Abstract
Background and Purpose
We sought to assess the efficacy and safety of donepezil in patients with vascular dementia (VaD) fulfilling National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences criteria.
Methods
This international, multicenter, 24-week trial was conducted from March 2003 to August 2005. Patients (N=974; mean age, 73.0 years) with probable or possible VaD were randomized 2:1 to receive donepezil 5 mg/d or placebo. Coprimary outcome measures were scores on the Vascular-Alzheimer Disease Assessment Scale–Cognitive Subscale and Clinician’s Interview–Based Impression of Change, plus carer interview. Analyses were performed for the intent-to-treat population with the last-observation-carried-forward method.
Results
Compared with placebo, donepezil-treated patients showed significant improvement from baseline to end point on the Vascular-Alzheimer Disease Assessment Scale–Cognitive Subscale (least-squares mean difference, −1.156; 95% CI, −1.98 to −0.33; P<0.01) but not on the Clinician’s Interview–Based Impression of Change, plus carer interview. Patients with hippocampal atrophy who were treated with donepezil demonstrated stable cognition versus a decline in the placebo-treated group; in those without atrophy, cognition improved with donepezil versus relative stability with placebo. Results on secondary efficacy measures were inconsistent. The incidence of adverse events was similar across groups. Eleven deaths occurred in the donepezil group (1.7%), similar to rates previously reported for donepezil trials in VaD, whereas no deaths occurred in the placebo group.
Conclusions
Patients treated with donepezil 5 mg/d demonstrated significant improvement in cognitive, but not global, function. Donepezil was relatively well tolerated; adverse events were consistent with current labeling. Mortality in the placebo group was unexpectedly low. The differential treatment response of VaD patients by hippocampal size suggests that hippocampal imaging warrants further investigation for understanding VaD.
Keywords: vascular dementia, donepezil, hippocampal atrophy, efficacy, safety
Vascular dementia (VaD) is the second most common type of dementia, but there are currently no medications approved for its treatment in most countries.1 The acetylcholinesterase inhibitor donepezil is indicated for the treatment of Alzheimer disease (AD), and it significantly improves cognition, global functioning, and activities of daily living.2,3 As with AD, cholinergic deficits and disruption of cholinergic pathways occurring in some patients with VaD may contribute to cognitive impairment.4
Two prior donepezil studies in VaD demonstrated significant cognitive improvement but inconsistent benefits in global functioning.5,6 Consequently, this large study was undertaken to further evaluate the potential benefits of donepezil in VaD. Several important methodological changes were incorporated into this trial, including the exclusive use of low-dose donepezil (5 mg/d) to reduce withdrawals due to adverse events (AEs) and the addition of 2 items to the AD Assessment Scale-Cognitive Subscale to better assess executive function, an area particularly affected in VaD.7 As in prior studies, VaD was diagnosed according to National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences criteria8 (Table 1 ). The current trial used a central neuroimaging reader to determine eligibility, ensure consistent application of the neuroimaging criteria, and assign a probable or possible VaD diagnosis.
Table 1.
Demographic and Clinical Characteristics at Baseline (Safety Population)
| Placebo (n=326) |
Donepezil (n=648) |
|
|---|---|---|
| Demographics, n (%) | ||
| Male, n (%) | 176 (54.0) | 398 (61.4) |
| Age, mean±SE (range), y | 72.3±0.5 (35–90) | 73.4±0.4 (40–94) |
| Age group, n (%), y | ||
| ≤65 | 72 (22.1) | 113 (17.4) |
| 66–74 | 100 (30.7) | 199 (30.7) |
| 75–79 | 75 (23.0) | 159 (24.5) |
| >79 | 79 (24.2) | 177 (27.3) |
| Medical history, n (%) | ||
| No history of evidence for prominent, progressive memory impairment before clinical stroke/TIA/VaD | 308 (94.5) | 610 (94.1) |
| No history of a diagnosis of AD preceding a clinical stroke/TIA/VaD | 318 (97.5) | 633 (97.7) |
| Strokes/TIAs | ||
| ≥1 stroke or TIA | 250 (76.7) | 502 (77.5) |
| ≥1 stroke or TIA before onset of dementia | 208 (63.8) | 417 (64.4) |
| Other features (present), n (%) | ||
| Onset of dementia within 3 months of a recognized clinical stroke | 170(52.1) | 340 (52.5) |
| Abrupt onset | 231 (70.9) | 453 (69.9) |
| Fluctuating course/stepwise progression | 273 (83.7) | 540 (83.3) |
| Early onset of gait disturbance | 116(35.6) | 267 (41.2) |
| Unsteadiness and frequent falls | 96 (29.4) | 231 (35.6) |
| Personality and mood changes | 195(59.8) | 365 (56.3) |
| Early onset of urinary tract problems | 62(19.0) | 101 (15.6) |
| Pseudobulbar palsy | 19 (5.8) | 46 (7.1) |
| Clinical characteristics, n (%) | ||
| Hachinski score (range 1–18), mean±SE* | 10.4±0.2 | 10.4±0.1 |
| Cardiovascular disease | 305 (93.6) | 591 (91.2) |
| Atherosclerosis | 179 (54.9) | 373 (57.6) |
| Hypertension | 254 (77.9) | 495 (76.4) |
| Smoking (history) | 178 (54.6) | 382 (59.0) |
| Focal neurologic signs | 226 (69.3) | 453 (69.9) |
| Focal neurologic symptoms | 180 (55.2) | 375 (57.9) |
| Impaired domains of cognition, n (%) | ||
| Memory | 326 (100.0) | 647 (99.8) |
| Executive function† | 275 (84.4) | 561 (86.6) |
| Concentration | 281 (86.2) | 538 (83.0) |
| Attention | 271 (83.1) | 513 (79.2) |
| Orientation | 248 (76.1) | 475 (73.3) |
| Calculation | 195 (59.8) | 404 (62.3) |
| Visuospatial function | 197 (60.4) | 353 (54.5) |
| Language‡ | 190 (58.3) | 361 (55.7) |
| Judgment | 174 (53.4) | 375 (57.9) |
| Motor control | 137 (42.0) | 272 (42.2) |
| Praxis | 142 (43.6) | 259 (40.0) |
| Agnosia | 46 (14.1) | 88 (13.6) |
| Assessment items (range), mean±SE | ||
| CIBIS (1= normal to 7=most severely ill) | 3.6±0.049 | 3.6±0.034 |
| V-ADAS-cog (0–80) | 21.72±0.62 | 21.75±0.44 |
| ADAS-cog (0–70) | 18.64±0.56 | 18.32±0.40 |
| MMSE§ (0–30) | 23.57±0.27 | 23.49±0.20 |
| CLOX-1§ (0–15) | 9.52±0.20 | 9.24±0.14 |
| CLOX-2§ (0–15) | 11.71 ±0.18 | 11.89±0.13 |
| EXIT25 (0–50) | 16.42±0.38 | 16.19±0.28 |
| DAD§ (0–100) | 70.71 ±1.25 | 71.63±0.90 |
| CDR-SB (0–18) | 5.44±0.15 | 5.32±0.11 |
| NCT (0–5)¶ | 3.00±0.07 | 3.02±0.05 |
| Maze (0, 5)# | 0.73±0.10 | 0.77±0.07 |
| Diagnostic assignment (ITT population: n=321 placebo; n=628 donepezil) | ||
| “Possible VaD” (central reader+clinical) | 228 (69.9) | 437 (67.4) |
| “Probable VaD” (central reader+clinical) | 98(30.1) | 211 (32.6) |
| Hippocampal assessment (adequate MRI scan: n=245 placebo; n=436 donepezil), n (%) | ||
| HA (Scheltens’ score ≥2) | 133 (54.3) | 246 (56.4) |
| NH (Scheltens’ score <2) | 112 (45.7) | 190 (43.6) |
TIA indicates transient ischemic attack; CIBIS, Clinician’s Interview Based Impression of Severity; MMSE, Mini Mental State Examination; and CDR-SB, Clinical Dementia Rating-Sum of the Boxes.
Measured at screening.
Executive function was considered impaired if abstract thinking, executive impairment, or impaired planning was reported.
Language was considered affected if the patient had impairment of any of the following: aphasia, verbal fluency, comprehension verbal, or comprehension written.
Low scores reflect impairment.
Scored continuously on a 0 to 5 scale with 5 reflecting the greatest impairment.
Scored categorically: 0 for completing the Maze without errors, 5 for incomplete or completing with errors.
In cases with adequate magnetic resonance imaging (MRI) studies, a prespecified subgroup analysis of hippocampal volume was performed according to Scheltens’ scores.9 This analysis was undertaken to investigate the extent to which hippocampal atrophy (HA) was present in this rigorously selected VaD population.
Methods
Study Design
This investigation was a randomized, double-blind, placebo-controlled, 24-week study conducted from March 2003 to August 2005 at 111 centers in 9 countries.
Patients
Participants were outpatients (age 35 to 94 years) with possible or probable VaD per National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences criteria (including brain imaging), had been stroke-free for ≥3 months, had not taken acetylcholinesterase inhibitors or memantine for at least 6 weeks, and did not have unstable medical conditions. Entry criteria were similar to those of prior studies of donepezil in VaD.5,6 Cholinomimetics and anticholinergics were not allowed; sympathomimetics and antihistamines were disallowed for 48 hours before visits.
Written, informed consent was obtained from participants in compliance with the Declaration of Helsinki and the independent ethics committee or institutional review board at each site.
Protocol
Participants were randomly assigned 2:1 to donepezil 5 mg or placebo once daily. Subjects without an MRI or CT scan in the previous 12 months had one during the screening period. A central reader from Synarc Inc evaluated and categorized all scans obtained for this study (Figure 1).* Scans were performed routinely at each site and were considered adequate if they were of sufficient technical quality to be accurately read for purposes of determining study inclusion. After receiving this imaging information, investigators considered the clinical history, examination, and laboratory evaluations to assign the designation of “probable VaD” or “possible VaD.” Semiquantitative rating of hippocampal volume according to Scheltens’ score was performed in cases with adequate MRI scans (all readings performed by C. DeCarli).9
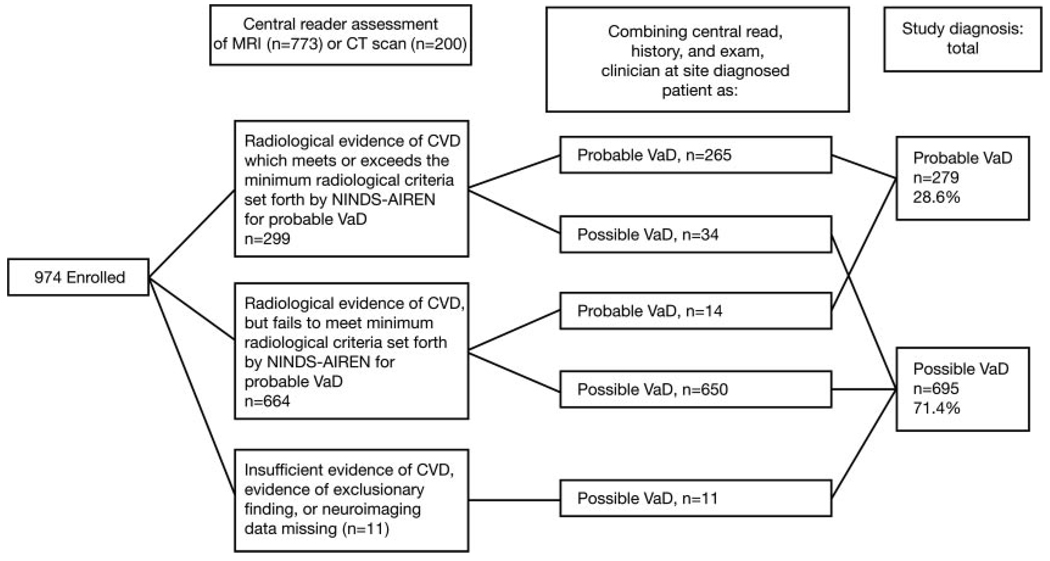
Figure 1.
Decision tree for classifying patients as having possible vs probable VaD. CT indicates computed tomography; CVD, cardiovascular disease; and NINDS-AIREN, National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche et l’Enseignement en Neurosciences.
Outcome Measures
Primary Efficacy Assessments
The coprimary efficacy outcome measures were scores on the Vascular AD Assessment Scale-Cognitive Subscale (V-ADAS-cog)10 and the Clinician’s Interview–Based Impression of Change, plus carer interview (CIBIC-Plus)11 performed at baseline and at weeks 6, 12, 18, and 24 (or at the end of the trial). V-ADAS-cog comprised the ADAS-cog12 plus the Maze and Number Cancellation test (NCT) to specifically assess executive function.13
Secondary Efficacy Assessments
Secondary efficacy end points included the ADAS-cog, Mini Mental State Examination (MMSE),14 executive clock-drawing task (CLOX 1/2),15 Executive Interview (EXIT25),16 Disability Assessment for Dementia (DAD),17 and Clinical Dementia Rating-Sum of Boxes (CDR-SB).18 MMSE and CDR-SB were performed at baseline and at weeks 12 and 24/trial end; EXIT25, CLOX, and DAD were performed at baseline and at week 24/trial end.
Safety
Safety and tolerability were assessed for all randomized patients who received the study drug (safety population). AEs were considered serious (SAEs) according to standard criteria.19 Safety assessments included vital signs, physical and neurologic examination findings, clinical laboratory test results, and ECG abnormalities. Prior and concomitant medications were recorded.
Statistical Analysis
Determination of sample size (N=880) was based on pooled results of the CIBIC-Plus from 2 trials of donepezil in VaD patients5,6 plus estimates of standard deviation from interim blinded data analyses. For a 2:1 donepezil:placebo randomization ratio, with a χ2 test (Cochran-Mantel-Haenszel) and an α significance level of 0.05 (2 tailed), this sample size provided 90% statistical power to distinguish between the groups when the proportions in the 7 categories of the CIBIC-Plus are characterized by an effect size of 0.024, and with an assumed common standard deviation of 1.13.
Analyses were performed according to the intent-to-treat (ITT) population, defined as all patients in the safety population having a baseline and at least 1 postbaseline assessment for at least 1 primary efficacy measure, and the last-observation-carried-forward method. Differences between treatment groups for continuous efficacy measures were assessed by ANCOVA models with type III sums of squares including baseline, treatment, and center as factors. CIBIC-Plus was analyzed with the Cochran-Mantel-Haenszel procedure, stratified by centers. ANCOVA was used to test for sensitivity of the Cochran-Mantel-Haenszel procedure. ANCOVA or Fisher’s exact tests were used to analyze changes in vital signs, clinical laboratory tests, and AEs.
Subgroup analyses of primary and selected secondary efficacy outcomes, including V-ADAS-cog total score, CIBIC-Plus overall change, ADAS-cog, DAD, and NCT, were performed with ANCOVA for patients grouped by hippocampal size (Scheltens’ scores <2 indicate relatively normal-size hippocampi [NH]; scores ≥2 indicate HA).9 SAS version 8 or higher was used for analyses. All statistical tests were 2 tailed and were performed at the 0.05 significance level.
Results
Subject Disposition
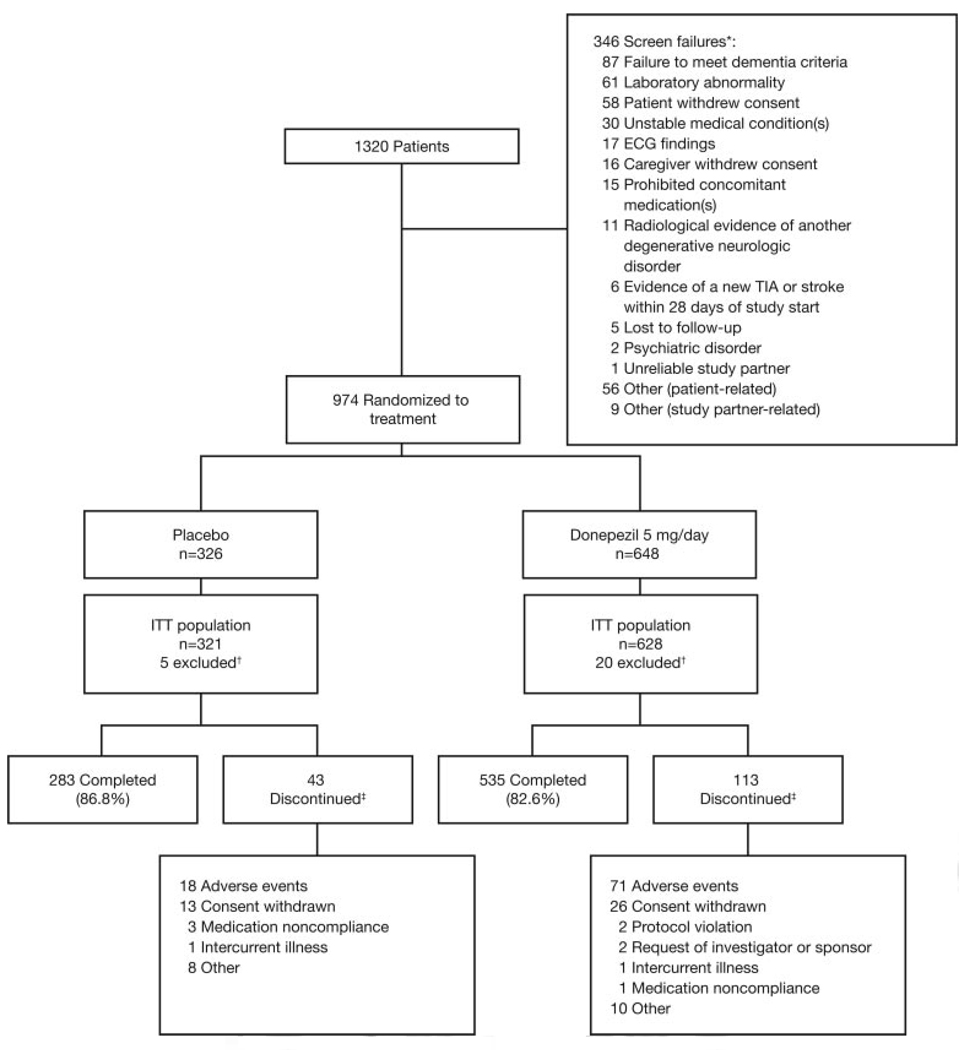
Of 1320 patients screened, 974 were randomly assigned to treatment with donepezil (n=648) or placebo (n=326) and received at least 1 dose of the study drug (safety population); 949 patients were in the ITT population (Figure 2). Overall, 84.0% of patients completed the study; mean±SE duration of exposure was 149.7± 1.8 days for donepezil and 156.5±2.0 days for placebo. Mean study medication compliance was 97% in both groups at week 24. A greater percentage of patients in the donepezil group discontinued treatment because of an AE (11.0% vs 5.5%).
Figure 2.
Flowchart of patients excluded from the study and included patients randomized to active treatment with 5 mg/d donepezil or placebo. TIA indicates transient ischemic attack. *Patients might have >1 reason for screen failure, and some patients were screened more than once. †Twenty-five patients were excluded from the ITT population because they did not have a baseline assessment in addition to at least 1 postbaseline assessment for at least 1 of the primary efficacy variables. ‡Eleven patients died during the study (all in the donepezil group).
Baseline Characteristics
Table 1 summarizes the baseline demographic and clinical characteristics of the safety population. The majority of patients had a clinical profile highly consistent with a diagnosis of VaD: high rates of atherosclerosis, hypertension, and stroke/transient ischemic attacks. Dementia severity was mild (mean Mini Mental State Examination score, 23.5); cardiovascular disease history and baseline efficacy assessments were similar in both groups (Table 1 and Supplemental Table I available online at http://stroke.ahajournals.org). The donepezil group had a higher proportion of men and patients age ≥75 years.
Figure 1 illustrates the central reader brain imaging classification. After incorporation of the history and examination results, 71.4% of patients had possible VaD and 28.6%, probable VaD, divided similarly in the placebo and donepezil groups. The percentage of patients with HA was slightly >50% in both groups (Table 1).
Efficacy
Primary Efficacy Measures
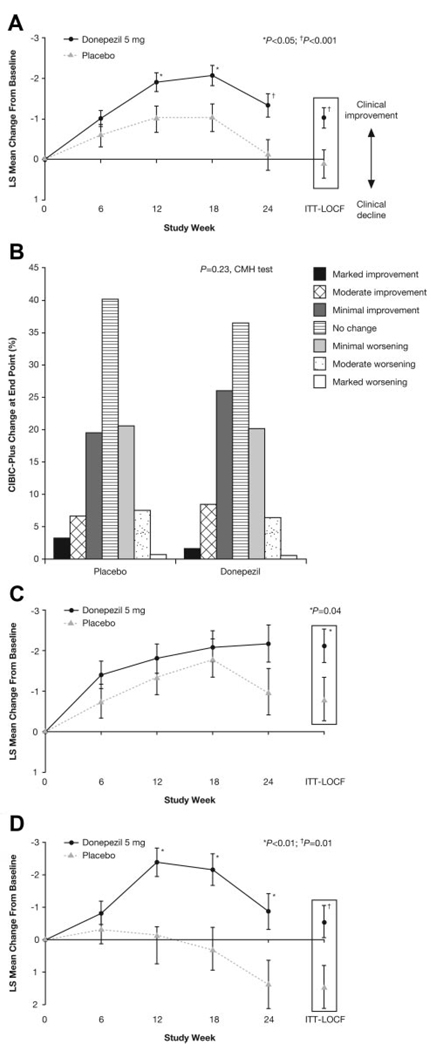
Patients treated with donepezil showed significant improvement compared with those taking placebo on the V-ADAS-cog at end point and at all time points except week 6 (Figure 3A). The least-squares mean±SE change from the baseline total score at end point was −1.03±0.25 (donepezil group) and 0.12±0.35 (placebo group), indicating a slight improvement in those receiving donepezil and relative stability in the placebo group.
Figure 3.
Primary outcome measures in donepezil and placebo patients. A, V-ADAS-cog least-squares (LS) mean change from baseline; B, CIBIC-Plus overall change at end point. C, V-ADAS-cog LS mean change from baseline for patients with Scheltens’ score <2. D, V-ADAS-cog LS mean change from baseline for patients with Scheltens’ score ≥2. CMH indicates Cochran-Mantel-Haenszel; LOCF, last observation carried forward.
No difference between donepezil and placebo was demonstrated for CIBIC-Plus at end point for the ITT population (P=0.23; Figure 3B), but Cochran-Mantel-Haenszel analysis of the distribution of CIBIC-Plus responses did favor donepezil at weeks 18 (P<0.001) and 24 (P<0.05). ANCOVA confirmed significance at week 18 but not at week 24 (data not shown).
HA Subgroup Analysis
Of the 681 subjects with adequate MRIs for hippocampal analysis, 369 (54%) had HA and 312 (46%) had NH. HA ratings were equally distributed between the donepezil and placebo groups (Table 1). There were no significant differences between placebo and donepezil in baseline scores on any of the outcome measures evaluated for this subgroup analysis (V-ADAS-cog, CIBIC-Plus, NCT, ADAS-cog, or DAD). However, mean baseline scores on each of these measures, except CIBIC-Plus, were consistently and significantly better in patients with NH than in those with HA (Supplemental Table II, available online at http://stroke.ahajournals.org).
A differential treatment response based on hippocampal volume was also found for V-ADAS-cog. In the NH group, a significant treatment difference at end point favoring donepezil was observed (donepezil, −2.11 ±0.42; placebo, −0.80±0.53; P=0.04; Figure 3C). In contrast, patients with HA showed worsening in the placebo group but slight improvement in the donepezil group, which also resulted in a significant treatment benefit at end point (placebo, 1.44±0.67; donepezil, −0.56±0.50; P=0.01; Figure 3D). There were no significant differences in the CIBIC-Plus on the basis of hippocampal volume at end point for either group, but in the NH group, significant treatment differences favoring donepezil were observed at weeks 12 and 18 (P=0.03; Supplemental Table II).
Secondary Efficacy Measures
Significant treatment differences favoring donepezil were demonstrated at end point for the ADAS-cog and Mini Mental State Examination (Table 2). DAD scores showed significantly greater improvement in the donepezil group at week 24 (least-squares mean difference=2.24; 95% CI, 0.36 to 4.12; P=0.02) and a trend at end point (P=0.06). At end point, a treatment difference favoring donepezil was demonstrated on the NCT. No significant differences were observed on the CLOX, EXIT25, Clinical Dementia Rating-Sum of Boxes, or Maze.
Table 2.
Secondary Efficacy Outcomes at End Point (ITT, Last Observation Carried Forward)
| Least Squares Mean Change From Baseline Scores (SE) |
Difference in Least Squares Mean (95% CI) |
|||
|---|---|---|---|---|
| Placebo | Donepezil | P | ||
| Cognitive assessments | ||||
| ADAS-cog* | −0.33 (0.29) | −1.04(0.21) | −0.707 (−1.40, −0.02) | 0.0464 |
| MMSE† | 0.18 (0.18) | 0.65 (0.13) | 0.472 (0.05, 0.89) | 0.0301 |
| Executive function tests | ||||
| EXIT25* | −0.70 (0.27) | −0.86 (0.20) | −0.160 (−0.80, 0.48) | 0.6255 |
| CLOX-1† | 0.17 (0.16) | 0.42 (0.12) | 0.243 (−0.13, 0.62) | 0.2104 |
| CLOX-2† | 0.07 (0.13) | −0.06 (0.09) | −0.131 (−0.44, 0.18) | 0.4090 |
| NCT* | −0.09 (0.05) | −0.22 (0.04) | 0.131 (−0.26, −0.01) | 0.0396 |
| Maze* | 0.45 (0.11) | 0.29 (0.08) | 0.157 (−0.41, 0.10) | 0.2327 |
| Daily functioning | ||||
| DAD† | −0.24 (0.77) | 1.53 (0.56) | 1.768 (−0.05, 3.59) | 0.0591 |
| Global assessment | ||||
| CDR-SB* | 0.03 (0.10) | 0.05 (0.07) | 0.020 (−0.20, 0.24) | 0.8654 |
MMSE indicates Mini Mental State Examination; and CDR-SB, Clinical Dementia Rating-Sum of the Boxes.
Negative change indicates improvement.
Positive change indicates improvement.
Safety
Incidence of AEs was similar in the donepezil (80.7%) and placebo (77.6%) groups; commonly occurring AEs included nausea, anorexia, abdominal pain, diarrhea, abnormal dreams, hypertonia, and leg cramps (Table 3). Most were transient and mild to moderate in severity. AEs were assessed by the investigator as probably/possibly related to the study drug in 29.5% of cases for donepezil and 26.4% of cases for placebo. The most common AEs in this category were diarrhea (donepezil, 8.1%; placebo, 3.1%) and nausea (donepezil, 7.1%; placebo, 2.4%).
Table 3.
Treatment-Emergent AEs
| AEs, n (%) | Placebo (n=326) |
Donepezil (n=648) |
|---|---|---|
| Any AE | 253 (77.6) | 523 (80.7) |
| Mild/moderate | 227 (66.6) | 441 (68.0) |
| Severe | 36 (11.0) | 82 (12.7) |
| Assessment of relation of AEs to study drug | ||
| Not related | 156(47.9) | 262 (40.4) |
| Possibly or probably related | 97 (29.8) | 261 (40.3) |
| AEs affecting ≥5% of donepezil-treated patients and at least 10% in the placebo group | ||
| Nausea | 14 (4.3) | 64 (9.9) |
| Anorexia | 9 (2.8) | 37 (5.7) |
| Abdominal pain | 8 (2.5) | 33 (5.1) |
| SAEs affecting ≥2% of patients in either group | ||
| At least 1 SAE* | 47 (14.4) | 94 (14.5) |
| Cardiovascular system | 15 (4.6) | 23 (3.5) |
| Infections and infestations | 10 (3.1) | 14 (2.2) |
| Injury, poisoning, and procedural complications | 10 (3.1) | 12 (1.9) |
| Nervous system | 13 (4.0) | 31 (4.8) |
| Respiratory system | 9 (2.8) | 12 (1.9) |
| Urogenital system | 7 (2.1) | 13 (2.0) |
| Death | 0 (0.0) | 11 (1.7) |
Including death.
Similar numbers of patients in both groups had at least 1 SAE (Table 3) without between-group differences in frequency of SAEs (donepezil, 6.6%; placebo, 5.8%; P=0.77). Overall, 59 patients experienced cardiovascular or cerebrovascular SAEs. No clinically meaningful changes from baseline in systolic and diastolic blood pressures, pulse, or ECG were observed in either group.
Eleven patients in the donepezil group and no patients in the placebo group died during the study or within 30 days of the last dose of the study drug. Ten of these deaths were preceded by a serious treatment-emergent AE, including 4 strokes and 3 cardiovascular events. Three of the 11 deaths were assessed by the investigator as possibly related to the study drug (bradycardia, myocardial infarction, and death of unknown etiology).
Analysis of the Antithrombotic Trialists’ Collaboration20 end point (comprising nonfatal myocardial infarction, nonfatal stroke, and vascular death) indicated no between-group difference (hazard ratio = 1.13; 95% CI, 0.50 to 2.59; P=0.83). Kaplan-Meier time-to-event analysis also demonstrated no between-group difference (hazard ratio = 1.17; 95% CI, 0.51 to 2.69; P=0.87).
Discussion
This study, the largest clinical trial of donepezil in VaD patients, did not demonstrate significance on both coprimary end points. Small but significant improvement was observed in donepezil-treated patients on the V-ADAS-cog, but no difference was seen on the CIBIC-Plus. Mild impairment at baseline may have created a ceiling effect on the CIBIC-Plus, an instrument that may lack the ability to detect small clinical improvements. Nonetheless, these results are consistent with 2 prior donepezil VaD trials,5,6 suggesting that donepezil may have a greater impact on cognitive than global outcomes in VaD. Results of galantamine and rivastigmine VaD trials also demonstrated significant improvement in cognition, more so than in activities of daily living.21,22
There were 2 main findings from the hippocampal volume subgroup analysis: first, that the NH group had better function at baseline, and second, that there was a differential response pattern in the NH subgroups compared with the HA subgroups. In the placebo HA subgroup, baseline-to-end-point mean V-ADAS-cog scores worsened (1.44±0.67), whereas in the placebo NH subgroup, scores improved slightly (−0.80±0.53). Baseline-to-end-point mean V-ADAS-cog scores improved in both donepezil subgroups, but more so in the NH subgroup (−2.11±0.42) than in the HA subgroup (−0.56±0.50). Thus, although there was a significant treatment effect in the HA (−2.00±0.72, P=0.01) and the NH (−1.31±0.56, P=0.04) groups, the effect was driven largely by a decline in the placebo HA group and benefit in the donepezil NH group. These results underscore the important role that HA may play in influencing disease course, as recently shown in another large sample of VaD patients23 as well as the pattern of treatment response, and support the growing body of evidence showing that mixed pathology is common in older patients with dementia.1,4,24 Although covert comorbid AD may be 1 source of HA in this rigorously selected VaD population, HA may also be a result of hippocampal sclerosis, cardiovascular disease itself, or other causes. This topic deserves further investigation.
We standardized the decision tree to determine probable versus possible VaD by including a central MRI/computed tomography reader and by making imaging the first step in classification. This gave more weight to imaging over clinical findings in the determination of possible versus probable VaD, in contrast to prior donepezil clinical trials that relied primarily on the principal investigator’s clinical judgment. This may explain the high ratio of possible to probable VaD cases in this study. Interestingly, results for both primary outcome measures were similar regardless of assignment to probable or possible VaD, calling into question the utility of this distinction.
Regarding executive dysfunction, no significant treatment effects were observed for EXIT25 or CLOX, whereas a significant difference favoring donepezil was observed on the NCT. No difference was seen on the Maze, most likely because of a ceiling effect. For activities of daily living, which are strongly predicated on executive function, a significant difference was observed on the DAD at week 24 but not at end point. These data indicate that donepezil treatment has a variable effect on executive function and activities of daily living in VaD patients, extending similar findings from prior donepezil trials in VaD.5,6
A recent study of donepezil in patients with cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy,25 a genetic form of pure subcortical ischemic VaD characterized primarily by executive dysfunction, showed that donepezil (5 to 10 mg/d) had no effect on V-ADAS-cog but had a significant effect on certain measures of executive function: EXIT25 and Trails B. These subjects were younger (mean age, 57 years), and ≈75% had no clinically significant memory dysfunction, which may partly explain the lack of benefit on the V-ADAS-cog. The positive effect on executive function, however, indicates some cholinergic deficit in executive dysfunction.
Eleven deaths occurred during the study or within 30 days of last dose, all in the donepezil group, which comprised two thirds of randomized patients. This was unexpected, necessitating a thorough analysis. First, we identified a difference between the placebo and donepezil populations that may have been associated with differential mortality, namely, that the percentage of patients age >79 years was higher in the donepezil group, and of the 11 fatalities, 8 were in this age range. Second, we examined whether the mortality rate in the donepezil group in this trial was elevated compared with either donepezil or placebo groups in prior VaD trials. The average mortality rate in the donepezil group in the 2 previous VaD studies, which included a 10-mg/d arm, was identical to that observed in this study (1.7%), whereas the rate in the placebo group was higher (2.0%).5,6 Third, we analyzed data from this study and the 2 previous VaD studies combined, which showed that (1) the difference in mortality rates between the donepezil group and the placebo group was nonsignificant (1.7% vs 1.1%) and (2) the difference in time to death between deceased patients who received donepezil versus placebo was also nonsignificant (Kaplan-Meier analysis). Fourth, we noted that of the 11 deaths in this study, only 3 were assessed by the investigator as possibly related to the study drug, including 1 case of bradycardia, which has been associated with acetylcholinesterase inhibitor therapy.26 Finally, we compared mortality in this study with that in the general population from World Health Organization all-cause annual mortality rates per 100 000 population for 2 representative countries in which this study was conducted.†27 We found that mortality in the done-pezil group was consistent with that in the general population (expected deaths, 19; observed, 11), whereas mortality in the placebo group was not (expected, 9; observed, 0). Taken together, these data suggest that the observed difference in mortality rates may have resulted primarily from the smaller sample size of the placebo group not adequately representing the actual mortality rate in this elderly population.
The rates of treatment-emergent AEs, AEs potentially attributable to the study drug, and SAEs were similar in both groups. AEs observed significantly more frequently in the donepezil group were consistent with its cholinergic activity, as described on the product label and in previous clinical trials.
This study had several limitations. First, the absence of a 10-mg/d donepezil group might have reduced the chance of obtaining more complete efficacy. However, in previous donepezil VaD studies, the marginal increase in early terminations owing to AEs in the 10-mg/d group was greater than the marginal increase in efficacy compared with the 5-mg/d group.5,6 Second, the large proportion of patients age ≥75 years probably increased the overall admixture of other potential sources of cognitive impairment, as demonstrated by hippocampal evaluation. Third, substantial variability in imaging information resulted from using both computed tomography and MRI images and site-based image-acquisition procedures. Ideally, all patients would have had an MRI scan at study entry according to a uniform image-acquisition protocol, including hippocampal assessment. Finally, strict adherence to the National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences neuroimaging criteria might have skewed the diagnosis of possible versus probable VaD; these criteria will require reevaluation.
In summary, patients treated with donepezil demonstrated small but significant improvements on primary and secondary measures of cognitive, but not global, functioning. AEs associated with treatment were consistent with donepezil labeling. Mortality in the placebo group was unexpectedly low. Although the clinical profile of patients in this study was characteristic of VaD and was notably different from that of patients enrolled in AD trials, a prespecified subgroup analysis showed a differential dementia severity and therapeutic response to donepezil based on the presence versus absence of HA. This finding suggests that evaluating hippocampal volume may be helpful in understanding VaD.
Supplementary Material
Acknowledgments
Rachelle Doody, MD, and Philip Scheltens, MD, PhD, contributed to the development of this article.
Sources of Funding
This study (NCT00165737)* was sponsored by Eisai Medical Research Inc. Editorial support was provided by B. Kadish, MD, at PAREXEL Inc, and was funded by Eisai Inc and Pfizer Inc.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
*The data from this study were originally released publicly on March 16, 2006.
One expert reader at Synarc evaluated approximately the first 500 patients in the study, in conjunction with an eligible reader. The remaining scans were read by the eligible reader, and these readings were validated on an ongoing basis by adjudication of 10% of randomly selected patients by the expert reader (random selection was done by the data entry system).
We averaged these rates (United States, age 65–74 years, men=2979.6, women=1921.1; age ≥75, men=9088.3, women=7657.9; Germany, age 65–74 years, men=28877, women=1475.7; age ≥75, men=9317.1, women=7788.1) to approximate the expected annual mortality per 100 000 for our cohort, obtained an estimate of 5388, and adjusted this rate on the basis of the period of observation (eg, placebo patients were enrolled for a median of 168 days and deaths were counted for 30 additional days). The resulting mortality rates correspond to 9 expected deaths in the placebo group and 19 in the donepezil group.
Disclosures
Gustavo C. Román, MD, none. Stephen Salloway, MD, has provided consulting services to Eisai, Pfizer, Forest, Medivation, Myriad, Elan, Sanofi-Aventis, and Merck. He received honoraria from Pfizer, Eisai, Novartis, Forest, and Elan. He has also received research support from Eisai, Pfizer, Forest, Janssen, Myriad, Elan, Neurochem, Wyeth, and Cephalon; the National Institutes of Health; and the Norman and Rosalie Fain Family Foundation. He was a study investigator. Sandra E. Black, MD, has been an investigator conducting clinical trials in dementia research for Eisai, Pfizer, Novartis, Myriad Pharmaceuticals, Sanofi-Aventis, Roche, and Sonexa; is an ad hoc consultant for Eisai, Pfizer, Novartis, Lundbeck, Janssen-Ortho, Myriad, Elan-Wyeth, and Schering-Plough; and has received speaker fees from Pfizer, Novartis, Janssen-Ortho, Myriad, and Lundbeck. Donald R. Royall, MD, was a consultant to Eisai for cognitive outcome assessments during this trial and holds the copyrights for the EXIT25 and CLOX scales. He also participated as principal investigator of the San Antonio site. Charles DeCarli, MD, was consultant to Eisai for MRI analysis during the time of the study. Michael W. Weiner, MD, has served on scientific advisory boards of Bayer Schering Pharma, Lilly, CoMentis, Neurochem, Eisai, Avid, Bristol Meyers, Forest, Pfizer, and Novartis. He has received commercial research support from Merck and Avid and public research support from the National Institutes of Health, Veterans Administration, and Department of Defense. He owns stock options in Elan and Synarc. Margaret Moline, PhD, and Dinesh Kumar, MS, are employees of Eisai Medical Research. Rachel Schindler, MD, is an employee of Pfizer Inc. Holly Posner, MD, MS, an employee of Pfizer Inc, was an employee of Eisai Medical Research during the study.
References
- 1.Román GC. Vascular dementia revisited: diagnosis, pathogenesis, treatment, and prevention. Med Clin North Am. 2002;86:477–499. doi: 10.1016/s0025-7125(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 2.Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 3.Winblad B, Kilander L, Eriksson S, Minthon L, Batsman S, Wetterholm A, Jansson Blixt C, Haglund A. Donepezil in patients with severe Alzheimer’s disease: double-blind, parallel-group, placebo-controlled study. Severe Alzheimer’s Disease Study Group. Lancet. 2006;367:1057–1065. doi: 10.1016/S0140-6736(06)68350-5. [DOI] [PubMed] [Google Scholar]
- 4.Román GC, Kalaria RN. Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol Aging. 2006;27:1769–1785. doi: 10.1016/j.neurobiolaging.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Black S, Román GC, Geldmacher DS, Salloway S, Hecker J, Burns A, Perdomo C, Kumar D, Pratt R. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Donepezil 307 Vascular Dementia Study Group. Stroke. 2003;34:2323–2330. doi: 10.1161/01.STR.0000091396.95360.E1. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson D, Doody R, Helme R, Taubman K, Mintzer J, Kertesz A, Pratt RD. Donepezil in vascular dementia: a randomized, placebo-controlled study. Donepezil 308 Study Group. Neurology. 2003;61:479–486. doi: 10.1212/01.wnl.0000078943.50032.fc. [DOI] [PubMed] [Google Scholar]
- 7.Chan M, Lim WS, Sahadevan S. Stage-independent and stage-specific phenotypic differences between vascular dementia and Alzheimer’s Disease. Dement Geriatr Cogn Disord. 2008;26:513–521. doi: 10.1159/000178755. [DOI] [PubMed] [Google Scholar]
- 8.Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43:250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 9.Scheltens P, Leys D, Barkhof D, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Valk J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohs RC, Knopman D, Petersen RC, Ferris SH, Ernesto C, Grundman M, Sano M, Bieliauskas L, Geldmacher D, Clark C, Thal LJ. Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 suppl 2:S13–S21. [PubMed] [Google Scholar]
- 11.Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH. Validity and reliability of the Alzheimer’s Disease Cooperative Study: Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 suppl 2:S22–S32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 12.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 13.Román GC, Royall DR. Executive control function: a rational basis for the diagnosis of vascular dementia. Alzheimer Dis Assoc Disord. 1999;13 suppl 3:S69–S80. doi: 10.1097/00002093-199912003-00012. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Royall DR, Cordes JA, Polk M. CLOX: an executive clock drawing task. J Neurol Neurosurg Psychiatry. 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 17.Gélinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the Disability Assessment for Dementia. Am J Occup Ther. 1999;53:471–481. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- 18.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24:637–639. [PubMed] [Google Scholar]
- 19.US Food and Drug Administration. [Accessed June 2, 2009];What is a serious adverse event? Available at: http://www.fda.gov/medwatch/report/DESK/advevnt.htm.
- 20.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auchus AP, Brashear HR, Salloway S, Korczyn AD, DeDeyn PP, Gassmann-Mayer C. Galantamine treatment of vascular dementia: a randomized trial. GAL-INT-26 Study Group. Neurology. 2007;69:448–458. doi: 10.1212/01.wnl.0000266625.31615.f6. [DOI] [PubMed] [Google Scholar]
- 22.Ballard C, Sauter M, Scheltens P, He Y, Barkhof F, van Straaten EC, van der Flier WM, Hsu C, Wu S, Lane R. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Curr Med Res Opin. 2008;24:2561–2574. doi: 10.1185/03007990802328142. [DOI] [PubMed] [Google Scholar]
- 23.Bastos-Leite AJ, van der Flier WM, van Straaten ECW, Staekenborg SS, Scheltens P, Barkhof F. The contribution of medial temporal lobe atrophy and vascular pathology to cognitive impairment in vascular dementia. Stroke. 2007;38:3182–3185. doi: 10.1161/STROKEAHA.107.490102. [DOI] [PubMed] [Google Scholar]
- 24.Jagust WJ, Zheng L, Harvey DJ, Mack WJ, Vinters HV, Weiner MW, Ellis WG, Zarow C, Mungas D, Reed BR, Kramer JH, Schuff N, DeCarli C, Chui HC. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008;63:72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dichgans M, Markus HS, Salloway S, Verkkoniemi A, Moline M, Wang Q, Posner H, Chabriat HS. Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol. 2008;7:310–318. doi: 10.1016/S1474-4422(08)70046-2. [DOI] [PubMed] [Google Scholar]
- 26.Gill SS, Anderson GM, Fischer HD, Belln CM, Li P, Normand SL, Rochon PA. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: a population-based cohort study. Arch Intern Med. 2009;169:867–873. doi: 10.1001/archinternmed.2009.43. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization: All cause mortality by age group. [Accessed March 23, 2009]; Available at: http://www.who.int/whosis/database/mort/table1_process.cfm.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.