Abstract
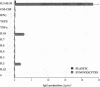
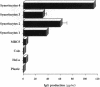
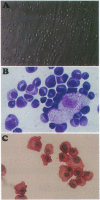
To understand the accumulation of plasma cells within RA synovium, the ability of rheumatoid synoviocytes to support the differentiation of B cells into plasma cells was explored. Tonsillar B lymphocytes cultured over confluent monolayers of synoviocytes, secreted threefold more Igs (mainly IgM) than B cells cultured directly on plastic well. More importantly, synoviocytes enhanced by 14-fold the production of Igs (mainly IgG) by B cells costimulated with Staphylococcus aureus Cowan (SAC) particles. IL-10 and, in a lower extent, IL-2 increased Ig secretion in cocultures, and their combination was synergistic. In the presence of SAC, IL-2, and IL-10, synoviocytes increased by 13-884-fold the production of IgG, which reached 0.19 ng/cell per day. RA as well as normal synoviocytes were more potent than other adherent cell lines to support terminal B cell differentiation. Synoviocyte activity involved both a support of B cell survival, and an induction of the terminal differentiation of B cells into mature plasma cells with typical morphology, high levels of intracytoplasmic Igs, and CD20- CD38high surface expression. The present observation should permit the identification of molecules involved in the maturation of B cells into plasma cells, and in their accumulation in rheumatoid synovium.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Armitage R. J., Conley M. E., Rosenblatt H., Jenkins N. A., Copeland N. G., Bedell M. A., Edelhoff S., Disteche C. M., Simoneaux D. K. CD40 ligand gene defects responsible for X-linked hyper-IgM syndrome. Science. 1993 Feb 12;259(5097):990–993. doi: 10.1126/science.7679801. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Farrington M., Hollenbaugh D., Li X., Milatovich A., Nonoyama S., Bajorath J., Grosmaire L. S., Stenkamp R., Neubauer M. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell. 1993 Jan 29;72(2):291–300. doi: 10.1016/0092-8674(93)90668-g. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Rousset F. Human B lymphocytes: phenotype, proliferation, and differentiation. Adv Immunol. 1992;52:125–262. doi: 10.1016/s0065-2776(08)60876-7. [DOI] [PubMed] [Google Scholar]
- Blanchard D., Gaillard C., Hermann P., Banchereau J. Role of CD40 antigen and interleukin-2 in T cell-dependent human B lymphocyte growth. Eur J Immunol. 1994 Feb;24(2):330–335. doi: 10.1002/eji.1830240209. [DOI] [PubMed] [Google Scholar]
- Brieva J. A., Roldán E., Rodríguez C., Navas G. Human tonsil, blood and bone marrow in vivo-induced B cells capable of spontaneous and high-rate immunoglobulin secretion in vitro: differences in the requirements for factors and for adherent and bone marrow stromal cells, as well as distinctive adhesion molecule expression. Eur J Immunol. 1994 Feb;24(2):362–366. doi: 10.1002/eji.1830240214. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. How B and T cells talk to each other. Nature. 1994 Feb 3;367(6462):425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- Dechanet J., Briolay J., Rissoan M. C., Chomarat P., Galizzi J. P., Banchereau J., Miossec P. IL-4 inhibits growth factor-stimulated rheumatoid synoviocyte proliferation by blocking the early phases of the cell cycle. J Immunol. 1993 Nov 1;151(9):4908–4917. [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Aubry J. P., Banchereau J. Interleukin 4 inhibits the proliferation but not the differentiation of activated human B cells in response to interleukin 2. J Exp Med. 1988 Oct 1;168(4):1321–1337. doi: 10.1084/jem.168.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Brière F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992 Mar 1;175(3):671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilosa R. M., Maeda K., Masuda A., Szakal A. K., Tew J. G. Germinal center B cells and antibody production in the bone marrow. J Immunol. 1991 Jun 15;146(12):4071–4077. [PubMed] [Google Scholar]
- Fluckiger A. C., Garrone P., Durand I., Galizzi J. P., Banchereau J. Interleukin 10 (IL-10) upregulates functional high affinity IL-2 receptors on normal and leukemic B lymphocytes. J Exp Med. 1993 Nov 1;178(5):1473–1481. doi: 10.1084/jem.178.5.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. J., Freedman A. S., Rabinowe S. N., Segil J. M., Horowitz J., Rosen K., Whitman J. F., Nadler L. M. Interleukin 6 gene expression in normal and neoplastic B cells. J Clin Invest. 1989 May;83(5):1512–1518. doi: 10.1172/JCI114046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerne P. A., Zuraw B. L., Vaughan J. H., Carson D. A., Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J Clin Invest. 1989 Feb;83(2):585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Kawano M. M., Huang N., Harada Y., Iwato K., Tanabe O., Tanaka H., Sakai A., Asaoku H., Kuramoto A. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood. 1993 May 15;81(10):2658–2663. [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hibi T., Dosch H. M. Limiting dilution analysis of the B cell compartment in human bone marrow. Eur J Immunol. 1986 Feb;16(2):139–145. doi: 10.1002/eji.1830160206. [DOI] [PubMed] [Google Scholar]
- Iguchi T., Ziff M. Electron microscopic study of rheumatoid synovial vasculature. Intimate relationship between tall endothelium and lymphoid aggregation. J Clin Invest. 1986 Feb;77(2):355–361. doi: 10.1172/JCI112312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsikis P. D., Chu C. Q., Brennan F. M., Maini R. N., Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994 May 1;179(5):1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Ziff M. Electron microscopic studies of lymphoid cells in the rheumatoid synovial membrane. Arthritis Rheum. 1973 Jul-Aug;16(4):471–486. doi: 10.1002/art.1780160407. [DOI] [PubMed] [Google Scholar]
- Koch G., Osmond D. G., Julius M. H., Benner R. The mechanism of thymus-dependent antibody formation in bone marrow. J Immunol. 1981 Apr;126(4):1447–1451. [PubMed] [Google Scholar]
- Korthäuer U., Graf D., Mages H. W., Brière F., Padayachee M., Malcolm S., Ugazio A. G., Notarangelo L. D., Levinsky R. J., Kroczek R. A. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993 Feb 11;361(6412):539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- Kozbor D., Moretta A., Messner H. A., Moretta L., Croce C. M. Tp44 molecules involved in antigen-independent T cell activation are expressed on human plasma cells. J Immunol. 1987 Jun 15;138(12):4128–4132. [PubMed] [Google Scholar]
- Kurosaka M., Ziff M. Immunoelectron microscopic study of the distribution of T cell subsets in rheumatoid synovium. J Exp Med. 1983 Oct 1;158(4):1191–1210. doi: 10.1084/jem.158.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. K., Bridges S. L., Jr, Kirkham P. M., Koopman W. J., Schroeder H. W., Jr Evidence of antigen receptor-influenced oligoclonal B lymphocyte expansion in the synovium of a patient with longstanding rheumatoid arthritis. J Clin Invest. 1994 Jan;93(1):361–370. doi: 10.1172/JCI116968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky P. E., Davis L. S., Cush J. J., Oppenheimer-Marks N. The role of cytokines in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1989;11(2):123–162. doi: 10.1007/BF00197186. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Johnson G. D., Gordon J., MacLennan I. C. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992 Jan;13(1):17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- Miossec P., Briolay J., Dechanet J., Wijdenes J., Martinez-Valdez H., Banchereau J. Inhibition of the production of proinflammatory cytokines and immunoglobulins by interleukin-4 in an ex vivo model of rheumatoid synovitis. Arthritis Rheum. 1992 Aug;35(8):874–883. doi: 10.1002/art.1780350805. [DOI] [PubMed] [Google Scholar]
- Natvig J. B., Randen I., Thompson K., Førre O., Munthe E. The B cell system in the rheumatoid inflammation. New insights into the pathogenesis of rheumatoid arthritis using synovial B cell hybridoma clones. Springer Semin Immunopathol. 1989;11(3):301–313. [PubMed] [Google Scholar]
- Olee T., Lu E. W., Huang D. F., Soto-Gil R. W., Deftos M., Kozin F., Carson D. A., Chen P. P. Genetic analysis of self-associating immunoglobulin G rheumatoid factors from two rheumatoid synovia implicates an antigen-driven response. J Exp Med. 1992 Mar 1;175(3):831–842. doi: 10.1084/jem.175.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randen I., Pascual V., Victor K., Thompson K. M., Førre O., Capra J. D., Natvig J. B. Synovial IgG rheumatoid factors show evidence of an antigen-driven immune response and a shift in the V gene repertoire compared to IgM rheumatoid factors. Eur J Immunol. 1993 Jun;23(6):1220–1225. doi: 10.1002/eji.1830230604. [DOI] [PubMed] [Google Scholar]
- Roldán E., García-Pardo A., Brieva J. A. VLA-4-fibronectin interaction is required for the terminal differentiation of human bone marrow cells capable of spontaneous and high rate immunoglobulin secretion. J Exp Med. 1992 Jun 1;175(6):1739–1747. doi: 10.1084/jem.175.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T., Narazaki M., Yasukawa K., Saito T., Miki D., Hamaguchi M., Davis S., Shoyab M., Yancopoulos G. D., Kishimoto T. Functional inhibition of hematopoietic and neurotrophic cytokines by blocking the interleukin 6 signal transducer gp130. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10998–11001. doi: 10.1073/pnas.89.22.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallé A., Zuber C. E., Defrance T., Djossou O., De Rie M., Banchereau J. Activation of human B lymphocytes through CD40 and interleukin 4. Eur J Immunol. 1989 Aug;19(8):1463–1467. doi: 10.1002/eji.1830190818. [DOI] [PubMed] [Google Scholar]
- Vernino L., McAnally L. M., Ramberg J., Lipsky P. E. Generation of nondividing high rate Ig-secreting plasma cells in cultures of human B cells stimulated with anti-CD3-activated T cells. J Immunol. 1992 Jan 15;148(2):404–410. [PubMed] [Google Scholar]