Abstract
Immunosenescence decreases influenza vaccine efficacy in older adults (age 65 and over). Strategies such as vaccine adjuvants are being developed to overcome immunosenescence. Our computer simulation model represented the decision to give an older adult either standard influenza vaccine or adjuvanted influenza vaccine and found the adjuvanted vaccine to be dominant in many scenarios, resulting in lowered cost and greater effectiveness. An adjuvanted vaccine that is 100% effective in overcoming immunosenescence remained dominant until its cost exceeded the standard vaccine cost by $65. In a single influenza season, the adjuvant would prevent 496,533 influenza cases, 171,981 hospitalizations, and 70,429 deaths.
Keywords: Influenza Vaccine Adjuvant, Computer Simulation, Older Adults
INTRODUCTION
Influenza is a significant problem in the older adult (age 65 and over) population. Although influenza affects all age groups, the rates of serious illness and death are highest among persons aged 65 years and greater.[1] Influenza vaccination is the primary method for preventing influenza and its severe complications.[2, 3] Studies have clearly demonstrated the clinical and cost-effectiveness of vaccinating older individuals.[1, 2, 4–7]
However, influenza vaccine efficacy appears to be significantly lower in older adults than in younger adults, even when the vaccine strains match the circulating influenza virus strains.[8] This “vaccine efficacy deficit” among older adults may be the result of immunosenescence, i.e., the aging immune system.[9, 10] An older and weaker immune system may not generate an adequate response to the influenza vaccine. The “vaccine efficacy deficit” leaves older adults more susceptible to influenza and related complications such as pneumonia, hospitalization, and death.
Vaccine adjuvants are one possible solution to this “vaccine efficacy deficit” among the older adult population. Adjuvants are vaccine components that enhance, accelerate, and/or prolong a body’s immune response to the vaccine and, in turn, may overcome immunosenescence. Various influenza vaccine adjuvants are currently under development. We developed a computer simulation model to predict the potential morbidity and mortality benefits as well as the economic impact of introducing adjuvanted influenza vaccine to the older adult population in the United Sates (U.S.). The aim of our model was to quantify the impact of immunosenescence, better understand the potential added value of an influenza vaccine adjuvant, and provide information that may help price this technology once it reaches the market. Since the ability of adjuvants to overcome immunosenesence has not been clearly quantified, we explored the hypothetical range of adjuvant effectiveness rather than base our model on results from any specific clinical study of influenza vaccine adjuvants.
METHODS
Cohort Model
Using TreeAge Pro 2005 (TreeAge Software, Williamstown, Massachusetts), we developed a decision analytic computer simulation model, with probabilistic sensitivity analyses, comparing the use of regular (standard) influenza vaccine with the use of an adjuvanted influenza vaccine in the U. S. older adult population. The model aimed to quantify the incremental morbidity, mortality, and cost-effectiveness associated with introducing the vaccine adjuvant to this population. The base case scenario assumed the societal perspective and accounted for direct and indirect costs of illness. An additional scenario took the third-party payer perspective and considered only the direct costs of illness.
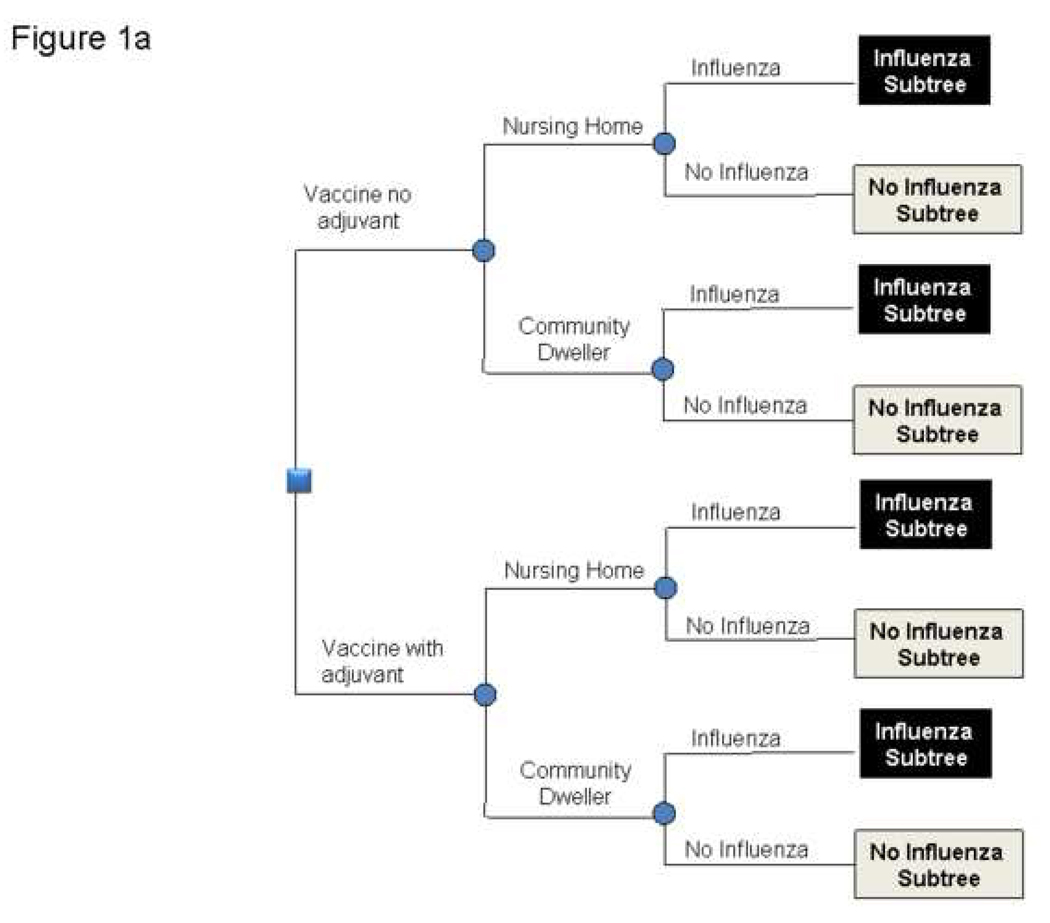
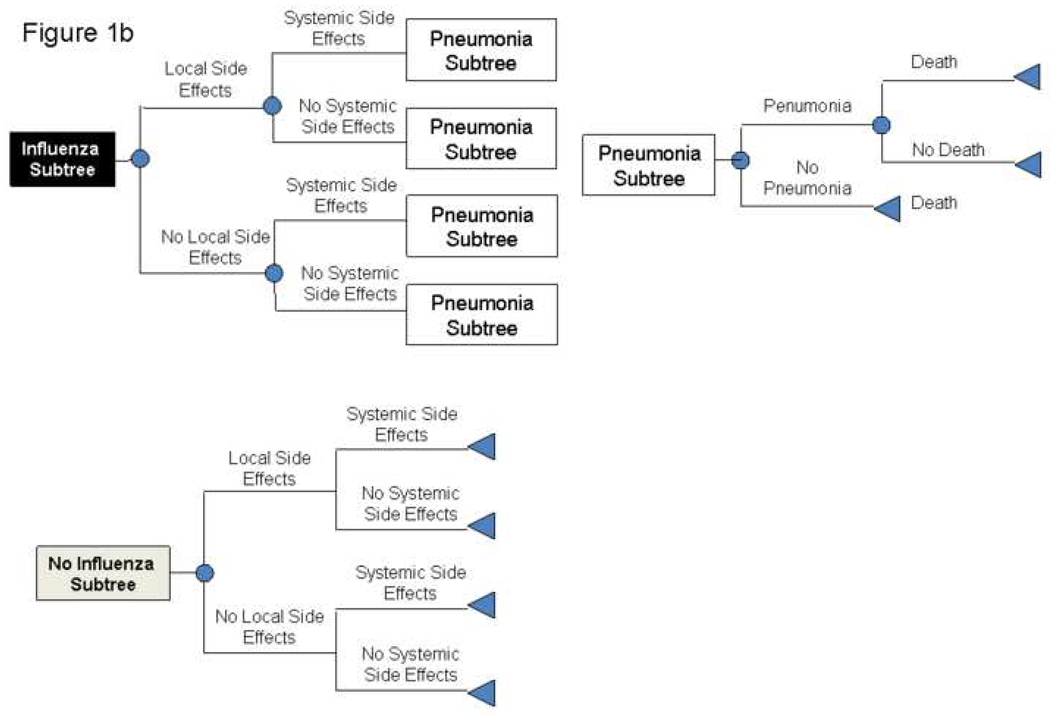
Figures 1a and 1b depict the general structure of the decision model. The time frame for the model was one year, i.e., a single influenza season. The model represented the decision to give a patient over 65 years of age either standard influenza vaccine or an influenza vaccine with adjuvant. Our model divided the older adults into two populations (those living in the general community and those residing in nursing homes), based on studies that these two populations may have different influenza incidences and outcomes.[11]
FIGURE 1.
1a and 1b: Structure of Model
After a person receives the vaccine, he or she could have a variety of different possible outcomes. This person may develop local side effects, which would require one day of ibuprofen treatment or systemic side effects, which would require 3 days of ibuprofen treatment. The patient may contract influenza during the subsequent year, depending on the effectiveness of the vaccine. Of the patients who develop influenza, a certain percentage requires hospitalization. Those who do not require hospitalization either just treat themselves with over-the-counter medications or visit an outpatient medical clinic. A subset of patients requiring hospitalization develops pneumonia, necessitating treatment. A subset of hospitalized patients does not survive.
Since we obtained distributions for most of our data inputs, we performed a probabilistic (Monte Carlo) sensitivity analysis, allowing us to simultaneously vary all parameters for cohorts who received either adjuvanted or non-adjuvanted influenza vaccine and accounting for the natural stochasticity of the subsequent outcomes.
Data Inputs
Table 1 lists the various data inputs for our model, dividing them into probabilities, costs, and utilities. The table also shows the distribution parameters used and the data sources for each variable. We used beta distributions for all of our utility variables and normal distributions for all other variables. Where possible, data inputs came from published meta-analyses.
TABLE 1.
Data Inputs for Model Variables
| Description (units) | Mean | 95% Range | Source | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| COSTS | ||||
| Standard Influenza Vaccine ($US) | 15.75 | 11.81 | 19.69 | [31] |
| Influenza Vaccine Adjuvant ($US) | 0 | 0 | 100.00 | |
| Influenza Treatment | ||||
| Over the Counter Medications ($US) | 14.71 | 11.03 | 18.39 | [31, 32] |
| Outpatient Visit ($US) | 95.88 | 71.91 | 119.85 | [33] |
| Productivity Loss for Outpatient Visit ($US) | 32.78 | 24.59 | 40.98 | [34] |
| Hospitalization ($US) | 5,402.73 | 4,052.05 | 6,753.41 | [35] |
| Death in Hospital ($US) | 5,000.00 | 3,750.00 | 6,250.00 | [36] |
| Treatment of Vaccine Side Effects | ||||
| Local Vaccine Side Effects ($US) | 0.72 | 0.54 | 0.90 | [31] |
| Systemic Vaccine Side Effects ($US) | 2.88 | 2.16 | 3.60 | [31] |
| DURATIONS | ||||
| Influenza (days) | 7.00 | 5.25 | 8.75 | [37–42] |
| Local Vaccine Side Effects (days) | 1.00 | 0.75 | 1.25 | Estimate |
| Systemic Vaccine Side Effects (days) | 2.00 | 1.50 | 2.50 | Estimate |
| Pneumonia (days) | 10.00 | 7.50 | 12.50 | [36] |
| UTILITIES | ||||
| One Year of Life for Older Adult (QALY) | 0.84 | 0.63 | 1.00 | [12] |
| Loss of Utility/Day | ||||
| Influenza (QALY) | 0.65 | 0.49 | 0.81 | [43] |
| Pneumonia (QALY) | 0.50 | 0.38 | 0.63 | [36] |
| Systemic Vaccine Side Effects (QALY) | 0.80 | 0.60 | 1.00 | Estimate |
| Local Vaccine Side Effects (QALY) | 0.95 | 0.71 | 1.00 | Estimate |
| PROBABILITIES | ||||
| General | ||||
| Older Adult Living in a Nursing Home | 0.05 | 0.03 | 0.06 | [44] |
| Outpatient Visit if Develop Influenza | 0.50 | 0 | 1.00 | Estimate |
| Community Dwelling Older Adults | ||||
| Death from Influenza-related Pneumonia | 0.38 | 0.28 | 0.47 | [11] |
| Nursing Home Residents | ||||
| Death from Influenza-related Pneumonia | 0.51 | 0.42 | 0.61 | [11] |
| Standard Influenza Vaccine | ||||
| Systemic Vaccine Side Effects | 0.11 | 0.08 | 0.14 | [11] |
| Local Vaccine Side Effects | 0.18 | 0.13 | 0.22 | [11] |
| Community Dwelling Older Adults | ||||
| Influenza | 0.04 | 0.03 | 0.05 | [2] |
| Pneumonia | 0.21 | 0.15 | 0.26 | [11] |
| Nursing Home Residents | ||||
| Influenza | 0.10 | 0.08 | 0.12 | [11] |
| Pneumonia | 0.43 | 0.33 | 0.52 | [11] |
| Influenza Vaccine with Adjuvant | ||||
| Adjuvant Potency | 0.50 | - | 1.00 | |
| Systemic Vaccine Side Effects | 0.25 | 0.23 | 0.28 | [45] |
| Local Vaccine Side Effects | 0.56 | 0.54 | 0.57 | [45] |
| Community Dwelling Older Adults | ||||
| Influenza | 0.02 | 0.01 | 0.02 | [46] |
| Pneumonia | 0.11 | 0.08 | 0.13 | [46] |
| Nursing Home Residents | ||||
| Influenza | 0.04 | 0.03 | 0.05 | [46] |
| Pneumonia | 0.21 | 0.16 | 0.27 | [46] |
Our base case scenario assumed that the adjuvant completely overcame immunosenescence. In other words, the adjuvant increased the effectiveness of standard influenza vaccine to the levels seen in the younger adult (ages 20–64) population: (as listed in Table 1) the probability of a community-dwelling older adult developing influenza decreased from 0.04 to 0.02 and developing pneumonia from 0.21 to 0.11while the probability of a nursing home resident developing influenza decreased from 0.10 to 0.04 and developing pneumonia 0.43 to 0.21. Subsequent sensitivity analyses varied the effectiveness of the vaccine adjuvant. In our model, the vaccine adjuvant not only lowered the patient’s probability of developing influenza but also lowered the chance of requiring hospitalization, developing pneumonia, and not surviving and raised the probability of local and systemic side effects. For example, the probability of a community dwelling older adult developing influenza when receiving vaccine with adjuvant is:
Probability of Community Dwelling Adult Developing InfluenzaVaccine without Adjuvant + [Adjuvant Potency × (Probability of Young Adult Developing InfluenzaStandard Vaccine − Probability of Community Dwelling Adult Developing InfluenzaVaccine without Adjuvant)]
All costs were in 2007 U.S. dollars. Our base case assumed that an adjuvanted vaccine would cost the same as standard vaccine. Additional analyses looked at the effects of increasing the cost of the adjuvanted vaccine.
Our model measured effectiveness in quality adjusted life-years (QALY). Patients who did not develop vaccine side effects or influenza throughout our model time frame accrued 0.84 QALYs, based on the quality of life utility obtained by Gold et al for persons aged 65 years or greater with no health conditions.[12] Vaccine side effects, influenza, hospitalization, and pneumonia each resulted in different decrements in QALY. We used the following formula to calculate the loss of QALYs from death:
Loss of QALYs from Death = (Age of Patient) × (Life Expectancy of Patient at that Age)
Table 2 shows the age distribution and corresponding life expectancy of persons age 65 and greater in the U.S. This table helped us develop the distribution of QALY loss from death.
TABLE 2.
Characteristics of Older Adult Population
| Age Group |
% of Older Adults |
Population Number in US 2000 |
Life Expectancy |
% Vaccinated | Estimated Number Vaccinated |
|
|---|---|---|---|---|---|---|
| Community | Nursing Home |
|||||
| 65–69 | 27.2% | 9,533,545 | 18.77 | 64% | 74% | 6,149,137 |
| 70–74 | 25.3% | 8,857,441 | 15.27 | 64% | 74% | 5,713,049 |
| 75–79 | 21.2% | 7,415,813 | 11.98 | 64% | 74% | 4,783,199 |
| 80–84 | 14.1% | 4,945,367 | 9.14 | 64% | 74% | 3,189,762 |
| 85–89 | 8.0% | 2,789,818 | 6.78 | 64% | 74% | 1,799,433 |
| 90–94 | 3.2% | 1,112,531 | 4.79 | 64% | 74% | 717,582 |
| 95+ | 1.0% | 337,238 | 3.35 | 64% | 74% | 217,519 |
| Total | 100.0% | 34,991,753 | 13.53 | 22,569,681 | ||
| Source | [47] | [47] | [48] | [49] | [50] | |
United States (U.S.) Model
Our U. S. model extended the Cohort model to the entire U. S. population to predict the national morbidity, mortality, and economic effects of the influenza vaccine adjuvant. The U.S. model used the data from Table 2.
Sensitivity Analyses
We examined the effects of varying different parameter values individually throughout the ranges listed in Table 1. Multi-dimensional sensitivity analyses were performed on selected parameters. In addition, we conducted probabilistic (Monte Carlo) sensitivity analyses.
RESULTS
Cohort Model
In the base case scenario in which both the adjuvanted and non-adjuvanted vaccines cost the same, employing the adjuvant was the dominant strategy. From the societal perspective, the adjuvant in one year per person resulted in lower costs and greater effectiveness (Table 3). The adjuvanted vaccine remained the dominant strategy even when the adjuvanted vaccine cost was increased to $10, $20, and $30 more than the standard vaccine.
TABLE 3.
Results from Cohort Model
| Societal Perspective | |||||
|---|---|---|---|---|---|
| Vaccine No Adjuvant | Vaccine with Adjuvant | ||||
| Adjuvant Cost | Cost | Effectiveness | Cost | Effectiveness | |
| $ | 0 | $76.13 +/− $12.48 | 0.79 +/− 0.10 | $39.26 +/− $5.51 | 0.81 +/− 0.10 |
| $ | 10.00 | $76.11 +/− $12.58 | 0.79 +/− 0.10 | $49.22 +/− $5.55 | 0.81 +/− 0.10 |
| $ | 20.00 | $76.13 +/− $12.58 | 0.78 +/− 0.10 | $59.24 +/− $5.50 | 0.81 +/− 0.10 |
| $ | 30.00 | $77.18 ± $12.66 | 0.79 +/− 0.10 | $69.78 ± $5.62 | 0.81 +/− 0.10 |
| Third Party Perspective | |||||
| Vaccine No Adjuvant | Vaccine with Adjuvant | ||||
| Adjuvant Cost | Cost | Effectiveness | Cost | Effectiveness | |
| $ | 0 | $75.77 ± $12.45 | 0.78 +/− 0.10 | $38.47 ± $5.53 | 0.81 +/− 0.10 |
| $ | 10.00 | $75.57 ± $12.69 | 0.78 +/− 0.10 | $48.35 ± $5.58 | 0.81 +/− 0.10 |
| $ | 20.00 | $75.90 ± $12.63 | 0.78 +/− 0.10 | $58.45 ± $5.61 | 0.81 +/− 0.10 |
| $ | 30.00 | $75.93 ± $12.73 | 0.78 +/− 0.10 | $68.51 ± $5.87 | 0.81 +/− 0.10 |
The adjuvanted vaccine remained dominant in similar scenarios from the third party perspective. In the base case scenario with adjuvanted vaccine costing the same as the standard vaccine, the vaccine with adjuvant was dominant and resulted in lower costs (Adjuvanted Vaccine, $38.47 ± 5.53; Standard Vaccine, $75.77 ± $12.45) and greater effectiveness (Adjuvanted Vaccine, 0.81 ± 0.1; Standard Vaccine, 0.78 ± 0.1). The adjuvanted vaccine remained the dominant strategy even when the adjuvanted vaccine cost was increased to $10 more than the standard vaccine (Adjuvanted Vaccine, $48.35 ± $5.58; Standard Vaccine, $75.57 ± $12.69), $20 more (Adjuvanted Vaccine, $58.45 ± $5.61; Standard Vaccine, $75.90 ± $12.63), and $30 more (Adjuvanted Vaccine, $68.51 ± $5.87; Standard Vaccine, $75.93 ± $12.73).
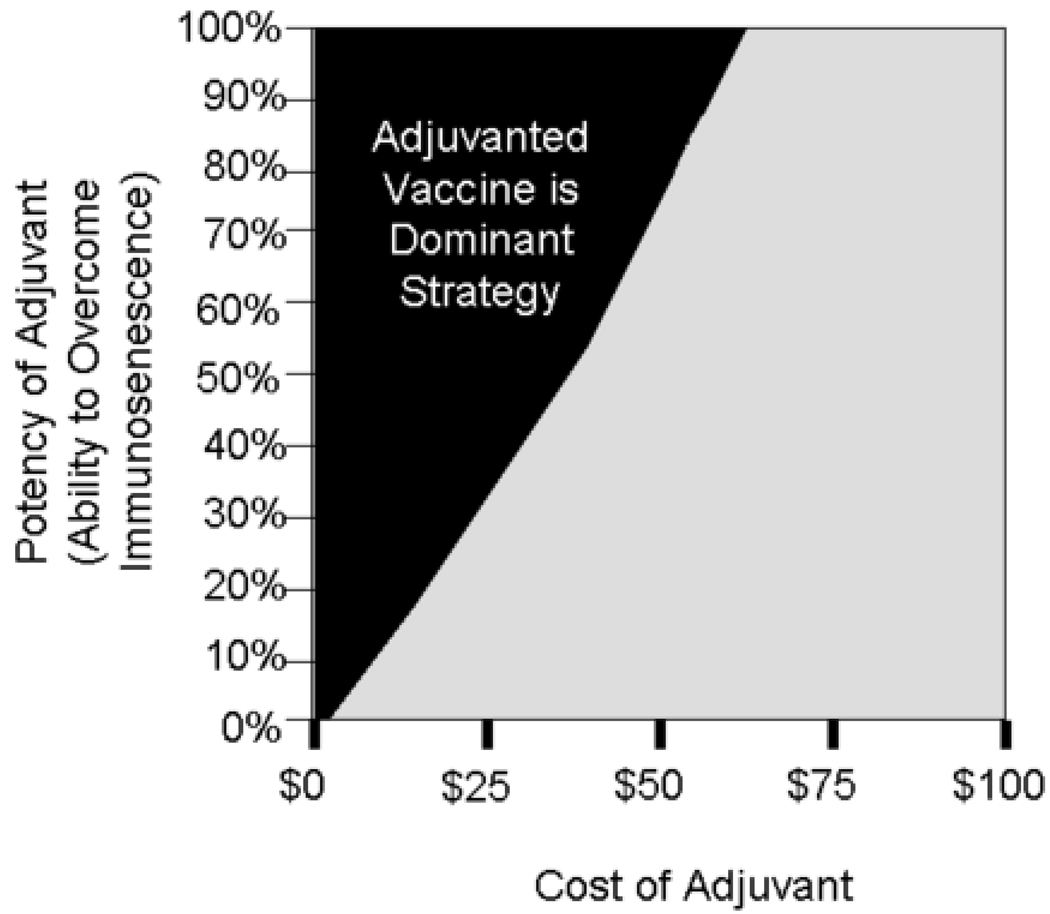
Figure 2 shows the effects of varying the cost and potency of the adjuvant and the cost-potency combinations that resulted in the adjuvant being the dominant strategy. The gains in cost savings and effectiveness increased as the potency of the adjuvant increased. The cost advantage decreased as the cost of the adjuvant increased. If the adjuvant were 100% effective in overcoming immunosenescence, then the adjuvant ceased to be the dominant strategy when priced $65 over the cost of standard influenza vaccine.
FIGURE 2.
Dominant Strategy at Different Vaccine Adjuvant Potency and Cost Combinations
United States (U.S.) Model
The base case U.S. model suggested that the adjuvant would result in considerable savings and health benefits. In one year, employing the adjuvant prevented 496,533 influenza cases, 171,981 hospitalizations, and 70,429 deaths. Employing the adjuvant would save society $824 million if the adjuvanted vaccine were to cost the same as standard vaccine, $595 million if it were to cost an extra $10, $369 million if it were to cost an additional $20, and $167 million if it were to cost an additional $30. Using the adjuvant would save third party payors $842 million if the adjuvanted vaccine were to cost the same as standard vaccine, $614 million if it were to cost an extra $10, $394 million if it were to cost an additional $20, and $167 million if it were to cost an additional $30.
Sensitivity Analyses
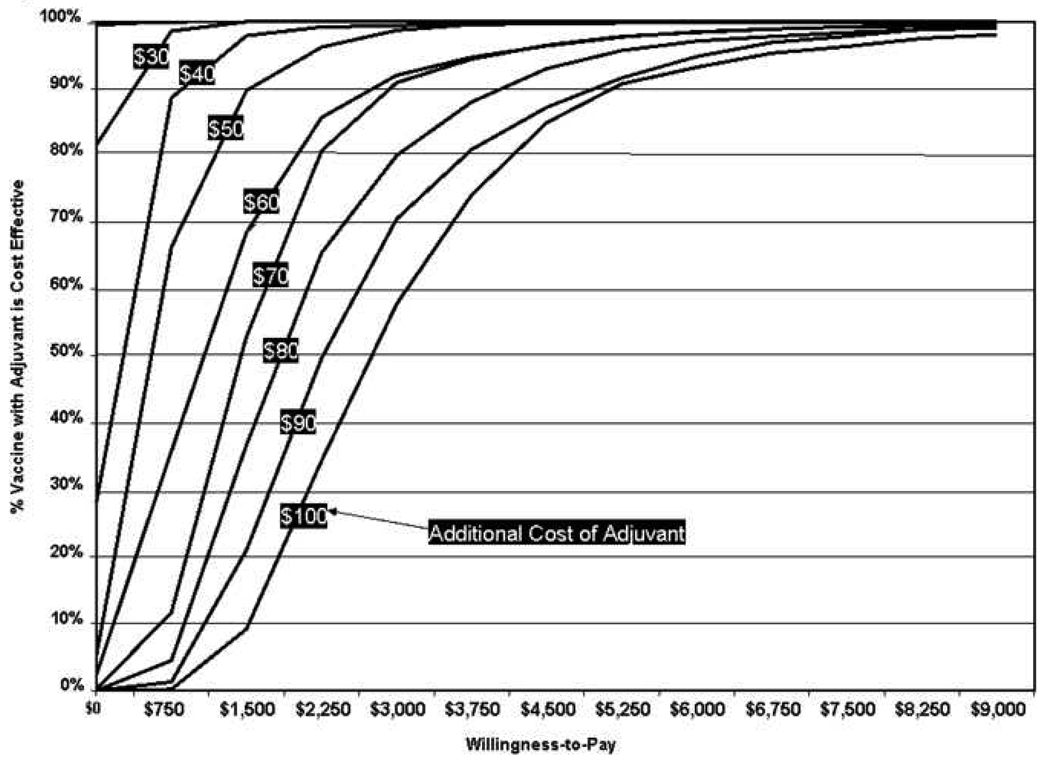
Figure 3 illustrate acceptability curves at different vaccine adjuvant costs. As long as the difference between the costs of the adjuvanted vaccine and standard vaccine is less than $20, the adjuvanted vaccine was always the dominant strategy regardless of the one’s willingness to pay. When the adjuvanted vaccine is $40 more than the standard vaccine, it ceases to be the favorable strategy when willingness to pay is less than $750 per QALY saved. When the adjuvanted vaccine is $60 more than the standard vaccine, the willingness-to-pay threshold is $1,500 per QALY saved. This threshold moves up to $2,250/QALY saved when the adjuvanted vaccine is $80 more and $3,000/QALY saved when the adjuvanted vaccine is $100 more.
FIGURE 3.
Acceptability Curves at Different Vaccine Adjuvant Costs
Some may speculate that increasing the conventional vaccine dose could increase its effectiveness, and hence blunt the advantage of an adjuvanted vaccine. A separate sensitivity analysis examined this possibility, postulating a doubled dose conventional influenza vaccination and assuming it has the same side effects profile as standard vaccination and half the potency of the adjuvanted vaccine in overcoming immunosenescence. If the adjuvanted vaccine has 25% potency in overcoming immunosenescence, it dominates the double dose vaccine if it costs < $18 more than the double dose; if 50% potent, it dominates if a cost difference of < $31 is present; and if 75% potent, adjuvanted vaccine dominates if it costs < $42 more than the doubled dose vaccine.
DISCUSSION
Our results suggest that introducing an adjuvanted influenza vaccine to the older adult (age 65 and over) population could save significant morbidity, mortality, and costs. Employing the adjuvant remained a dominant strategy (i.e., results in both cost and health savings) throughout a variety of adjuvant cost and potency scenarios. The magnitude of the health benefits depended heavily on how well the adjuvant overcame immunosenescence, while the magnitude of the cost savings depends largely on the cost of the adjuvanted vaccine. Our analyses suggested that pricing the adjuvanted vaccine at less than 533% of the standard influenza vaccine will result in net cost savings. This information may be useful when it is time to establish the price and reimbursement for adjuvanted vaccines should they reach the market.
Our analyses highlight the significant annual burden of immunosenescence in the U.S. This problem will continue to grow as the life expectancy and number of older individuals continues to increase.[13, 14] In fact, our model may have underestimated influenza incidence and consequently the burden of immunosenescence. Many influenza cases may go unreported, and influenza may be the underlying cause behind many exacerbations of chronic pulmonary, cardiac, or diabetic conditions.[15, 16] As a result, existing data may underreport hospitalizations and deaths due to influenza in the older adult population. Moreover, immunosenescence may play a large but underappreciated factor in influenza pandemic planning. The older adult population may continue to contract and spread the pandemic virus despite being vaccinated against the pandemic strain. Ignoring this potential problem could represent a gaping hole in our public health preparedness.
All of this supports the value of investing time, effort, and resources into developing influenza vaccine adjuvants and other ways of overcoming immunosenescence. Although most influenza vaccines currently do not contain any adjuvants, adding adjuvants to influenza vaccines may be on the horizon.[17] The U.S. Food and Drug Administration (FDA) has approved the use of alum as an adjuvant for several vaccines. MF59 has been used as an influenza vaccine adjuvant outside the U.S., and its use in the U.S. is currently under evaluation.[18–21] Several other naturally-occurring and synthetic compounds are in various stages of development.[22–24]
Our study is not limited to influenza vaccine adjuvants. Any intervention that overcomes immunosenescence could easily replace the adjuvant in our analysis. Evidence suggests that improving diet, increasing exercise, providing social support, decreasing stress, and limiting alcohol intake may help enhance the immune response in older individuals.[25] Other potential strategies include using higher doses of influenza vaccine or other medications in conjunction with influenza vaccination.[26, 27]
The problem of immunosenescence extends beyond influenza and influenza vaccination. The aging immune system and its attenuated response make older individuals more susceptible to a wide variety of infectious diseases, including infections of the respiratory tract, gastrointestinal tract, and central nervous system.[28–30] It also may decrease the response or the persistence of an immune response to other vaccines.
However, as we demonstrate in our consideration of a hypothetical doubled dose influenza vaccine, effectiveness comparisons between these interventions and an adjuvanted vaccination strategy would be a key feature in determining the relative cost-effectiveness of these differing strategies.
Limitations
Every computer model is a simplification of real life. No model can fully represent every single event and outcome that may ensue in the year after an influenza vaccination. For example, in our model, no individuals sought complementary and alternative therapies or visited the emergency room without being hospitalized. Moreover, our model did not account for the significant co-morbidities that exist in many of the older population. Such co-morbidities may increase a person’s risk of influenza and influenza-related complications and even change the type of complications that may occur. For example, a patient with severe chronic obstructive pulmonary disease (COPD) may require intubation and mechanical ventilation.
Additionally, such computer models usually do not fully represent the heterogeneity of the population. Considerable diversity exists within the older adult population. Community-dwelling older adults range from those with full-time jobs to those who spend considerable time in health care and nursing facilities. Nursing home residents range from individuals requiring round-the-clock medical care to those in very low acuity settings. Our model does not look at how potential differences in race, ethnicity, and socioeconomic status may affect probability of hospitalization, complications, and death and ultimately the cost and effectiveness of the adjuvant.
Finally, based on convention, we arbitrarily chose 65 years as the lower age limit for our older adult population. There is no evidence that immune systems change overnight between 64 and 65 years of age. Immunosenescence is more likely a gradual process that depending on the individual may start well before or after age 65 and proceed at different rates.
Conclusions and Future Directions
Our study quantified the burden of immunosenescence to society and third party payors and suggested that effective influenza vaccine adjuvants could save considerable money, morbidity, and mortality. As long as the adjuvanted vaccine costs less than 533% of the standard vaccine, employing the adjuvanted vaccine would dominate employing the standard vaccine in the older adult population. Our findings portray the significant problem posed by immunosenescence and the importance of finding ways to overcome this problem. Future studies will take a closer look at using the adjuvant in different subpopulations, such as patients on immunosuppressive therapy, patients with immunological diseases, younger adults with chronic disease, and subpopulations of the older adult population (e.g., diabetics, age 90 and over).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fleming DM, Watson JM, Nicholas S, Smith GE, Swan AV. Study of the effectiveness of influenza vaccination in the elderly in the epidemic of 1989-90 using a general practice database. Epidemiol Infect. 1995 Dec;115(3):581–589. doi: 10.1017/s095026880005874x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994 Dec 7;272(21):1661–1665. [PubMed] [Google Scholar]
- 3.Nichol KL. The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine. 2003 May 1;21(16):1769–1775. doi: 10.1016/s0264-410x(03)00070-7. [DOI] [PubMed] [Google Scholar]
- 4.Fedson DS, Wajda A, Nicol JP, Hammond GW, Kaiser DL, Roos LL. Clinical effectiveness of influenza vaccination in Manitoba. JAMA. 1993 Oct 27;270(16):1956–1961. [PubMed] [Google Scholar]
- 5.Nichol KL, Wuorenma J, von Sternberg T. Benefits of influenza vaccination for low-, intermediate-, and high-risk senior citizens. Arch Intern Med. 1998 Sep 14;158(16):1769–1776. doi: 10.1001/archinte.158.16.1769. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003 Nov 22;362(9397):1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordin J, Mullooly J, Poblete S, et al. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J Infect Dis. 2001 Sep 15;184(6):665–670. doi: 10.1086/323085. [DOI] [PubMed] [Google Scholar]
- 8.Treanor JJ, Campbell JD, Brady RC, et al. Rapid licensure of a new, inactivated influenza vaccine in the United States. Hum Vaccin. 2005 Nov–Dec;1(6):239–244. doi: 10.4161/hv.1.6.2376. [DOI] [PubMed] [Google Scholar]
- 9.McElhaney JE. The unmet need in the elderly: designing new influenza vaccines for older adults. Vaccine. 2005 Jul 8;23 Suppl 1:S10–S25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Murasko DM, Bernstein ED, Gardner EM, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002 Jan–Mar;37(2–3):427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 11.Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3:CD004876. doi: 10.1002/14651858.CD004876.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses: using national measures to create condition-specific values. Med Care. 1998 Jun;36(6):778–792. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Garrett N, Martini EM. The boomers are coming: a total cost of care model of the impact of population aging on the cost of chronic conditions in the United States. Dis Manag. 2007 Apr;10(2):51–60. doi: 10.1089/dis.2006.630. [DOI] [PubMed] [Google Scholar]
- 14.Martini EM, Garrett N, Lindquist T, Isham GJ. The boomers are coming: a total cost of care model of the impact of population aging on health care costs in the United States by Major Practice Category. Health Serv Res. 2007 Feb;42(1 Pt 1):201–218. doi: 10.1111/j.1475-6773.2006.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yap FH, Ho PL, Lam KF, Chan PK, Cheng YH, Peiris JS. Excess hospital admissions for pneumonia, chronic obstructive pulmonary disease, and heart failure during influenza seasons in Hong Kong. J Med Virol. 2004 Aug;73(4):617–623. doi: 10.1002/jmv.20135. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen-Van-Tam JS, Brockway CR, Pearson JC, Hayward AC, Fleming DM. Excess hospital admissions for pneumonia and influenza in persons > or = 65 years associated with influenza epidemics in three English health districts: 1987-95. Epidemiol Infect. 2001 Feb;126(1):71–79. doi: 10.1017/s0950268801005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palese P. Making better influenza virus vaccines? Emerg Infect Dis. 2006 Jan;12(1):61–65. doi: 10.3201/eid1201.051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldo V, Baldovin T, Floreani A, Carraro AM, Trivello R. MF59-adjuvanted influenza vaccine confers superior immunogenicity in adult subjects (18–60 years of age) with chronic diseases who are at risk of post-influenza complications. Vaccine. 2007 May 16;25(20):3955–3961. doi: 10.1016/j.vaccine.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 19.Atmar RL, Keitel WA, Patel SM, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006 Nov 1;43(9):1135–1142. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 20.Lin J, Zhang J, Dong X, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006 Sep 16;368(9540):991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 21.Iob A, Brianti G, Zamparo E, Gallo T. Evidence of increased clinical protection of an MF59-adjuvant influenza vaccine compared to a non-adjuvant vaccine among elderly residents of long-term care facilities in Italy. Epidemiol Infect. 2005 Aug;133(4):687–693. doi: 10.1017/s0950268805003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambhara S, Kurichh A, Miranda R, et al. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol. 2001 Aug 1;211(2):143–153. doi: 10.1006/cimm.2001.1835. [DOI] [PubMed] [Google Scholar]
- 23.Fritz JH, Brunner S, Birnstiel ML, et al. The artificial antimicrobial peptide KLKLLLLLKLK induces predominantly a TH2-type immune response to co-injected antigens. Vaccine. 2004 Sep 3;22(25–26):3274–3284. doi: 10.1016/j.vaccine.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Ichinohe T, Watanabe I, Ito S, et al. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J Virol. 2005 Mar;79(5):2910–2919. doi: 10.1128/JVI.79.5.2910-2919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002 Sep;57(9):M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- 26.Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med. 2006 May 22;166(10):1121–1127. doi: 10.1001/archinte.166.10.1121. [DOI] [PubMed] [Google Scholar]
- 27.Hamazaki K, Sawazaki S, Itomura M, et al. No effect of a traditional Chinese medicine, Hochu-ekki-to, on antibody titer after influenza vaccination in man: a randomized, placebo-controlled, double-blind trial. Phytomedicine. 2007 Jan;14(1):11–14. doi: 10.1016/j.phymed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol. 2001 Jul;21(4):264–271. doi: 10.1023/a:1010979225018. [DOI] [PubMed] [Google Scholar]
- 29.Choi C. Bacterial meningitis in aging adults. Clin Infect Dis. 2001 Oct 15;33(8):1380–1385. doi: 10.1086/322688. [DOI] [PubMed] [Google Scholar]
- 30.McElhaney JE. Overcoming the challenges of immunosenescence in the prevention of acute respiratory illness in older people. Conn Med. 2003 Sep;67(8):469–474. [PubMed] [Google Scholar]
- 31.Red Book. 2007 ed. Montvale, NJ: Thompson Healthcare Inc.; 2007. [Google Scholar]
- 32. [cited 2008 January 25];Sudafed Non-drowsy Maximum Strength Nasal Decongestant Tablets. Available from: http://www.drugstore.com/products/prod.asp?pid=11509&catid=382.
- 33. [cited 2008 February 4];Centers for Medicare & Medicaid Services. 2008 Available from: http://www.cms.hhs.gov/ [PubMed]
- 34.Labor USDo. 2007. Jun, National Compensation Survey: Occupational Wages in the United States, June 2006. ed. [Google Scholar]
- 35.Levit K, Ryan K, Elixhauser A, Stranges E, Kassed C, R C. HCUP Facts and Figures: Statistics on Hospital-based Care in the United States in 2005. 2007:1–66. [Google Scholar]
- 36.Smith KJ, Roberts MS. Cost-effectiveness of newer treatment strategies for influenza. Am J Med. 2002 Sep;113(4):300–307. doi: 10.1016/s0002-9343(02)01222-6. [DOI] [PubMed] [Google Scholar]
- 37.Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1999 Apr 30;48(RR-4):1–28. [PubMed] [Google Scholar]
- 38.Neuraminidase inhibitors for treatment of influenza A and B infections. MMWR Recomm Rep. 1999 Dec 17;48(RR-14):1–9. [PubMed] [Google Scholar]
- 39.Gubareva LV, Kaiser L, Hayden FG. Influenza virus neuraminidase inhibitors. Lancet. 2000 Mar 4;355(9206):827–835. doi: 10.1016/S0140-6736(99)11433-8. [DOI] [PubMed] [Google Scholar]
- 40.Hayden FG, Osterhaus AD, Treanor JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenzavirus infections.GG167 Influenza Study Group. N Engl J Med. 1997 Sep 25;337(13):874–880. doi: 10.1056/NEJM199709253371302. [DOI] [PubMed] [Google Scholar]
- 41.Jefferson TO, Demicheli V, Deeks JJ, Rivetti D. Amantadine and rimantadine for preventing and treating influenza A in adults. Cochrane Database Syst Rev. 2000;(2):CD001169. doi: 10.1002/14651858.CD001169. [DOI] [PubMed] [Google Scholar]
- 42.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. Jama. 2000 Feb 23;283(8):1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 43.Sackett DL, Torrance GW. The utility of different health states as perceived by the general public. J Chronic Dis. 1978;31(11):697–704. doi: 10.1016/0021-9681(78)90072-3. [DOI] [PubMed] [Google Scholar]
- 44.He Wan, Sengupta Manisha, Velkoff VictoriaA, DeBarros KimberlyA. Bureau USC. 65+ in the United States: 2005 Current Population Reports. US Census Bureau. Current Population Reports 2005. 2005 December;:1–243. (P23-209) [Google Scholar]
- 45.Govaert TM, Dinant GJ, Aretz K, Masurel N, Sprenger MJ, Knottnerus JA. Adverse reactions to influenza vaccine in elderly people: randomised double blind placebo controlled trial. Bmj. 1993 Oct 16;307(6910):988–990. doi: 10.1136/bmj.307.6910.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007;(2):CD001269. doi: 10.1002/14651858.CD001269.pub3. [DOI] [PubMed] [Google Scholar]
- 47.Hetzel L, Smith A. The 65 Years and Over population. Commerce Do. 2000 ed. [Google Scholar]
- 48.Wilmoth JR, Shkolnikov V. HMD United States of America University of California, Berkeley (USA), and Max Plank Insititute for Demographic Research (Germany) [Google Scholar]
- 49.Survey NHI. Annual percentage of adults aged 50 years and over who had received an influenza vaccination during the past 12 months, by age group and sex: United States, 1997–2006. Survey ERoSEBoDftNHI, ed. 2007 Receipt of influenza vaccination.
- 50.Monto AS, Hornbuckle K, Ohmit SE. Influenza vaccine effectiveness among elderly nursing home residents: a cohort study. Am J Epidemiol. 2001 Jul 15;154(2):155–160. doi: 10.1093/aje/154.2.155. [DOI] [PubMed] [Google Scholar]