I will be asking which drug is the most potent, least toxic, easiest to administer, and most compatible with other drugs for combination therapy. Both drugs are enormously versatile and can be used with all of the established immunosuppressants, including steroids and the antilymphoid preparations. The discovery of the spectacular synergism between FK 506 and old or new antimetabolite drugs has opened the immediate possibilities of xenotransplantation.1
Because both FK 506 and cyclosporine (CyA) are metabolized by the liver, there is a particular risk of overdosage in patients with hepatic dysfunction, resulting in astronomical blood level(s) in FK 506-treated patients whose liver grafts do not function well.2 This phenomenon has been more completely studied with FK 5063 and may be more extreme, but it also occurs with CyA.
The ease of administration nod may go to FK 506. Its oral absorption is not dependent upon bile, making adjustments or overlapping of intravenous and oral dosage unnecessary with bile fistulas or other abnormalities of biliary drainage. Also in contrast to CyA, FK 506 is so little affected by malabsorption and diarrhea that the doses and blood levels are not much different after intestinal grafting than after any other kind of whole-organ transplantation.4
The relative toxicities of FK 506 vs CyA were accurately summarized in San Francisco 2 years ago (Table 1). The nephrotoxicity, neurotoxicity, and diabetogenicity of the two drugs were thought then (and still are) to be comparable and strictly dose related. There are differences. Hypertension and hypercholesterolemia are less with FK 506. Gingival hyperplasia, hirsutism, and the coursening of features evident with CyA do not occur with FK 506. This is slightly puzzling because FK 506 is at least as potent as CyA in promoting certain kinds of growth, such as hepatic regeneration, and in preventing the hepatocyte atrophy caused in experimental models by Eck fistula.
Table 1.
Nonimmunologic Profile (4 + Worst) (All Dose Related)
| FK 506 | CyA | |
|---|---|---|
| Nephrotoxicity | ++* | ++ |
| Neurotoxicity | + | + |
| Diabetogenicity | + | + |
| Growth effects | ||
| Hirsutism | 0 | +++ |
| Gingival hyperplasia | 0 | ++ |
| Facial brutilization | 0 | + |
| Hepatotrophic effects | ++++ | +++ |
| Gynecomastia | 0 | + |
| Other metabolic effects | ||
| Cholesterol increase | 0† | ++ |
| Uric acid increase | +? | ++ |
Reprinted with permission.2
Less hypertension.
In rats, Van Thiel has shown an increase in cholesterol synthesis and serum concentration.
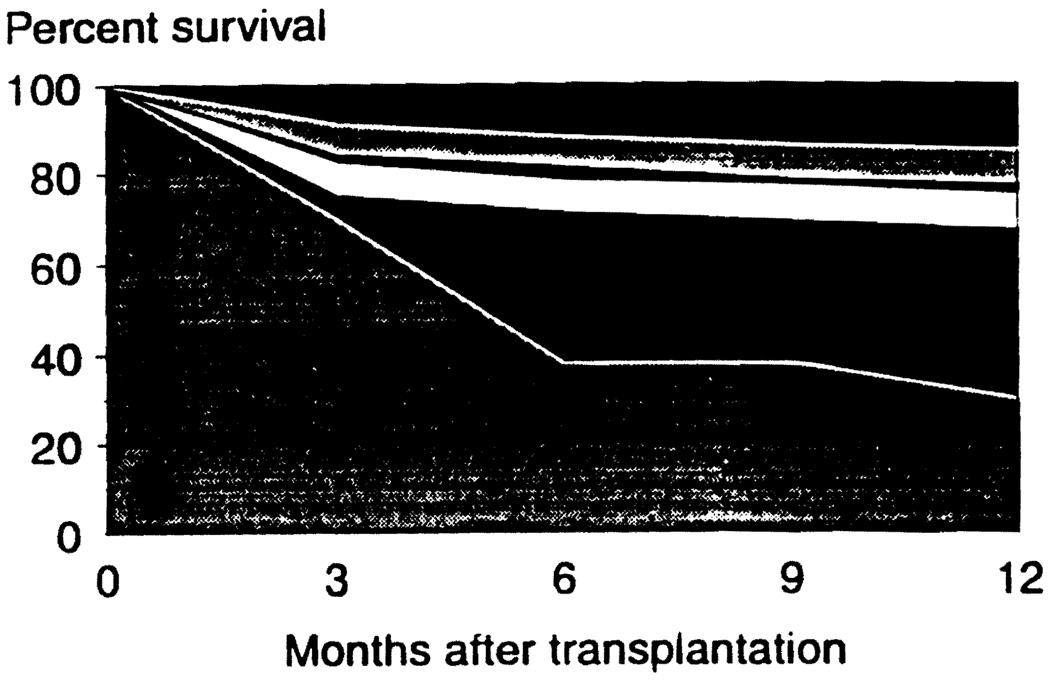
These so-called hepatotrophic or ”liver-healing“ properties of both drugs5 could help explain why they had such an extraordinary effect on the prognosis after liver transplantation (Fig 1). In a Pittsburgh randomized trial of FK 506 vs CyA, the liver transplant and recipient survival curves in the FK 506 and CyA arms were not significantly different when analyzed by intent to treat. However, in this trial, a crossover was permitted based on physician judgment that a patient had rejection that could not be controlled at all or could not be controlled without the excessive use of steroids. This resulted in a flight from CyA to the extent that more than half of the recipients started on the CyA limb were switched to FK 506 within 6 months. The crossover phenomenon could have reflected a bias on the part of the physicians allowing the crossover. However, a more important explanation came from the quality of life studies (physical, psychological, and social well-being) of Simmons in heart6 as well as liver recipients. In all 16 indices measured, the FK 506 patients had better scores and in most of these, the differences were statistically significant.
Fig 1.
Survival after liver transplantation in azathioprine, CyA, and FK 506 eras. During the azathioprine era (lower line), patient and graft survival were synonymous. The middle band (CyA) and upper band (FK 506) have patient survival as the upper border and graft survival as the lower border.
This doctor-initiated, as well as patient-driven crossover phenomena seen with the liver, also affected the kidney randomized trials in that about 20% of the patients started on CyA ended up with good results by virtue of FK 506 rescue as reported in another session at this meeting by Jordan. These observations caused an abandonment of these trials more than a year ago. As in the liver studies, it was proved that patient and graft survival curves based on intent to treat could be made equal, providing crossover rescue was allowed.
Since then, double versus triple-drug FK 506–based protocols have been evaluated in cadaver kidney recipients, all comers—primary and retransplantation—the immunologically difficult retransplant cohort being 32% of the total. Patient survival has been 99% (one death in 125 cases). Graft survival in recipients treated with FK 506 and prednisone (double drug) have fared the same as those treated with the triple-drug combination of FK 506, prednisone, and azathioprine (85% 1-year graft survival). These trials are presented in detail elsewhere at this congress.
In our heart program, 1-year graft and patient survival has been approximately 90%. As with the other organs, the ability to quickly reduce or stop steroids has been a particular advantage in children. There has been no evidence of the coronary arteritis reported by Morris in rats.
Leaving aside the debating rhetoric, a new drug stands or falls by allowing (or failing to allow) accomplishments that were not attainable before. FK 506 has been a tool on both sides of the Atlantic with which a large number of patients who have failed treatment with CyA have been rescued. The ability to cross over in the randomized trials admittedly has blurred the distinction between CyA and FK 506, but it has helped the patients and has allowed us to succeed more often today than was possible 3½ years ago.
To move on, we can point today to a collection of patients whom I thought I might not see in my lifetime: recipients of complete cadaveric small bowel, either transplanted alone, with the liver, or as part of a multivisceral graft.4 Another sight that I thought I might never see is that of a well recipient of a baboon liver. This was accomplished in June under the same FK 506-based cocktail as we use routinely for liver allotransplantation with the addition of nontoxic doses of cyclophosphamide. This patient, who died after 70 days without experiencing serious rejection, is reported elsewhere.
What has emerged from the actual testing of FK 506 is a step up the ladder of progress that has already been taken. Perhaps that has placed the value of FK 506 beyond the boundary of a debatable issue. In fact, FK 506 and CyA are both great drugs that have changed the face of medicine. Debating their fate is uncomfortably like determining which of fraternal twins should be allowed to live. Perhaps we will conclude “Why not both?”
REFERENCES
- 1.Murase N, Starzl TE, Demetris AJ, et al. Transplantation. (in press) [Google Scholar]
- 2.Starzl TE, Abu-Elmagd K, Tzakis A, et al. Transplant Proc. 1991;23:914. [PubMed] [Google Scholar]
- 3.Abu-Elmagd K, Fung JJ, Alessiani M, et al. Transplantation. 1991;52:71. doi: 10.1097/00007890-199107000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todo S, Tzakis AG, Abu-Elmagd K, et al. Ann Surg. 1992;216:223. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Porter KA, Mazxzaferro V, et al. Transplantation. 1991;51:67. doi: 10.1097/00007890-199101000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dew MA, Harris RC, Simmons RG, et al. Transplant Proc. 1991;23:3061. [PubMed] [Google Scholar]