Abstract
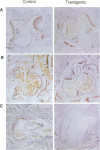
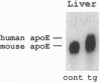
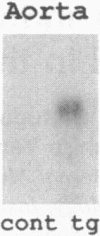
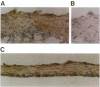
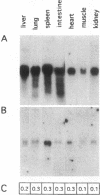
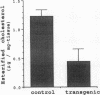
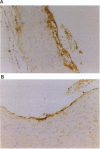
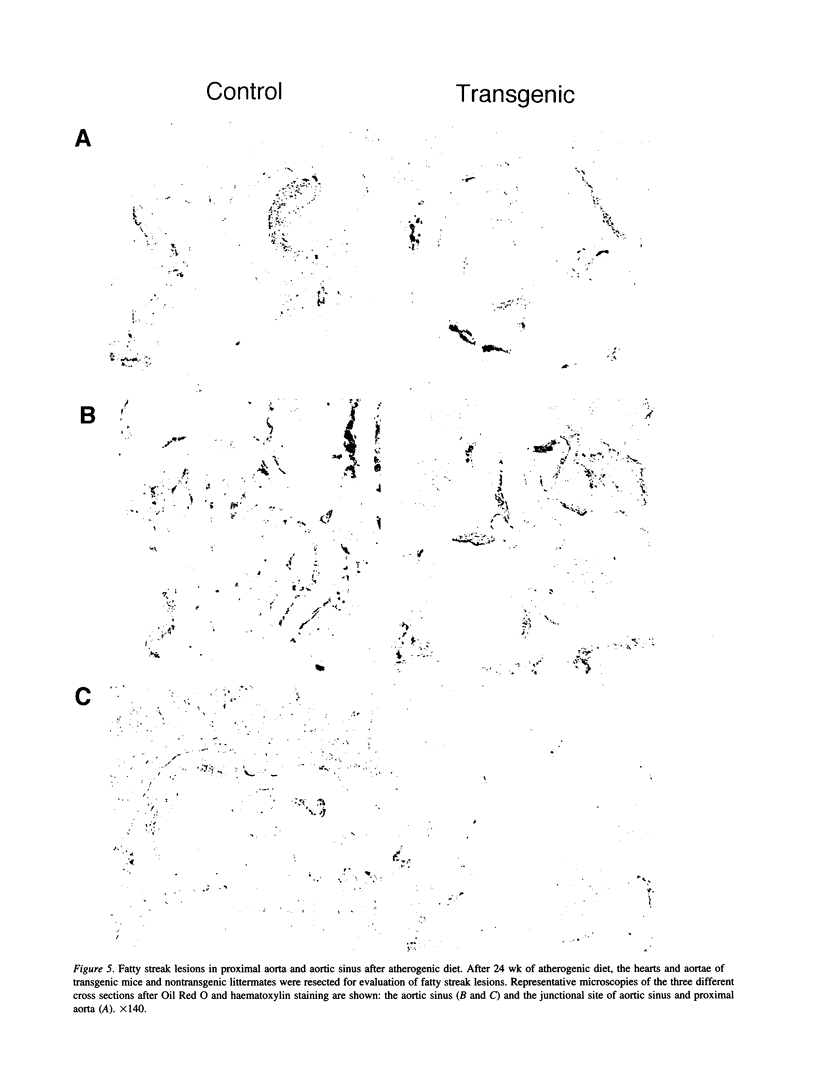
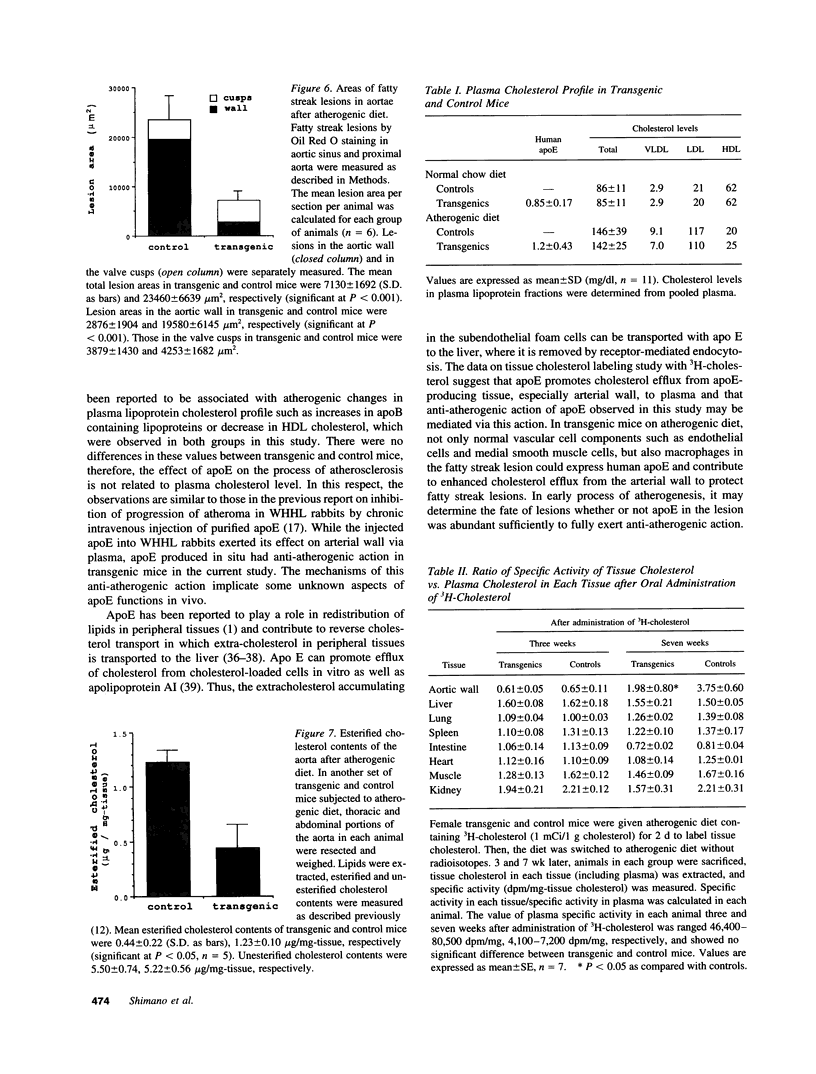
Apolipoprotein E (apoE) plays a crucial role in lipoprotein metabolism both in plasma and in peripheral tissues. To test whether apoE in the vascular wall has a direct and local effect on atherogenesis, we established transgenic mice expressing human apoE under control of H2 Ld promoter. Studies on mRNA levels and immunohistochemistry demonstrated that this line was characterized by high expression of human apoE in the arterial wall while its expression was relatively low in other tissues as compared with the respective endogenous expression of mouse apoE. They showed no difference in plasma cholesterol levels and lipoprotein profile from controls when fed both normal and atherogenic diets. However, after 24 wk of an atherogenic diet, the formation of fatty streak lesions in proximal aorta was markedly inhibited in transgenic mice as compared with controls. Both lesion area and esterified cholesterol content were < 30% of those in controls. In a tissue cholesterol labeling study with 3H-cholesterol, the specific activity of aorta cholesterol was much less in transgenic mice, suggesting that apoE enhances cholesterol efflux from the aortic wall into plasma. Thus, apoE has anti-atherogenic action which is mediated via enhancing reverse cholesterol transport from arterial wall.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Babaev V. R., Dergunov A. D., Chenchik A. A., Tararak E. M., Yanushevskaya E. V., Trakht I. N., Sorg C., Smirnov V. N. Localization of apolipoprotein E in normal and atherosclerotic human aorta. Atherosclerosis. 1990 Dec;85(2-3):239–247. doi: 10.1016/0021-9150(90)90116-z. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Brown M. S. Independent pathways for secretion of cholesterol and apolipoprotein E by macrophages. Science. 1983 Feb 18;219(4586):871–873. doi: 10.1126/science.6823554. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Breslow J. L., McPherson J., Nussbaum A. L., Williams H. W., Lofquist-Kahl F., Karathanasis S. K., Zannis V. I. Identification and DNA sequence of a human apolipoprotein E cDNA clone. J Biol Chem. 1982 Dec 25;257(24):14639–14641. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crespo P., Ros M. A., Ordovás J. M., Rodriguez J. C., Ortiz J. M., León J. Foam cells from aorta and spleen overexpress apolipoprotein E in the absence of hypercholesterolemia. Biochem Biophys Res Commun. 1992 Mar 16;183(2):514–523. doi: 10.1016/0006-291x(92)90512-j. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Friedman G., Vogel T. Enhanced metabolism of normolipidemic human plasma very low density lipoprotein in cultured cells by exogenous apolipoprotein E-3. Arteriosclerosis. 1988 Sep-Oct;8(5):480–487. doi: 10.1161/01.atv.8.5.480. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fielding C. J., Fielding P. E. Cholesterol transport between cells and body fluids. Role of plasma lipoproteins and the plasma cholesterol esterification system. Med Clin North Am. 1982 Mar;66(2):363–373. doi: 10.1016/s0025-7125(16)31425-0. [DOI] [PubMed] [Google Scholar]
- Hara H., Yokoyama S. Interaction of free apolipoproteins with macrophages. Formation of high density lipoprotein-like lipoproteins and reduction of cellular cholesterol. J Biol Chem. 1991 Feb 15;266(5):3080–3086. [PubMed] [Google Scholar]
- Kano M., Koizumi J., Jadhav A., Thompson G. R. Plasma exchange and low density lipoprotein apheresis in Watanabe heritable hyperlipidemic rabbits. Arteriosclerosis. 1987 May-Jun;7(3):256–261. doi: 10.1161/01.atv.7.3.256. [DOI] [PubMed] [Google Scholar]
- Landias D., Beck B. N., Buerstedde J. M., Degraw S., Klein D., Koch N., Murphy D., Pierres M., Tada T., Yamamoto K. The assignment of chain specificities for anti-Ia monoclonal antibodies using L cell transfectants. J Immunol. 1986 Nov 1;137(9):3002–3005. [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Hussain M. M., Greenman B., Fisher M., Vogel T., Gorecki M. Intravenous infusion of apolipoprotein E accelerates clearance of plasma lipoproteins in rabbits. J Clin Invest. 1989 Jun;83(6):2125–2130. doi: 10.1172/JCI114126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokuno H., Yamada N., Shimano H., Ishibashi S., Mori N., Takahashi K., Oka T., Yoon T. H., Takaku F. The enhanced cellular uptake of very-low-density lipoprotein enriched in apolipoprotein E. Biochim Biophys Acta. 1991 Feb 26;1082(1):63–70. doi: 10.1016/0005-2760(91)90300-7. [DOI] [PubMed] [Google Scholar]
- Ozato K., Wan Y. J., Orrison B. M. Mouse major histocompatibility class I gene expression begins at midsomite stage and is inducible in earlier-stage embryos by interferon. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2427–2431. doi: 10.1073/pnas.82.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen B., Ishida B. Y., Verstuyft J., Winters R. B., Albee D. Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis. 1990 Mar-Apr;10(2):316–323. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- Paigen B., Morrow A., Brandon C., Mitchell D., Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985 Oct;57(1):65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Arnold K. S., Mahley R. W. Rate and equilibrium constants for binding of apo-E HDLc (a cholesterol-induced lipoprotein) and low density lipoproteins to human fibroblasts: evidence for multiple receptor binding of apo-E HDLc. Proc Natl Acad Sci U S A. 1979 May;76(5):2311–2315. doi: 10.1073/pnas.76.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Rajavashisth T. B., Kaptein J. S., Reue K. L., Lusis A. J. Evolution of apolipoprotein E: mouse sequence and evidence for an 11-nucleotide ancestral unit. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8085–8089. doi: 10.1073/pnas.82.23.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Butler S., Ord V. A., Lipton B. A., Dyer C. A., Curtiss L. K., Palinski W., Witztum J. L. Abundant expression of apoprotein E by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb. 1993 Sep;13(9):1382–1389. doi: 10.1161/01.atv.13.9.1382. [DOI] [PubMed] [Google Scholar]
- Rubin E. M., Krauss R. M., Spangler E. A., Verstuyft J. G., Clift S. M. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991 Sep 19;353(6341):265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- Shimano H., Fukazawa C., Shibasaki Y., Mori N., Gotoda T., Harada K., Shimada M., Yamada N., Yazaki Y., Takaku F. The effect of apo E secretion on lipoprotein uptake in transfected cells. Biochim Biophys Acta. 1991 Nov 27;1086(3):245–254. doi: 10.1016/0005-2760(91)90166-f. [DOI] [PubMed] [Google Scholar]
- Shimano H., Namba Y., Ohsuga J., Kawamura M., Yamamoto K., Shimada M., Gotoda T., Harada K., Yazaki Y., Yamada N. Secretion-recapture process of apolipoprotein E in hepatic uptake of chylomicron remnants in transgenic mice. J Clin Invest. 1994 May;93(5):2215–2223. doi: 10.1172/JCI117218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H., Yamada N., Katsuki M., Shimada M., Gotoda T., Harada K., Murase T., Fukazawa C., Takaku F., Yazaki Y. Overexpression of apolipoprotein E in transgenic mice: marked reduction in plasma lipoproteins except high density lipoprotein and resistance against diet-induced hypercholesterolemia. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1750–1754. doi: 10.1073/pnas.89.5.1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H., Yamada N., Katsuki M., Yamamoto K., Gotoda T., Harada K., Shimada M., Yazaki Y. Plasma lipoprotein metabolism in transgenic mice overexpressing apolipoprotein E. Accelerated clearance of lipoproteins containing apolipoprotein B. J Clin Invest. 1992 Nov;90(5):2084–2091. doi: 10.1172/JCI116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H., Yamada N., Shimada M., Ohsawa N., Fukazawa C., Yazaki Y., Takaku F., Katsuki M. Hepatic and renal expression of rat apolipoprotein E under control of the metallothionein promoter in transgenic mice. Biochim Biophys Acta. 1991 Aug 27;1090(1):91–94. doi: 10.1016/0167-4781(91)90041-j. [DOI] [PubMed] [Google Scholar]
- Sugita K., Miyazaki J., Appella E., Ozato K. Interferons increase transcription of a major histocompatibility class I gene via a 5' interferon consensus sequence. Mol Cell Biol. 1987 Jul;7(7):2625–2630. doi: 10.1128/mcb.7.7.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Shames D. M., Havel R. J. Effect of low density lipoprotein receptor deficiency on the metabolism of apolipoprotein B-100 in blood plasma. Kinetic studies in normal and Watanabe heritable hyperlipidemic rabbits. J Clin Invest. 1987 Aug;80(2):507–515. doi: 10.1172/JCI113099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Shames D. M., Stoudemire J. B., Havel R. J. Metabolism of lipoproteins containing apolipoprotein B-100 in blood plasma of rabbits: heterogeneity related to the presence of apolipoprotein E. Proc Natl Acad Sci U S A. 1986 May;83(10):3479–3483. doi: 10.1073/pnas.83.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Shames D. M., Takahashi K., Havel R. J. Metabolism of apolipoprotein B-100 in large very low density lipoproteins of blood plasma. Kinetic studies in normal and Watanabe heritable hyperlipidemic rabbits. J Clin Invest. 1988 Dec;82(6):2106–2113. doi: 10.1172/JCI113832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N., Shimano H., Mokuno H., Ishibashi S., Gotohda T., Kawakami M., Watanabe Y., Akanuma Y., Murase T., Takaku F. Increased clearance of plasma cholesterol after injection of apolipoprotein E into Watanabe heritable hyperlipidemic rabbits. Proc Natl Acad Sci U S A. 1989 Jan;86(2):665–669. doi: 10.1073/pnas.86.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]