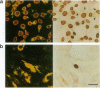
Abstract
Vascular smooth muscle cells (SMC) isolated from embyronic and early fetal (e13-e18) rat aortas exhibit an "embryonic growth phenotype" in culture (Cook, C. L., M. C. M. Weiser, P. E. Schwartz, C. L. Jones, and R. A. Majack. 1994. Circ. Res. 74:189-196). Cells in this growth phenotype exhibit autonomous, serum-independent replication, in contrast to SMC in the "adult" growth phenotype, whose proliferation in culture is dependent on exogenous mitogens. To determine which of these two phenotypes is genetically dominant, heterokaryons were constructed between adult and embryonic (day e17) rat aortic SMC. The fused cells were maintained in serum-free medium for 3 d, then were labeled with bromodeoxyuridine (BrdU) for an additional 24 h. Under these conditions, parental e17 SMC exhibited a high rate of self-driven DNA synthesis (73-85% BrdU-positive cells), while parental adult SMC showed minimal replication (13-21% BrdU-positive cells). Homokaryons of parental cells exhibited parental growth phenotypes and showed the expected mitogenic response when stimulated with serum. Heterokaryons between e17 and adult SMC exhibited a nonautonomous growth phenotype; the "adult" growth phenotype was calculated to be dominant in > 89% of all true heterokaryons. The data suggest that adult SMC express molecules capable of genetically extinguishing or otherwise inhibiting the autonomous replication of embryonic SMC.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blau H. M., Baltimore D. Differentiation requires continuous regulation. J Cell Biol. 1991 Mar;112(5):781–783. doi: 10.1083/jcb.112.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M. Differentiation requires continuous active control. Annu Rev Biochem. 1992;61:1213–1230. doi: 10.1146/annurev.bi.61.070192.010025. [DOI] [PubMed] [Google Scholar]
- Clegg C. H., Hauschka S. D. Heterokaryon analysis of muscle differentiation: regulation of the postmitotic state. J Cell Biol. 1987 Aug;105(2):937–947. doi: 10.1083/jcb.105.2.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Mechanisms of stenosis after arterial injury. Lab Invest. 1983 Aug;49(2):208–215. [PubMed] [Google Scholar]
- Cook C. L., Weiser M. C., Schwartz P. E., Jones C. L., Majack R. A. Developmentally timed expression of an embryonic growth phenotype in vascular smooth muscle cells. Circ Res. 1994 Feb;74(2):189–196. doi: 10.1161/01.res.74.2.189. [DOI] [PubMed] [Google Scholar]
- Dipasquale B., Youle R. J. Programmed cell death in heterokaryons. A study of the transfer of apoptosis between nuclei. Am J Pathol. 1992 Dec;141(6):1471–1479. [PMC free article] [PubMed] [Google Scholar]
- Drummond I. A., Madden S. L., Rohwer-Nutter P., Bell G. I., Sukhatme V. P., Rauscher F. J., 3rd Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992 Jul 31;257(5070):674–678. doi: 10.1126/science.1323141. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kocher O., Bloom W. S., Vandekerckhove J., Weber K. Actin expression in smooth muscle cells of rat aortic intimal thickening, human atheromatous plaque, and cultured rat aortic media. J Clin Invest. 1984 Jan;73(1):148–152. doi: 10.1172/JCI111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gashler A. L., Bonthron D. T., Madden S. L., Rauscher F. J., 3rd, Collins T., Sukhatme V. P. Human platelet-derived growth factor A chain is transcriptionally repressed by the Wilms tumor suppressor WT1. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10984–10988. doi: 10.1073/pnas.89.22.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova M. A., Frid M. G., Shekhonin B. V., Balabanov Y. V., Koteliansky V. E. Expression of fibronectin variants in vascular and visceral smooth muscle cells in development. Dev Biol. 1990 Sep;141(1):193–202. doi: 10.1016/0012-1606(90)90114-x. [DOI] [PubMed] [Google Scholar]
- Harris H. The analysis of malignancy by cell fusion: the position in 1988. Cancer Res. 1988 Jun 15;48(12):3302–3306. [PubMed] [Google Scholar]
- Jones K. W., Shapero M. H., Chevrette M., Fournier R. E. Subtractive hybridization cloning of a tissue-specific extinguisher: TSE1 encodes a regulatory subunit of protein kinase A. Cell. 1991 Sep 6;66(5):861–872. doi: 10.1016/0092-8674(91)90433-y. [DOI] [PubMed] [Google Scholar]
- Killary A. M., Fournier R. E. A genetic analysis of extinction: trans-dominant loci regulate expression of liver-specific traits in hepatoma hybrid cells. Cell. 1984 Sep;38(2):523–534. doi: 10.1016/0092-8674(84)90507-5. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani G. Expression of actin mRNAs in rat aortic smooth muscle cells during development, experimental intimal thickening, and culture. Differentiation. 1986;32(3):245–251. doi: 10.1111/j.1432-0436.1986.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Kocher O., Skalli O., Bloom W. S., Gabbiani G. Cytoskeleton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest. 1984 Jun;50(6):645–652. [PubMed] [Google Scholar]
- Kocher O., Skalli O., Cerutti D., Gabbiani F., Gabbiani G. Cytoskeletal features of rat aortic cells during development. An electron microscopic, immunohistochemical, and biochemical study. Circ Res. 1985 Jun;56(6):829–838. doi: 10.1161/01.res.56.6.829. [DOI] [PubMed] [Google Scholar]
- Lombardi D. M., Reidy M. A., Schwartz S. M. Methodologic considerations important in the accurate quantitation of aortic smooth muscle cell replication in the normal rat. Am J Pathol. 1991 Feb;138(2):441–446. [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Peehl D. M., Stanbridge E. J. The role of differentiation in the suppression of tumorigenicity in human cell hybrids. Int J Cancer. 1982 Jul 15;30(1):113–120. doi: 10.1002/ijc.2910300119. [DOI] [PubMed] [Google Scholar]
- Wright W. E. Control of differentiation in heterokaryons and hybrids involving differentiation-defective myoblast variants. J Cell Biol. 1984 Feb;98(2):436–443. doi: 10.1083/jcb.98.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E. Synthesis of rat myosin light chains in heterokaryons formed between undifferentiated rat myoblasts and chick skeletal myocytes. J Cell Biol. 1981 Oct;91(1):11–16. doi: 10.1083/jcb.91.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]