (Tachibana et al., 1993) as well as the slow component of PMCA-dependent Ca2+ extrusion in turtle hair cells (~10 s; Tucker and Fettiplace, 1995). Such slow decay of residual [Ca2+]i may regulate an adaptational process with a time constant of 10–30 s.
Depolarization increased calcium in both rod and cone ISs lacking synaptic terminals. We thus hypothesize that the voltage-dependent calcium channels mediating influx are not localized to synaptic terminals but are expressed in ISs as well, as has been also shown to be the case for retinal bipolar cells (Heidelberger and Matthews, 1992).
PMCA-Mediated Calcium Extrusion Is Well Suited for Signaling under Light-Adapted Conditions
Light responses are reliably signaled to postsynaptic neurons over a range of background illumination that spans several orders of magnitude. At any level of background light, [Ca2+]i in ISs and synaptic terminals would be determined by the balance of influx, primarily through the L-type calcium channels, and extrusion via the PMCA. In brighter backgrounds, light-evoked hyperpolarizations as small as 10–50 μV are reliably signaled to postsynaptic cells (Fain et al., 1977; Copenhagen et al., 1990). Under these conditions, [Ca2+]i would be reduced to well below 100 nM, which is close to the Michaelis constant of PMCAs but is 10–20 times lower than the Michaelis constant for the Na+/K+, Ca2+ exchangers measured in rod outer segments (Chambers et al., 1990; Carafoli, 1991; Lagnado et al., 1992; Schnetkamp, 1995). Therefore, the higher affinity PMCA appears well suited to the signaling needs at the photoreceptor output at low calcium levels.
The Separation of Calcium Homeostatic Apparati Allows for Differential Regulation of Ca2+ Influx and Extrusion in Inner and Outer Segments
At least two signal transduction mechanisms found in the IS are absent from the OS: the calcium/calmodulin-dependent nitric oxide synthase/guanylate cyclase cascade (Koch et al., 1994) and the D2/D4 dopamine receptor linked to a cAMP cascade (Iuvone et al., 1990; Muresan and Besharse, 1993). Indeed, second messengers, such as cAMP, can modulate the affinity and Vmax of the PMCA (Carafoli, 1991). Our results also suggest that the activity of the PMCA itself may affect synaptic transmission at the photoreceptor synapse via an acidification associated with Ca2+ extrusion. Since protons can act directly on L-type calcium currents (Dixon et al., 1993), this property of the pump may result in modulation of the release of the synaptic transmitter (Barnes et al., 1993).
Thus, the possibility of differential regulation of calcium and the need for a high affinity extrusion mechanism in inner segments argue for the advantage and need for PMCA in this compartment.
Experimental Procedures
Preparation of Isolated Cells
Neotenic tiger salamanders (Ambystoma tigrinum) were decapitated and pithed. Retinas were dissected from the eyecup at room temperature (20°C–22°C) and in room light, incubated on a shaker in 0 Ca/papain (7 U/ml) saline for 25 min and triturated with a BSA-coated flame-constricted pipette. In some experiments, cells were isolated mechanically without papain. All photoreceptors were clearly identifiable as rods or cones by the characteristic shape of their ellipsoid, their synaptic terminals, and their outer segments. Coverslips containing Fura 2–loaded cells were superfused continually with the amphibian saline solution, containing (in mM) 97 NaCl, 2 KCl, 2 CaCl2, 2 MgCl2, 10 HEPES, 20 glucose, 1 pyruvic acid, 0.3 ascorbic acid, and 1 glutathione at 240 mOsm. pH was adjusted to 7.6 with NaOH or KOH. In Na+-free solutions, Na+ was substituted by equiosmolar amounts of Li+, N-methyl-D-glucamine, or choline, whereas in high K+ solutions, Na+ was replaced by appropriate amounts of K+. The recording chamber was superfused through a multibarrel quartz tube system controlled by electromagnetic valves, which permitted exchange of solutions within several hundred milliseconds.
Chemicals
Calmidazolium (both from Sigma, St. Louis, MO), W-13, cyclopiazonic acid, W-13, and trifluoperazine (RBI, Natick, MA) were dissolved in DMSO. Dilutions were made fresh with final concentrations of DMSO of <0.001%. In several experiments in which DMSO-soluble drugs were used, equal amounts of DMSO were added to the control salines to rule out potential artifactual effects of DMSO.
[Ca2+] Measurement and Calibration
Photoreceptors were loaded with 3–5 μM Fura 2–AM supplemented with 10% Pluronic F-127 for 7 min in L-15 solution (all from Molecular Probes, Eugene, OR) and washed for 20 min. The concentration of Fura 2–AM in loaded photoreceptors was estimated to be 40–70 μM using filled 20 mm path-length microcapillaries (#5002, Vitrocom, Mountain Lakes, NJ). Autofluorescence in unloaded cells was not detectable; addition of 10 mM Mn2+ to cells depolarized by 60 mM KCl quenched both 340 nm and 380 nm fluorescence by 90%–100%, indicating that compartmentalization of the dye into intracellular organelles under our experimental conditions is negligible. A few experiments were duplicated using the low affinity mag–Fura 5 dye with similar results. Ratios were calculated after subtraction of the background fluorescence, and Ca2+ levels were calibrated in vivo over regions of interest using the equation:
in which R is the measured ratio, S380b/S340u are the extremes of calcium-bound and calcium-free Fura 2–AM at 380 nm and 340 nm, respectively, and Kd is the Fura 2–AM dissociation constant for Ca2+ (224 nM). Since we did not directly determine the Kd, our calibrated values should be considered as estimates. Any error in our Kd estimate would involve only a difference in scale and would not affect the interpretation of the results. Rmin, Rmax, and S380b/S340u were determined by addition of 10 μM ionomycin to the Ca2+-free (0 Ca/3 mM EGTA) superfusate and to the saturating Ca2+ (10 mM) solution, respectively. Rmin was 0.43 ± 0.01, Rmax 8.39 ± 0.67, and S380b/S340u 8.28 ± 0.78. Under the conditions of our experiments, all cGMP-gated channels in OSs should be closed. Using Kd = 224 nM, we estimate that under these light-adapted conditions [Ca2+]i averages 26 ± 3 nM (cone ISs, n = 44), 51 ± 4 nM (rod ISs, n = 49), and 62 ± 23 nM (rod OSs, n = 7).
[H+]i Measurement and Calibration
Cells were incubated in 5 μM BCECF-AM (Molecular Probes) for 20 min and washed in L-15 for 30 min. Excitation light was 490 (10) nm and 440 (10) nm. Calibration was performed with 50 μM nigericin (Molecular Probes) in 90 mM KCl as described before (Dixon et al., 1993).
Image Acquisition
Excitation light was emitted from a 75 W xenon arc lamp passing through 340 (10) nm and 380 (10) nm bandpass filters (Chroma, Battleboro, VT) mounted in a filter wheel (Lambda-10, Sutter Instruments, Novato, CA) and delivered via a scrambler (Technical Video, Woods Hole, MA) to an inverted microscope. A dichroic mirror (Nikon DM400) was used together with a water immersion 40× objective (Nikon Fluor, NA 0.85) or an oil immersion 100× objective (Nikon Fluor NA 1.4). The acquired fluorescence signals were integrated on chip by a cooled 12 bit digital CCD camera (PXL, Photometrics, Tucson, AZ). Image acquisition, background subtraction, and ratio measurements were controlled by Metafluor software (Universal Imaging, West Chester, PA).
Data Analysis
Data analysis and curve fitting were performed with Igor Pro 3.03 software (WaveMetrics, Lake Oswego, OR) on a Macintosh computer. Data are expressed as mean ± SEM. Images and photographs were prepared with Adobe Photoshop 3.0.
Immunocytochemistry
Isolated Cells
Dissociated cells were washed in phosphate buffered saline (PBS [pH = 7.4]), fixed in 4% (w/v) paraformaldehyde for 30 min at room temperature, and permeabilized by 0.1% (v/v) Triton X-100. After three rinses in PBS, cells were incubated with a blocking solution (10% [w/v] mouse serum in PBS) and exposed for 2 hr to the primary anti-PMCA antibody (1:100, clone number 5F10, Sigma Immunochemicals) supplemented with 1% (w/v) goat serum. This was followed by rinsing in PBS and incubation with the secondary goat anti-mouse antibody (1:2000, Cy3-conjugated goat anti-mouse; Jackson ImmunoResearch, West Grove, PA) for 1.5 hr at room temperature. After the final rinse in PBS and distilled water, the cells were mounted in DABCO/Gelvatol (DuPont) and photographed using the 100× oil immersion objective (Nikon) and DeltaVision software (Seattle, WA). Parallel coverslips were processed in an identical way without being exposed to the primary antibody.
Slices
Salamander eyecups were immersion fixed at room temperature in 4% paraformaldehyde and washed in PBS containing 0.02% sodium azide. The retinae were dissected out and cryoprotected in a series of 10% (w/v) and 20% (w/v) sucrose in PBS, followed by an overnight incubation in 30% (w/v) sucrose in PBS. Vertical sections were preincubated with a 10% (w/v) normal goat serum, 1% (w/v) bovine serum albumin, 0.5% (v/v) Triton X-100 in PBS for 1 hr at room temperature and incubated overnight in the primary anti-PMCA antibody (1:50). Following a wash in PBS, the slices were incubated for 1 hr in goat anti-mouse IgG conjugated to Cy3 (1:1000) for 1 hr at room temperature.
Acknowledgments
We thank Amy Berntson and Catherine Morgans for help with immunolabeling, David Sretavan for the use of the DeltaVision Imaging microscope, and Juan Korenbrot, Ward Petersen, David Reichling, and Paul Witkovsky for comments on the manuscript. This research was supported by the ARVO/CIBA Fellowship (D. K.) and the National Institutes of Health (D. R. C.). Additional support was provided by That Man May See, Incorporated, and Research To Prevent Blindness.
References
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-triphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Adamo HP, Caride AJ, Penniston JT. Use of expression mutants and monoclonal antibodies to map the erythrocyte Ca2+ pump. J Biol Chem. 1992;267:14244–14249. [PubMed] [Google Scholar]
- Barnes S, Hille B. Ionic channels of the inner segment of tiger salamander cone photoreceptors. J Gen Physiol. 1989;94:719–743. doi: 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci USA. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Hodgkin AL. The effect of cyanide on the efflux of sodium from squid axons. J Physiol. 1969;200:497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Calcium pump of the plasma membrane. Physiol Rev. 1991;71:129–153. doi: 10.1152/physrev.1991.71.1.129. [DOI] [PubMed] [Google Scholar]
- Chambers JP, Kumar P, Tsin ATC, Valdes JJ. Partial characterization of a high affinity [Ca2+ + Mg2+]-dependent adenosinetriphosphatase from bovine retina. Exp Eye Res. 1990;50:127–134. doi: 10.1016/0014-4835(90)90222-g. [DOI] [PubMed] [Google Scholar]
- Copenhagen DR, Hemilä S, Reuter T. Signal transmission through the dark adapted retina of the toad (Bufo marinus) J Gen Physiol. 1990;95:717–732. doi: 10.1085/jgp.95.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Dubinsky JM, Schwartz EA. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J Physiol. 1984;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DB, Takahashi K, Copenhagen DR. L-glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron. 1993;11:267–277. doi: 10.1016/0896-6273(93)90183-r. [DOI] [PubMed] [Google Scholar]
- Fain GL, Schroder WH. Light-induced calcium release and re-uptake in toad rods. J Neurosci. 1990;10:2238–2249. doi: 10.1523/JNEUROSCI.10-07-02238.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain GL, Granda AM, Maxwell JH. Voltage signal of photoreceptors at visual threshold. Nature. 1977;265:181–183. doi: 10.1038/265181a0. [DOI] [PubMed] [Google Scholar]
- Gietzen K, Wuthrich A, Bader H. R 24571: a new powerful inhibitor of red blood cell Ca2+-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981;101:418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Gray-Keller MP, Detwiler PB. The calcium feedback signal in the phototransduction cascade of vertebrate rods. Neuron. 1994;13:849–861. doi: 10.1016/0896-6273(94)90251-8. [DOI] [PubMed] [Google Scholar]
- Haase W, Friese W, Gordon RD, Muller H, Cook NJ. Immunological characterization and localization of the Na+, Ca2+-exchanger in bovine retina. J Neurosci. 1990;10:1486–1494. doi: 10.1523/JNEUROSCI.10-05-01486.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins WA, Penn RD, Yoshikami S. Dark current and photocurrent in rods. Biophys J. 1970;10:380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberger R, Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol. 1992;447:235–256. doi: 10.1113/jphysiol.1992.sp019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscher CJ, Rega AF. Pre–steady-state kinetic study of the mechanism of inhibition of the plasma membrane Ca2+-ATPase by lanthanum. Biochemistry. 1996;35:14917–14922. doi: 10.1021/bi961879r. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Avendano G, Butler BJ, Adler R. Cyclic-AMP–dependent induction of serotonin N-acetyltransferase activity in photoreceptor-enriched chick retinal cell cultures: characterization and inhibition by dopamine. J Neurochem. 1990;55:673–682. doi: 10.1111/j.1471-4159.1990.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Zhuo M, Bito H, Tsien RW. Dendritic Ca2+ channels characterized by recordings from isolated hippocampal dendritic segments. Neuron. 1997;18:651–663. doi: 10.1016/s0896-6273(00)80305-0. [DOI] [PubMed] [Google Scholar]
- Koch KW, Lambrecht HG, Haberecht M, Redburn D, Schmidt HH. Functional coupling of a Ca2+-calmodulin–dependent nitric oxide synthase and a soluble guanylyl cyclase in vertebrate photoreceptor cells. EMBO J. 1994;13:3312–3320. doi: 10.1002/j.1460-2075.1994.tb06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. Ca2+ flux in retinal rod and cone outer segments: differences in Ca2+ selectivity of the cGMP-gated ion channels and Ca2+ clearance rates. Cell Calcium. 1995;18:285–300. doi: 10.1016/0143-4160(95)90025-x. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Podolsky RJ. Ionic mobility in muscle cells. Science. 1969;166:1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Lagnado L, Cervetto L, McNaughton PA. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Roberts WM. Calcium signaling in hair cells: multiple roles in a compact cell. Curr Opin Neurobiol. 1994;4:496–502. doi: 10.1016/0959-4388(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Madison DV, Poenie M, Reuter H, Tsien R, Tsien R. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci USA. 1988;85:2398–2402. doi: 10.1073/pnas.85.7.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricq AV, Korenbrot JI. Calcium and calcium-dependent chloride currents generate action potentials in solitary cone photoreceptors. Neuron. 1988;1:503–515. doi: 10.1016/0896-6273(88)90181-x. [DOI] [PubMed] [Google Scholar]
- McCarthy ST, Younger JP, Owen WG. Dynamic, spatially nonuniform calcium regulation in frog rods exposed to light. J Neurophysiol. 1996;76:1991–2004. doi: 10.1152/jn.1996.76.3.1991. [DOI] [PubMed] [Google Scholar]
- McNaughton PA. Light response of vertebrate photoreceptors. Physiol Rev. 1990;70:847–883. doi: 10.1152/physrev.1990.70.3.847. [DOI] [PubMed] [Google Scholar]
- Milanick MA. Proton fluxes associated with the Ca pump in human red blood cells. Am J Physiol. 1990;258:C552–C562. doi: 10.1152/ajpcell.1990.258.3.C552. [DOI] [PubMed] [Google Scholar]
- Miller DL, Korenbrot JI. Kinetics of light-dependent Ca fluxes across the plasma membrane of rod outer segments. J Gen Physiol. 1987;90:397–425. doi: 10.1085/jgp.90.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan Z, Besharse JC. D2-like dopamine receptors in amphibian retina: localization with fluorescent ligands. J Comp Neurol. 1993;331:149–160. doi: 10.1002/cne.903310202. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Yau KW. Calcium and light adaptation in retinal rods and cones. Nature. 1988;334:69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Niggli V, Siegel E, Carafoli E. The purified Ca2+ pump of human erythrocyte membranes catalyzes an electroneutral Ca2+-H+ exchange in reconstituted liposomal systems. J Biol Chem. 1982;257:2350–2356. [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Reichling DB, MacDermott AB. Lanthanum actions on excitatory amino acid–gated currents and voltage-gated calcium currents in rat dorsal horn neurons. J Physiol. 1991;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Schwartz EA. Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J Physiol. 1996;493:1–8. doi: 10.1113/jphysiol.1996.sp021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarikoski J, Ruusuvuori E, Koskelainen A, Donner K. Regulation of intracellular pH in salamander retinal rods. J Physiol. 1997;498:61–72. doi: 10.1113/jphysiol.1997.sp021841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapf JL, Copenhagen DR. Differences in the kinetics of rod and cone synaptic transmission. Nature. 1982;296:862–864. doi: 10.1038/296862a0. [DOI] [PubMed] [Google Scholar]
- Schnetkamp PP. How does the retinal rod Na+-Ca2+, K+ exchanger regulate cytosolic free Ca2+? J Biol Chem. 1995;270:13231–13239. doi: 10.1074/jbc.270.22.13231. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Okada T, Arimura T, Kobayashi K, Piccolino M. Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina. J Neurosci. 1993;13:2898–2909. doi: 10.1523/JNEUROSCI.13-07-02898.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Luckermann M, Kaila K, Ballanyi L. Acidosis of hippocampal neurones mediated by a plasmalemmal Ca2+/H+ pump. Neuroreport. 1996;7:2000–2004. doi: 10.1097/00001756-199608120-00029. [DOI] [PubMed] [Google Scholar]
- Tucker T, Fettiplace R. Confocal imaging of calcium microdomains and calcium extrusion in turtle hair cells. Neuron. 1995;15:1323–1335. doi: 10.1016/0896-6273(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Yau KW, Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984;311:661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Yuste R, Gutnick MJ, Saar D, Delaney KR, Tank DW. Ca2+ accumulations in dendrites of neocortical pyramidal neurons: an apical band and evidence for two functional compartments. Neuron. 1994;13:23–43. doi: 10.1016/0896-6273(94)90457-x. [DOI] [PubMed] [Google Scholar]