Abstract
Protein tyrosine phosphatase SHP-1 is an essential regulatory molecule in many different signaling pathways. The biological importance of SHP-1 is underscored by the motheaten mutant mouse strains with immunological disorders involving multiple organs and by the close association of aberrant SHP-1 expression with several human diseases. Recent studies provided some compelling evidence that supports a role of SHP-1 in regulating mast cell development and function and also in regulating type 2 allergic inflammatory responses in both innate and adaptive immune responses. In this article, we summarize the recent advancement of our understanding of this interesting phosphatase in the important area of allergic inflammation.
Keywords: Phosphatase, Mast cells, Th2 cytokines, Allergic inflammatory response, Allergy, Asthma
Introduction
Protein and lipid phosphorylation is an essential mechanism for cell signaling. On the other hand, phosphatase-mediated dephosphorylation as the opposing process is equally important. To date, more than 100 phosphatases have been identified in the human genome and some are well researched. However, very little is known about the majority of these enzymes. Src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP-1) is one of the phosphatases that have been extensively studied. Currently, there are nearly 1,000 original research publications and 100 reviews on topics related to SHP-1. The biology of SHP-1 has been well summarized in many reviews. In the March 2009 volume of Immunological Reviews, Gilfillan et al. discussed the function of SHP-1 in relation to the IgE-FcεRI activation in mast cells and Lorenz reviewed studies on the function of SHP-1 in T cells [1, 2]. Yet much more remains to be learned about this interesting and essential phosphatase in different areas. From some of the recent studies, evidence of a critical role of SHP-1 in regulating type 2 inflammatory responses, particularly in the lung, has started to emerge.
In this article, we will summarize the recent development in our understanding of the functions of SHP-1 in immunological homeostasis in the lung, in adaptive allergic responses to aeroallergen challenge, and in regulation of mast cell differentiation and functions in relation to allergic inflammatory and anaphylactic responses.
SHP-1 in immunological homeostasis of the lung
The first report of a mutant motheaten (me) mouse strain by Green and Shultz was published in 1975 [3]. Subsequently, a similar mutant strain, viable motheaten (mev) mouse, was described. Both mutations were found to be at the SHP-1 gene, though at different positions with different degrees of protein deficiency [4, 5]. Phenotypically, both me and mev mice have been found to have immunodeficiency but also with autoimmune diseases. Early in life, these mice develop spontaneous inflammatory abnormalities in multiple organs, including the skin, kidney, joints, and the lung [3, 6–9]. The pulmonary pathology in these mice has been described as unusual pneumonia, pneumonitis, or interstitial lung disease with progressive infiltration of macrophages, neutrophils, and lymphocytes [3, 6–8]. However, the nature of the inflammation in the lung was not clear, since the concept of different types of inflammatory responses had not been well established at the time.
To understand the function of SHP-1 in the immunological homeostasis in the lung, we determined the specific type of the inflammation in the lung of mev homozygous mice in a systematic approach. For this study, we used mev mice, not me mice, because mev mice have a relatively milder pathology and a longer life span, providing a larger window for research [7]. Wild type and mev mice at age of 7–9 weeks were examined and compared under a specific pathogen-free environment without any known stimulation.
Evaluation of the pulmonary inflammation by bronchoalveolar lavage (BAL) and lung histopathology showed that mev mice have significant inflammatory cell infiltration in the airways and in the lung parenchyma, ranging in degrees from focal to diffuse to massive, with consolidated lobes in some mice. The numbers of macrophages and eosinophils are highly increased, and lymphocytes and neutrophils are also increased. Goblet cell metaplasia and mucus hyperproduction are readily seen in the airway epithelium of mev mice by PAS or Alcian blue staining for mucin. Increased collagen deposition in subepithelial and parenchymal areas is revealed by Masson’s trichrome staining. Furthermore, mev mice have altered lung physiology as pulmonary function tests (PFT) show that these mice have significantly increased airway resistance in the absence of any stimulation and markedly increased airway hyperresponsiveness (AHR) upon methacholine challenge. Some of the mev mice died at low-dose methacholine challenge. Together, these features are indicative of an allergic-type inflammatory response usually seen in allergic asthma. At the molecular level, Th2 cytokines IL-4, IL-5, and IL-13, but not Th1 cytokine IFN-γ, are found to be significantly upregulated in the lung of mev mice. Correspondingly, Th2-related chemokines are also highly upregulated, including CCL2/MCP-1 and CCL11/Eotaxin, which are able to recruit monocytes and eosinophils. Consistent with these observations, phosphorylation of STAT6 is significantly enhanced in the lung tissue of mev mice. To prove more definitively that the Th2 signaling pathway plays a critical role in the mev lung phenotype, we took the genetic approach and cross-bred mev mice to those with targeted gene deletion at the IL-4, IL-13, or STAT6 gene locus and examined the phenotypical changes of their offspring. Selective gene deletion in mev mice showed that IL-13 and STAT6 play a critical role in pulmonary inflammation and mucus hyperproduction, whereas IL-4 plays only a minor or no role [10]. Similarly, deletion of the IL-4Rα gene in mev mice also significantly reduced the inflammation and mucus hyperproduction in the mev lung (Oh et al. unpublished data). Interestingly, there is still residual inflammation in the lung of mev mice on IL-13 or STAT6 knockout background, indicating that other signaling pathways may also be involved in the phenotype generation.
Taken together, these studies demonstrate that the spontaneous pulmonary pathology of mev mice is a Th2-dominated allergic-type inflammatory disorder, which is largely dependent on the Th2 signaling, particularly IL-13, STAT6, and IL-4Rα, with an unidentified source of Th2 cytokine production.
SHP-1 in adaptive immune response to allergen challenge in the airway
The role of SHP-1 in adaptive immune response to allergen stimulation was investigated using heterozygous me/+ mice in an ovalbumin (OVA) plus adjuvant-induced allergic asthma model [11]. The SHP-1 protein in the T cells of heterozygous me/+ mice is about one-third of that of wild type mice. The OVA/adjuvant immunization and challenge method is a strong protocol that induces a robust Th2-dominated inflammatory response in wild type mice. In SHP-1-deficient state, when sensitized and challenged with OVA allergen, me/+ mice displayed enhanced eosinophilic inflammation, mucus hyperproduction, and AHR that were correlated with increased generation of Th2 cells and specific cytokine production by bone marrow-derived mast cells (BMMC) [11]. These results indicate that SHP-1 may play an inhibitory role in adaptive immune response to allergens, possibly through the regulation of T cell and mast cell functions.
Our group investigated the function of SHP-1 in adaptive immune response in an aeroallergen tolerance model [12]. To avoid compounding effects, heterozygous mev/+ mice were used in this study since the SHP-1 enzyme activity in the lung tissue of these mice is about 60% of that of wild type mice and mev/+ mice are phenotypically normal. An OVA allergen tolerance model was used in which OVA allergen alone was given through inhalation, once a day for 5 days. The airway inflammation and histology were examined on day 7. This protocol normally elicits no noticeable inflammatory response in wild type mice. In mev/+ mice, however, a significantly enhanced eosinophil-dominated inflammation can be seen in the airways and in the lung parenchyma along with increased OVA-specific IgE in the serum and increased chemokine CCL20/MIP-3α in the lung. Interestingly, these responses are attenuated by anti-oxidant N-acetylcystein (NAC). These results indicate that SHP-1 deficiency leads to decreased tolerance to aeroallergen, which may be partially through oxidant stress [12].
It has been noted that SHP-1 and other phosphatases containing cysteine residues in the catalytic sites are susceptible to oxidant suppression. For instance, increased intracellular levels of H2O2 could inactivate phosphatases transiently [13–15]. Also, oxidant stress as in H2O2 induced a pro-type 2 inflammatory response in RBL-2H3 cells and in mouse BMMC [16, 17]. Indeed, other experiments in our study provided further evidence to support the notion of increased oxidant stress in mev/+ mice, including decreased capacity of alveolar macrophages to clear H2O2 load, increased nuclear translocation of the anti-oxidant transcription factor Nrf2 in splenocytes, and reduced tolerance to oxidant paraquat in the airways [12].
These studies defined a critical role of SHP-1 in adaptive immune response to allergen stimulation, specifically in controlling the degree of a robust Th2 response and the threshold of tolerance to aeroallergens. Potentially, some of these responses involve oxidants, and SHP-1 plays a regulatory role in this process.
SHP-1 in mast cell differentiation and function
Although it is well known that the mutant me and mev mice have immunological abnormalities, the cell types that are directly responsible for the inflammatory phenotype are not clear. Surprisingly, studies to identify the cell types showed that the motheaten pathology does not require mature B or T cells [18], but to a large extent, depend on myeloid cells [19, 20]. These studies provided first evidence that innate immune cells play an important role in the disease generation in these mice. With the spontaneous nature of the phenotype and a lack of identifiable allergen stimulation, it is reasonable to speculate that the type 2 allergic inflammatory response in the lung of these mice is initiated by cells other than B and T cells.
Since Th2 cytokines and signaling pathways are critical in the generation of the pulmonary pathology of mev mice, our attention was directed to the cell types that are able to produce Th2 cytokines and are able to initiate the Th2 inflammatory response. Mast cells, basophils, and eosinophils are good candidates, since these cells are able to produce significant amounts of Th2 cytokines, including IL-4 and IL-13, and these cell types have been known for their prominent roles in allergic responses, although mostly as effector cells [21–27]. In the next study, we focused on elucidating the roles of SHP-1 in regulating mast cell development and function.
SHP-1 has long been implicated in the development, signaling, and function of mast cells. Using genetic approaches, it has been shown that SHP-1 deficiency partially restores the mast cell numbers in c-Kit-deficient Wv/+ mice [19, 20]. In RBL-2H3 mast cells, SHP-1 constitutively binds FcεRI [28] and phosphorylation of SHP-1 is increased upon FcεRI activation [29]. However, studies on the role of SHP-1 in mast cell signaling and function have shown some conflicting results. In murine BMMC, SHP-1 negatively regulates SLP-76, linker for activation of T cells (LAT) and MAP kinases but may positively influence the interaction between SLP-76 and PLC-γ and subsequent calcium mobilization and degranulation [30]. Regarding proinflammatory cytokines, it was shown that in BMMC, SHP-1 negatively regulates mast cell production of IL-6 and IL-13 in response to IgE-FcεRI activation [11]. In contrast, in transfected RBL-2H3 cells, SHP-1 shows positive regulation of JNK activation and TNF-α production [31], although in vivo studies showed that the levels of TNF-α are highly elevated in the serum and in the lung tissue of SHP-1-deficient mev and me mice which are possibly responsible for some of the pathological changes in these mice [32, 33].
In the next series of experiments, we determined the functions of SHP-1 in mast cells in two main aspects: (1) Mast cell development, including differentiation, proliferation, and survival of BMMC from SHP-1-deficient mev mice; (2) Mast cell function and response to stimulation, including cytokine production and mediator release in response to stimuli, such as oxidants, bacterial product LPS, and allergen-induced IgE-FcεRI activation, in BMMC in vitro and in tissues of mev mice in vivo. Furthermore, we explored some potential molecular mechanisms that are involved in these processes.
Flow cytometry analysis showed that bone marrow cells from mev mice contain a significantly increased population of c-Kit/FcεRI double-positive cells, maturation markers for mast cells. When cultured in the IL-3-containing medium (WEHI conditioned medium), the percentage of this population is rapidly increasing in the first few days and the difference between mev cells and wild type cells is maintained until day 16 in culture. These results suggest that SHP-1 normally controls the number of mast cell progenitors in the bone marrow and SHP-1 regulates mast cell maturation.
Paradoxically, the proliferation rate of mev mast cells is significantly reduced compared to that of wild type cells when seeded either as total bone marrow cells or as mature mast cells in the same densities and determined by the XTT assay. One possible explanation for this observation is the up-regulation of anti-apoptotic gene Bcl-2 seen in mev mast cells. It has been reported that diminished cell proliferation was associated with increased Bcl-2, in parallel with increased cell survival. A possible mechanism is the accumulation of cells in the G0/G1 phase of the cell cycle when Bcl-2 expression is increased [34]. Indeed, compared to wild type cells, mev BMMC are more resistant to apoptosis. But the effect requires the presence of growth factor IL-3, and the SHP-1 deficiency itself does not engender survival signaling, indicating that the regulatory function of SHP-1 is associated with growth factor-induced receptor activation.
To determine the role of SHP-1 in mast cell functions, we measured the cytokine production and mediator release by BMMC in response to various stimuli. Our experiments showed that wild type mast cells are able to produce IL-4 when stimulated with H2O2, similar to what was described previously [17]. However, mev BMMC produce increased IL-4 and IL-13, but not IFN-γ, even in the absence of H2O2 and this can be blocked by anti-oxidant NAC, suggesting a heightened oxidative state in mast cells deficient in SHP-1 that favors Th2 cytokine production. Similarly, BMMC from mev mice produce significantly more IL-13 in response to LPS, which cannot be blocked by anti-oxidant NAC or c-Kit kinase inhibitor Gleevec, suggesting other signaling pathways such as MAP kinases may be involved [21]. A more general stimulation with PMA induced significantly increased IL-13, TNF-α, and IFN-γ in mev BMMC. These results have important implications that SHP-1-deficient mast cells are able to produce larger amounts of Th2 cytokines, particularly IL-13, in response to commonly seen environmental stimuli, such as LPS and oxidants.
Allergen-induced mast cell degranulation is an important process in allergic responses that involves mast cell activation by allergen-induced IgE-FcεRI ligation and release of proinflammatory mediators. Compared to BMMC from wild type mice, the total amount of β-hexosaminidase in the BMMC from mev mice is significantly higher, indicating increased production of mediators. In the absence of stimulation, mev BMMC release more β-hexosaminidase spontaneously compared to wild type BMMC. When stimulated by antigen DNP-HSA, IgE pre-sensitized mev BMMC release more β-hexosaminidase, which is further enhanced by stem cell factor (SCF; c-Kit ligand). Using immunoprecipitation and Western blotting with specific antibodies, we show that upon DNP-HSA stimulation of wild type BMMC, SHP-1 binds linker for activation of T cells 2 (LAT2) and LAT2 can be phosphorylated, which is further enhanced in the absence of SHP-1 in mev BMMC. These results suggest that mev BMMC produce and release higher amounts of mediators spontaneously and after FcεRI activation, which involves the regulatory molecule LAT2. These observations and some of the potential mechanisms by which SHP-1 interacts with components in the signaling pathways in mast cells and regulates mast cell functions are depicted in Fig. 1.
Fig. 1.
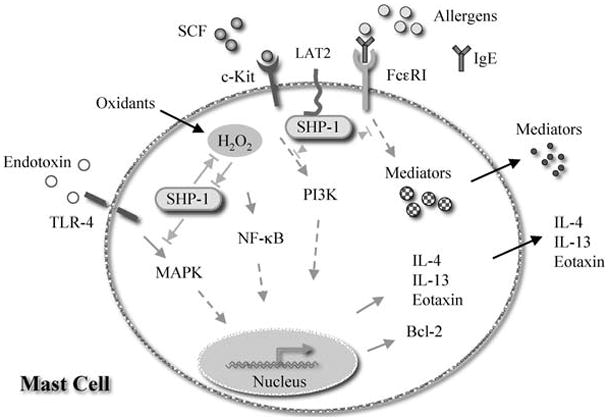
Schematic illustration of potential mechanisms by which SHP-1 regulates mast cell development and function. SHP-1 can bind to FcεRI and down-regulates its signaling after cross-linking induced by antigen and IgE, thus to control mast cell degranulation. In the process, SHP-1 may bind the adapter molecule LAT2. SHP-1 is also recruited to c-Kit when its ligand SCF is recognized. Through this interaction, SHP-1 is able to regulate mast cell differentiation, maturation, and survival, since c-Kit is essential for mast cells. The c-Kit signaling is largely through the PI3 kinase pathway. SHP-1 may be a target for intracellular oxidants, but SHP-1 may also be able to control oxidant-induced Th2 cytokine production by mast cells. This process is potentially regulated by the NF-κB signaling pathway. Finally, SHP-1 is a negative regulator of endotoxin (LPS)-induced mast cell production of IL-13. However, this regulation is independent of oxidant stress and NF-κB but possibly involves the MAP kinase signaling pathway. The up-regulation of anti-apoptotic gene Bcl-2 in the absence of SHP-1 regulation is able to increase the survival of mast cells, probably by a mechanism that keeps the cells in the G0/G1 phase of the cell cycle that slows down cell proliferation
These in vitro studies demonstrate mast cells are hyperresponsive in the absence of SHP-1 regulation. We asked the questions whether this is also the case in vivo and whether the alterations in mast cells are responsible for the allergic inflammatory phenotype in the lung of mev mice. In the next set of experiments, we determined the mast cell numbers and activities in the tissues and the relationship between mast cells and the pulmonary phenotype of mev mice. Lung histology with toluidine blue staining revealed that the number of mast cells in the lung tissue of mev mice is markedly higher than that in wild type mice and this is coincided with increased total IgE in the BAL fluid of mev mice. IgE has been reported to upregulate FcεRI, at least in mouse BMMC [35]. Measurement of histamine release showed that significantly increased amount of total and spontaneous release of histamine can be seen in the lung and spleen tissues of mev mice, consistent with the increased number of mast cells in the tissues. Furthermore, mev splenocytes produced more IL-13, IL-4, and CCL3/MIP-1α in response to FcεRIα ligation, indicating involvement of cell types that express FcεRI. On the other hand, compared to wild type cells, mev splenocytes produce more IP-10, same amount of IFN-γ, and decreased IL-12, regardless of FcεRI stimulation, indicating involvement of other cell types. Finally, backcross of mev mice to mast cell-deficient Kitw-sh genetic background leads to a dramatic reduction in the lung inflammation and decreased production of Th2 cytokines and chemokines, indicating SHP-1-deficient mast cells as a major source of Th2 cytokines and as an initiator of the mev lung phenotype [36].
Thus, these studies on bone marrow-derived mast cells in culture and tissue mast cells in mev mice demonstrate that SHP-1 is critical in regulating mast cell development and survival and SHP-1 is an essential regulator of mast cell functions in response to allergic and environmental stimuli in vivo (Table 1).
Table 1.
SHP-1 Regulation of mast cells
| Process | Examined | Regulation |
|---|---|---|
| Development | ||
| Differentiation | c-Kit/FcεRI | − |
| Proliferation | Growth | + |
| Survival | Apoptosis | − |
| Stimulation/response | ||
| Oxidant stress | IL-4/IL-13 | − |
| LPS | IL-13 | − |
| PMA/Ionomycin | IL-4/IL-13 | − |
| IgE-FcεRI | Mediators | − |
| Mast cells in lung tissue | ||
| Number | Cell count | − |
| Function | Cytokine/meditor | − |
Role of SHP-1 in anaphylaxis and food allergy
Anaphylaxis is an extreme form of Th2-mediated allergic responses to food or drugs that involves the production of IgE in the sensitization phase and activation of mast cells in the response phase. Without proper treatment, anaphylaxis could be fatal. Whether SHP-1 has a role in allergic anaphylactic responses is not known.
As discussed earlier, SHP-1 is intimately linked to mast cells in many aspects. In addition, several studies hinted a potential role of SHP-1 in anaphylaxis. A mouse Fcγ-Fcε fusion protein was able to block IgE-dependent anaphylaxis mediated by mast cells, and the process involved phosphorylation of inhibitory receptor FcγRIIB and recruitment of phosphatases SHIP-1, SHP-1, and SHP-2 [37], although the importance of SHP-1 in relation to FcγRIIB in mast cell activation is still debatable [38]. Also, recruitment of SHP-1 to the ITIM motifs is part of the function of another inhibitory receptor gp49B1, in regulating mast cell activation and IgE-FcεRI mediated local and systemic anaphylactic responses [39, 40]. However, since other inhibitory receptors exist with similar functions in regulating FcεRI activation that may also involve other phosphatases, whether the function of SHP-1 is unique or whether SHP-1 can be substituted by other phosphatases in these situations is not clear. More important, a direct functional link between SHP-1 and anaphylactic responses has not been established.
To investigate these possibilities, our laboratory initiated experiments using SHP-1-deficient me and mev mice in passive and active anaphylaxis models, including OVA allergen-induced and peanut allergen-induced systemic anaphylaxis. By measuring body temperature change and clinical scores, our preliminary experiments show that SHP-1 deficiency significantly enhances passive systemic anaphylaxis, correlating with enhanced mast cell responses in vitro as discussed earlier. Also, when sensitized and challenged with allergens, the SHP-1-deficient mice show prolonged OVA or peanut allergen-induced active systemic anaphylactic responses (Oh et al. Unpublished data). These findings begin to depict a direct role of SHP-1 in regulating mast cell responses and in anaphylaxis in vivo.
SHP-1 in human diseases
It is well documented that decreased expression of SHP-1 as a result of hypermethylation of its promoter regions is associated with lymphomas and leukemias in humans [41–46]. Decreased expression of SHP-1 phosphatase in erythrocyte progenitors was found in a large percentage of polycythemia vera patients in one study [47]. However, in two earlier reports, the levels of SHP-1 protein in peripheral blood granulocytes or CD34+ cells were normal and no abnormalities in the SHP-1 gene were found [48, 49]. Decreased levels of SHP-1 protein were associated with the progression of chronic myeloid leukemia [50]. Conversely, demethylation of SHP-1 promoter 2 and high levels of SHP-1 isoform II were found in epithelial tissues of patients with psoriasis [51]. Most recently, SHP-1 deficiency, increased inflammatory gene expression and enhanced activation of transcription factor STAT6, STAT1, and NF-κB were found in PBMC and macrophages of patients with multiple sclerosis [52, 53]. This is supported by observations of an enhanced inflammatory response in SHP-1-deficient mev/+ mice in an EAE model [54].
So far, altered expression of SHP-1 in cells or tissues of allergic or asthmatic patients has not been reported. Considering our accumulating knowledge of the functions of SHP-1 in regulating Th2 cytokine production and allergic inflammatory responses in mast cells and in animal models, carefully designed studies of allergic and asthmatic patient population should be worthy of pursuing to determine the involvement of phosphatase SHP-1 in these human diseases.
Conclusion
It is clear that SHP-1, as a critical regulatory molecule in a variety of signaling pathways, plays important roles in different diseases in humans and in various disorders in mice. The basic function of SHP-1 in dephosphorylation to dampen down signals in a variety of pathways determines its function in limiting the extent, rather than the direction, of the activation. Accumulating evidence supports the notion that the functions of SHP-1 in immunological responses involve both innate and adaptive immunity and the regulation is not restricted to the specific types of inflammation.
Still it is remarkable that SHP-1-deficient mice develop a mast cell-dependent and Th2 signaling-dependent inflammatory phenotype in the lung, even though the disorders in other tissues of these mice may have different characteristics. The underlying mechanisms for the SHP-1-deficient mast cells to favor Th2 responses and the Th2 propensity of the lung still remain to be elucidated.
Acknowledgments
This work was supported by the NIH grants HL079349 to ZZ and AI075025 to TZ and a grant from the China Scholarship Council to LZ. We thank Phyllis Zhu for proofreading the manuscript.
Abbreviations
- BMMC
Bone marrow-derived mast cells
- FcεRI
High-affinity Fc receptor for IgE
- SCF
Stem cell factor or c-Kit ligand
- me
Motheaten
- mev
Viable motheaten
- LAT2
Linker for activation of T cells 2, also called NTAL or LAB
References
- 1.Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–69. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228:342–59. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green MC, Shultz LD. Motheaten, an immunodeficient mutant of the mouse. I. Genetics and pathology. J Hered. 1975;66:250–8. doi: 10.1093/oxfordjournals.jhered.a108625. [DOI] [PubMed] [Google Scholar]
- 4.Shultz LD, et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–54. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 5.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet. 1993;4:124–9. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 6.Ward JM. Pulmonary pathology of the motheaten mouse. Vet Pathol. 1978;15:170–8. doi: 10.1177/030098587801500203. [DOI] [PubMed] [Google Scholar]
- 7.Shultz LD, Coman DR, Bailey CL, Beamer WG, Sidman CL. “Viable motheaten”, a new allele at the motheaten locus. I. Pathology. Am J Pathol. 1984;116:179–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi GA, Hunninghake GW, Kawanami O, Ferrans VJ, Hansen CT, Crystal RG. Motheaten mice–an animal model with an inherited form of interstitial lung disease. Am Rev Respir Dis. 1985;131:150–8. doi: 10.1164/arrd.1985.131.1.150. [DOI] [PubMed] [Google Scholar]
- 9.Kovarik J, Kuntz L, Ryffel B, Borel JF. The viable motheaten (mev) mouse–a new model for arthritis. J Autoimmun. 1994;7:575–88. doi: 10.1006/jaut.1994.1042. [DOI] [PubMed] [Google Scholar]
- 10.Oh SY, et al. A critical role of SHP-1 in regulation of type 2 inflammation in the lung. Am J Respir Cell Mol Biol. 2009;40:568–74. doi: 10.1165/rcmb.2008-0225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamata T, et al. src homology 2 domain-containing tyrosine phosphatase SHP-1 controls the development of allergic airway inflammation. J Clin Invest. 2003;111:109–19. doi: 10.1172/JCI15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho YS, Oh SY, Zhu Z. Tyrosine phosphatase SHP-1 in oxidative stress and development of allergic airway inflammation. Am J Respir Cell Mol Biol. 2008;39:412–9. doi: 10.1165/rcmb.2007-0229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunnick JM, Dorsey JF, Mei L, Wu J. Reversible regulation of SHP-1 tyrosine phosphatase activity by oxidation. Biochem Mol Biol Int. 1998;45:887–94. doi: 10.1002/iub.7510450506. [DOI] [PubMed] [Google Scholar]
- 14.Meng TC, Fukada T, Tonks NK. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell. 2002;9:387–99. doi: 10.1016/s1097-2765(02)00445-8. [DOI] [PubMed] [Google Scholar]
- 15.Heneberg P, Draber P. Regulation of cys-based protein tyrosine phosphatases via reactive oxygen and nitrogen species in mast cells and basophils. Curr Med Chem. 2005;12:1859–71. doi: 10.2174/0929867054546636. [DOI] [PubMed] [Google Scholar]
- 16.Frossi B, De Carli M, Daniel KC, Rivera J, Pucillo C. Oxidative stress stimulates IL-4 and IL-6 production in mast cells by an APE/Ref-1-dependent pathway. Eur J Immunol. 2003;33:2168–77. doi: 10.1002/eji.200323995. [DOI] [PubMed] [Google Scholar]
- 17.Frossi B, Rivera J, Hirsch E, Pucillo C. Selective activation of Fyn/PI3K and p38 MAPK regulates IL-4 production in BMMC under nontoxic stress condition. J Immunol. 2007;178:2549–55. doi: 10.4049/jimmunol.178.4.2549. [DOI] [PubMed] [Google Scholar]
- 18.Yu CC, Tsui HW, Ngan BY, Shulman MJ, Wu GE, Tsui FW. B and T cells are not required for the viable motheaten phenotype. J Exp Med. 1996;183:371–80. doi: 10.1084/jem.183.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulson RF, Vesely S, Siminovitch KA, Bernstein A. Signalling by the W/Kit receptor tyrosine kinase is negatively regulated in vivo by the protein tyrosine phosphatase Shp1. Nat Genet. 1996;13:309–15. doi: 10.1038/ng0796-309. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz U, et al. Genetic analysis reveals cell type-specific regulation of receptor tyrosine kinase c-Kit by the protein tyrosine phosphatase SHP1. J Exp Med. 1996;184:1111–26. doi: 10.1084/jem.184.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda A, Yoshikai Y, Aiba K, Matsuguchi T. Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distinctly regulated by c-Jun N-terminal kinase and p38 pathways. J Immunol. 2002;169:3801–10. doi: 10.4049/jimmunol.169.7.3801. [DOI] [PubMed] [Google Scholar]
- 22.Pawankar R, Okuda M, Yssel H, Okumura K, Ra C. Nasal mast cells in perennial allergic rhinitics exhibit increased expression of the Fc epsilonRI, CD40L, IL-4, and IL-13, and can induce IgE synthesis in B cells. J Clin Invest. 1997;99:1492–9. doi: 10.1172/JCI119311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346:1699–705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 24.MacGlashan D, Jr, White JM, Huang SK, Ono SJ, Schroeder JT, Lichtenstein LM. Secretion of IL-4 from human basophils. The relationship between IL-4 mRNA and protein in resting and stimulated basophils. J Immunol. 1994;152:3006–16. [PubMed] [Google Scholar]
- 25.Gibbs BF, et al. Purified human peripheral blood basophils release interleukin-13 and preformed interleukin-4 following immunological activation. Eur J Immunol. 1996;26:2493–8. doi: 10.1002/eji.1830261033. [DOI] [PubMed] [Google Scholar]
- 26.Redrup AC, Howard BP, MacGlashan DW, Jr, Kagey-Sobotka A, Lichtenstein LM, Schroeder JT. Differential regulation of IL-4 and IL-13 secretion by human basophils: their relationship to histamine release in mixed leukocyte cultures. J Immunol. 1998;160:1957–64. [PubMed] [Google Scholar]
- 27.Rumbley CA, Sugaya H, Zekavat SA, El Refaei M, Perrin PJ, Phillips SM. Activated eosinophils are the major source of Th2-associated cytokines in the schistosome granuloma. J Immunol. 1999;162:1003–9. [PubMed] [Google Scholar]
- 28.Kimura T, Zhang J, Sagawa K, Sakaguchi K, Appella E, Siraganian RP. Syk-independent tyrosine phosphorylation and association of the protein tyrosine phosphatases SHP-1 and SHP-2 with the high affinity IgE receptor. J Immunol. 1997;159:4426–34. [PubMed] [Google Scholar]
- 29.Ozawa T, Nakata K, Mizuno K, Yakura H. Negative autoregulation of Src homology region 2-domain-containing phosphatase-1 in rat basophilic leukemia-2H3 cells. Int Immunol. 2007;19:1049–61. doi: 10.1093/intimm/dxm070. [DOI] [PubMed] [Google Scholar]
- 30.Nakata K, et al. Positive and negative regulation of high affinity IgE receptor signaling by Src homology region 2 domain-containing phosphatase 1. J Immunol. 2008;181:5414–24. doi: 10.4049/jimmunol.181.8.5414. [DOI] [PubMed] [Google Scholar]
- 31.Xie ZH, Zhang J, Siraganian RP. Positive regulation of c-Jun N-terminal kinase and TNF-alpha production but not histamine release by SHP-1 in RBL-2H3 mast cells. J Immunol. 2000;164:1521–8. doi: 10.4049/jimmunol.164.3.1521. [DOI] [PubMed] [Google Scholar]
- 32.Thrall RS, Vogel SN, Evans R, Shultz LD. Role of tumor necrosis factor-alpha in the spontaneous development of pulmonary fibrosis in viable motheaten mutant mice. Am J Pathol. 1997;151:1303–10. [PMC free article] [PubMed] [Google Scholar]
- 33.Su X, Zhou T, Yang P, Edwards CK, III, Mountz JD. Reduction of arthritis and pneumonitis in motheaten mice by soluble tumor necrosis factor receptor. Arthritis Rheum. 1998;41:139–49. doi: 10.1002/1529-0131(199801)41:1<139::AID-ART17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 34.Borner C. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J Biol Chem. 1996;271:12695–8. doi: 10.1074/jbc.271.22.12695. [DOI] [PubMed] [Google Scholar]
- 35.Hsu C, MacGlashan D., Jr IgE antibody up-regulates high affinity IgE binding on murine bone marrow-derived mast cells. Immunol Lett. 1996;52:129–34. doi: 10.1016/0165-2478(96)02599-0. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Oh SY, Wu X, Oh MH, Wu F, Schroeder JT, et al. SHP-1 deficient mast cells are hyperresponsive to stimulation and critical in initiating allergic inflammation in the lung. J Immunol. 2009 doi: 10.4049/jimmunol.0901972. ePub 2010/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mertsching E, et al. A mouse Fcgamma-Fcepsilon protein that inhibits mast cells through activation of FcgammaRIIB, SH2 domain-containing inositol phosphatase 1, and SH2 domain-containing protein tyrosine phosphatases. J Allergy Clin Immunol. 2008;121:441–7. doi: 10.1016/j.jaci.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 38.Fong DC, Malbec O, Arock M, Cambier JC, Fridman WH, Daeron M. Selective in vivo recruitment of the phosphatidylinositol phosphatase SHIP by phosphorylated Fc gammaRIIB during negative regulation of IgE-dependent mouse mast cell activation. Immunol Lett. 1996;54:83–91. doi: 10.1016/s0165-2478(96)02654-5. [DOI] [PubMed] [Google Scholar]
- 39.Lu-Kuo JM, Joyal DM, Austen KF, Katz HR. gp49B1 inhibits IgE-initiated mast cell activation through both immunoreceptor tyrosine-based inhibitory motifs, recruitment of src homology 2 domain-containing phosphatase-1, and suppression of early and late calcium mobilization. J Biol Chem. 1999;274:5791–6. doi: 10.1074/jbc.274.9.5791. [DOI] [PubMed] [Google Scholar]
- 40.Daheshia M, Friend DS, Grusby MJ, Austen KF, Katz HR. Increased severity of local and systemic anaphylactic reactions in gp49B1-deficient mice. J Exp Med. 2001;194:227–34. doi: 10.1084/jem.194.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Q, Raghunath PN, Vonderheid E, Odum N, Wasik MA. Lack of phosphotyrosine phosphatase SHP-1 expression in malignant T-cell lymphoma cells results from methylation of the SHP-1 promoter. Am J Pathol. 2000;157:1137–46. doi: 10.1016/S0002-9440(10)64629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oka T, et al. Reduction of hematopoietic cell-specific tyrosine phosphatase SHP-1 gene expression in natural killer cell lymphoma and various types of lymphomas/leukemias: combination analysis with cDNA expression array and tissue microarray. Am J Pathol. 2001;159:1495–505. doi: 10.1016/S0002-9440(10)62535-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chim CS, Wong KY, Loong F, Srivastava G. SOCS1 and SHP1 hypermethylation in mantle cell lymphoma and follicular lymphoma: implications for epigenetic activation of the Jak/STAT pathway. Leukemia. 2004;18:356–8. doi: 10.1038/sj.leu.2403216. [DOI] [PubMed] [Google Scholar]
- 44.Khoury JD, Rassidakis GZ, Medeiros LJ, Amin HM, Lai R. Methylation of SHP1 gene and loss of SHP1 protein expression are frequent in systemic anaplastic large cell lymphoma. Blood. 2004;104:1580–1. doi: 10.1182/blood-2004-03-1151. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Q, Wang HY, Marzec M, Raghunath PN, Nagasawa T, Wasik MA. STAT3- and DNA methyltransferase 1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes. Proc Natl Acad Sci U S A. 2005;102:6948–53. doi: 10.1073/pnas.0501959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy J, et al. Differential methylation of genes that regulate cytokine signaling in lymphoid and hematopoietic tumors. Oncogene. 2005;24:732–6. doi: 10.1038/sj.onc.1208032. [DOI] [PubMed] [Google Scholar]
- 47.Wickrema A, et al. Defective expression of the SHP-1 phosphatase in polycythemia vera. Exp Hematol. 1999;27:1124–32. doi: 10.1016/s0301-472x(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 48.Asimakopoulos FA, et al. The gene encoding hematopoietic cell phosphatase (SHP-1) is structurally and transcriptionally intact in polycythemia vera. Oncogene. 1997;14:1215–22. doi: 10.1038/sj.onc.1200942. [DOI] [PubMed] [Google Scholar]
- 49.Andersson P, LeBlanc K, Eriksson BA, Samuelsson J. No evidence for an altered mRNA expression or protein level of haematopoietic cell phosphatase in CD34+ bone marrow progenitor cells or mature peripheral blood cells in polycythaemia vera. Eur J Haematol. 1997;59:310–7. doi: 10.1111/j.1600-0609.1997.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 50.Amin HM, Hoshino K, Yang H, Lin Q, Lai R, Garcia-Manero G. Decreased expression level of SH2 domain-containing protein tyrosine phosphatase-1 (Shp1) is associated with progression of chronic myeloid leukaemia. J Pathol. 2007;212:402–10. doi: 10.1002/path.2178. [DOI] [PubMed] [Google Scholar]
- 51.Ruchusatsawat K, Wongpiyabovorn J, Shuangshoti S, Hirankarn N, Mutirangura A. SHP-1 promoter 2 methylation in normal epithelial tissues and demethylation in psoriasis. J Mol Med. 2006;84:175–82. doi: 10.1007/s00109-005-0020-6. [DOI] [PubMed] [Google Scholar]
- 52.Christophi GP, et al. SHP-1 deficiency and increased inflammatory gene expression in PBMCs of multiple sclerosis patients. Lab Invest. 2008;88:243–55. doi: 10.1038/labinvest.3700720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christophi GP, et al. Macrophages of multiple sclerosis patients display deficient SHP-1 expression and enhanced inflammatory phenotype. Lab Invest. 2009;89:742–59. doi: 10.1038/labinvest.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng C, et al. Expression of the tyrosine phosphatase SRC homology 2 domain-containing protein tyrosine phosphatase 1 determines T cell activation threshold and severity of experimental autoimmune encephalomyelitis. J Immunol. 2002;168:4511–8. doi: 10.4049/jimmunol.168.9.4511. [DOI] [PubMed] [Google Scholar]


