C–H bond functionalization enables strategically new approaches to complex organic compounds including biologically active agents, research probes and functional organic materials.1 Catalytic coupling of sp3 C–H bonds and alkenes is an attractive strategy as it provides an overall alkylation of saturated carbon centers under nonbasic conditions. To complement transition metal-catalyzed processes,2 we have developed a conceptually different approach to direct coupling of sp3 C–H bonds and alkenes based on Lewis acid-promoted hydride transfer (Scheme 1A).3 Although acid-triggered hydride transfer reactions are known, there are only a few processes where hydride transfer is coupled to C–C bond formation in a predictable manner.4 We here report a simple and economical method, based on the generation of highly activated alkenyl-oxocarbenium intermediates, which expands the scope and efficiency of hydride transfer-initiated cyclization reactions and avoids the use of transition metal catalysts.
Scheme 1.
Compensating Lower Reactivity of C–H Bonds by Increasing the Activation of the Alkene
a Isolated yield of diastereomeric mixtures. Diastereomeric ratio determined by 1H NMR. b Contained varying amounts (<5%) of hydrolyzed products.
We have previously found that in the cyclization reactions shown in Scheme 1A, substrates with less reactive C–H bonds (reactivity can be estimated by considering the cation-stabilizing ability of adjacent groups) required the use of expensive transition metal Lewis acids such as PtCl4, or were altogether resistant to the hydride transfer.3 We now provide additional evidence for this finding, illustrated by substrate 1 which undergoes a very slow cyclization under the preferred conditions (BF3•Et2O, CH2Cl2, RT), taking four days to afford a low yield of product 2 (Scheme 1B). To address this problem and avoid the use of expensive Lewis acids, we examined the reactivity of the corresponding alkenyl acetals, inspired by the high reactivity of alkenyl-oxocarbenium intermediates toward Diels-Alder reactions and other transformations.5 When submitted to the standard reaction conditions, acetal 3 was consumed within one hour, providing a good yield of the cyclic product 4; direct comparison of acetal 3 to the corresponding aldehyde 1 revealed a dramatic increase in both reactivity and chemical yield, as well as an improvement in diastereoselectivity. Remarkably, a primary ether can undergo alkylation in the α-position at room temperature!
The mechanistic rationale is depicted in Scheme 2; boron trifluoride etherate opens the cyclic acetal, generating the oxocarbenium intermediate II, which activates the conjugated double bond for the hydride abstraction. Subsequent to the hydride transfer step, the resulting oxocarbenium-enolether intermediate III undergoes rapid C–C bond formation and the new oxocarbenium species reforms the acetal, producing the desired product V and the Lewis acid catalyst. The observed stereoselectivity can be explained by the favorable transition state, IV, where all substituents are in equatorial positions.
Scheme 2.
Proposed Mechanistic Rationale
We next examined the reactivity of different C–H bonds (in the α-position to the ether oxygen) in the context of alkenyl acetal and ketal substrates (Table 1). These compounds are readily available in few synthetic steps where the alkenyl acetal moiety is installed by the cross-metathesis between the homoallylic ether or alcohol and commercially available 2-vinyl-1,3-dioxolane (or 2-methyl-2-vinyl-1,3-dioxolane, Supporting Information). The activated benzyl ether 5 affords the cyclized product in excellent yield and diastereoselectivity (90%, >20:1, Table 1). The acetal moiety also enabled efficient cyclization of the allyl ether 7 and the crotyl ether 9 (Table 1, entries 2 and 3).6 As indicated by the cyclization of the ethyl ether 3 (Scheme 1B), less reactive alkyl ethers also underwent the desired cyclization in high yield and excellent stereoselectivity, including the more hindered isopropyl ether 11 and cyclohexyl ether 13, both readily available from 2-propanol and cyclohexanol, respectively (Table 1, entries 4 and 5). Comparable yields and stereoselectivity were obtained with lower catalyst loading, which required longer reaction times (5 mol% BF3•Et2O, Table 1, entry 4). Other Lewis and protic acids were examined (e.g., TMSOTf, TiCl4, Bi(OTf)3, MsOH) and proved inferior to BF3•Et2O in terms of obtained yields (by >20%).
Table 1.
Cyclization of Alkenyl Acetal/Ketal Substrates
Reactions performed at 0.02–0.03 M in CH2Cl2 with BF3•Et2O (0.5 equiv.) at room temperature (<1 h). The major diastereomer is shown.
Isolated yield as an average of three runs.
Diastereomeric ratio determined by 1H NMR or GC.
Isolated yield using 0.05 equivalents of BF3•Et2O after 3 h.
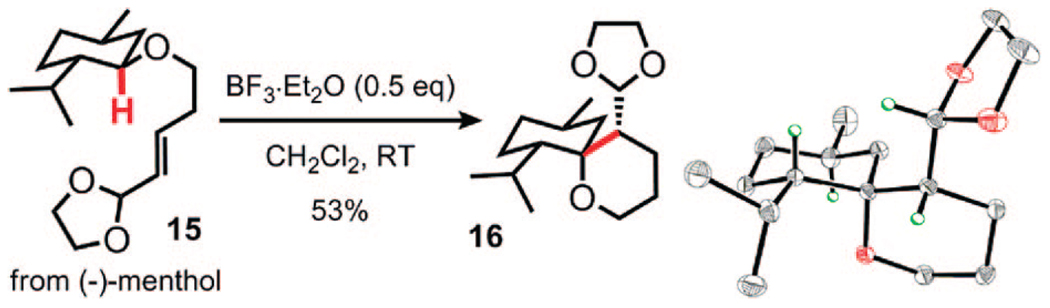
To explore the alkylation reaction in a more complex structural context and to examine the effect of other chirality elements in the molecule, we synthesized substrate 15 from (–)-menthol (Figure 1). Under the standard reaction conditions, this compound gave product 16 as the only observed diastereomer. The molecular structure was confirmed by X-ray analysis (Figure 1). Apparently, the stereoselectivity of the C–C bond forming step is controlled by the adjacent center bearing the isopropyl group. This example illustrates the synthetic power of the hydride transfer-triggered cyclization: a tertiary center is transformed in one step into a quaternary ether center under mild conditions and with excellent stereocontrol, providing a novel and structurally complex spirocyclic product from a readily available terpene.
Figure 1.
Cyclization of menthol-derived alkenyl acetal 15. Molecular structure of product 16 as revealed by X-ray analysis. Selected hydrogen atoms have been added for clarity.
We next considered the idea of generating the key alkenyl-oxocarbenium intermediate (such as II, Scheme 2) from a ketone and ethylene glycol in situ, which would eliminate the need for preparation of the corresponding ketal. Indeed, addition of ethylene glycol to boron trifluoride etherate in dichloromethane had a dramatic effect on the reaction rate as demonstrated in the cyclization of enone 17; the reaction was complete in less than 12 h, while the same conditions in the absence of ethylene glycol required 96 h to reach completion (Table 2, the rate plot is shown in the Supporting Information). Optimization of the reaction conditions showed that best results were obtained with 0.2 equivalents of ethylene glycol under standard conditions; other diols including chiral diols were investigated and found to be less effective than ethylene glycol (Supporting Information). Examining the ethylene glycol effect with the benzyl ether substrate 19 showed not only a 5-fold increase in rate, but also an improvement in the isolated yield and stereoselectivity (Table 2, entry 2). Finally, the slow trans-annular cyclization of cyclohexenone 21 was accelerated by the addition of ethylene glycol, affording bicyclic product 22 in 79% yield as a single diastereoisomer.
Table 2.
Ethylene Glycol Serves as an Organocatalyst.
 | |||||
|---|---|---|---|---|---|
| substrate | product | conditionsa | time(h) | yield | dr |
 |
 |
A | 96 | 86% | 1.5:1 |
| B | 12 | 83% | 2.4:1 | ||
 |
 |
Ab | 14 | 88% | 8:1 |
| Bb | 3 | 95% | 14:1 | ||
 |
 |
A | 96 | 81% | - |
| B | 24 | 79% | - | ||
Conditions A: BF3•Et2O (0.5 equiv). Conditions B: BF3•Et2O (0.5 equiv) and ethylene glycol (0.2 equiv). Reactions performed at 50 °C. Isolated yield as an average of three runs. Diastereomeric ratio determined by NMR or GC.
Reaction run at room temperature.
The use of boron trifluoride etherate as the Lewis acid and ethylene glycol as the organocatalyst provides a highly active catalytic system, presumably via the in situ formation of alkenyl-oxocarbenium intermediates, which eliminates the need for expensive transition metal Lewis acids or the preparation of acetal/ketal substrates.7,8 This binary catalytic system expands the scope and improves the efficiency of the hydride transfer-initiated alkylation reactions.
Supplementary Material
Acknowledgment
This work was supported by the National Institute of General Medical Sciences (NIGMS). We thank Dr. S. J. Pastine for helpful discussions and Professor G Parkin’s group for the X-ray analysis (Columbia University, CHE-0619638).
Footnotes
Supporting Information Available: Experimental procedures and spectroscopic data for starting materials and products. X-ray data for compound 16 and 22. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Godula K, Sames D. Science. 2006;312:67–72. doi: 10.1126/science.1114731. [DOI] [PubMed] [Google Scholar]; (b) Davies HML. Angew. Chem., Int. Ed. 2006;45:6422–6425. doi: 10.1002/anie.200601814. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kakiuchi F, Chatani N. Adv. Synth. Catal. 2003;345:1077–1101. [Google Scholar]; (b) DeBoef B, Pastine SJ, Sames D. J. Am. Chem. Soc. 2004;126:6556–6557. doi: 10.1021/ja049111e. [DOI] [PubMed] [Google Scholar]; (c) Shi L, Tu Y-Q, Wang M, Zhang F-M, Fan C-A, Zhao Y-M, Xia W-J. J. Am. Chem. Soc. 2005;127:10836–10837. doi: 10.1021/ja0528331. [DOI] [PubMed] [Google Scholar]
- 3.Pastine SJ, McQuaid KM, Sames D. J. Am. Chem. Soc. 2005;127:12180–12181. doi: 10.1021/ja053337f. [DOI] [PubMed] [Google Scholar]
- 4.The tert-amino effect cyclizations: Verboom W, Reinhoudt DN, Visser R, Harkema S. J. Org. Chem. 1984;49:269–276.. Recent review: Mátyus P, Éliás O, Tapolcsányi P, Polonka-Bálint A, Halász-Dajka B. Synthesis. 2006;16:2625–2639.. Formation of carbocycles: Atkinson RS, Green RH. J. Chem. Soc, Perkin Tans. 1974;1:401..
- 5. Gassman PG, Singleton DA, Wilwerding JJ, Chavan SP. J. Am. Chem. Soc. 1987;109:2182–2184. Roush WR, Gillis HR, Essenfeld AP. J. Org. Chem. 1984;49:4674–4682.. Review: Harmata M, Rashatasakhon P. Tetrahedron. 2003;59:2371–2395..
- 6.For oxidative alkylation of benzyl and allyl ethers using an external oxidant. see: Tu W, Liu L, Floreancig PE. Angew. Chem., Int. Ed. 2008;47:4184–4187. doi: 10.1002/anie.200706002..
- 7.For enantioselective hydride reduction of α,β-unsaturated aldehydes and ketones using amine organocatalysts, see: Ouellet SG, Turtle JB, MacMillan DWC. J. Am. Chem. Soc. 2005;127:32–33. doi: 10.1021/ja043834g. Ouellet SG, Walji AM, MacMillan DWC. Acc. Chem. Res. 2007;40:1327–1339. doi: 10.1021/ar7001864..
- 8.Unfortunately, the amine organocatalysts were not able to catalyze the intramolecular alkylation reactions discussed in this paper.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.