Abstract
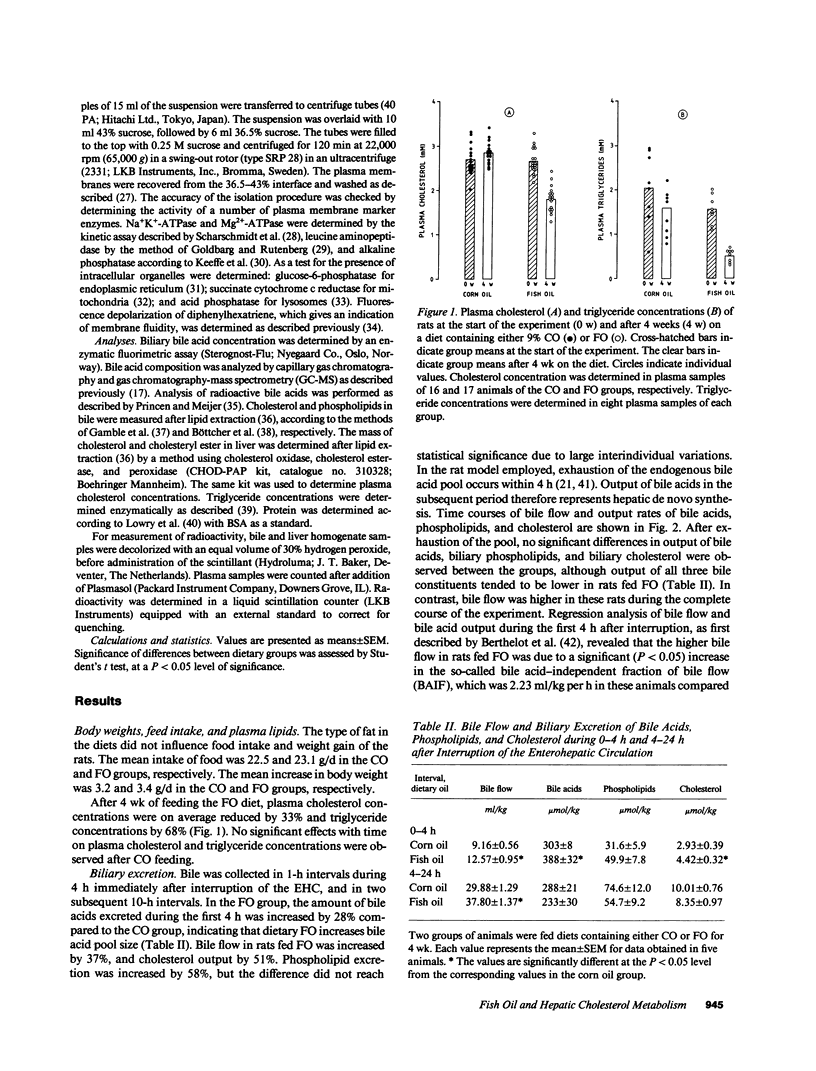
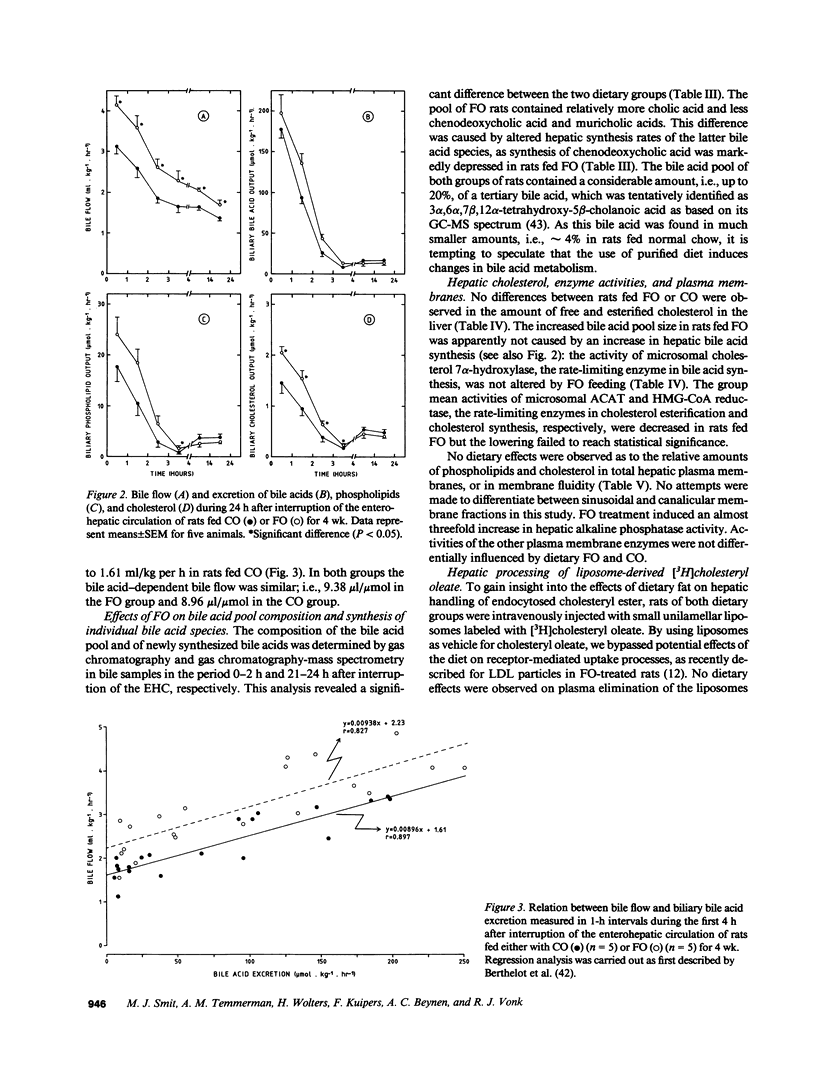
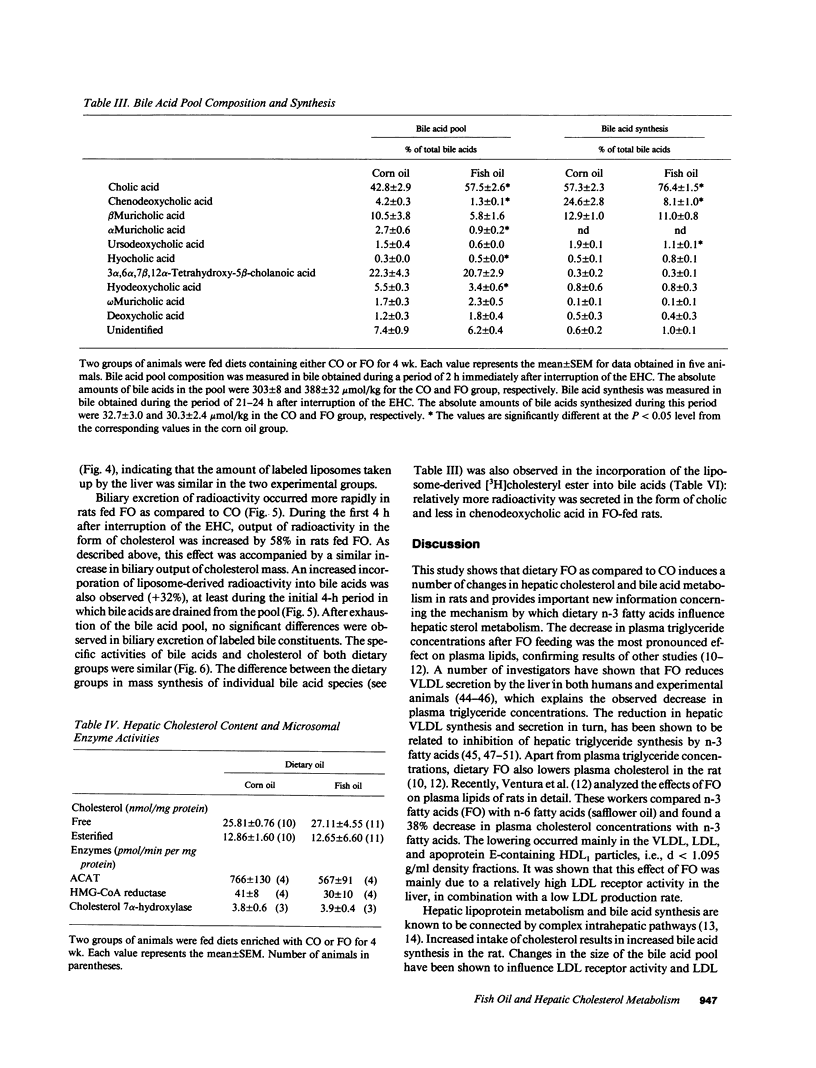
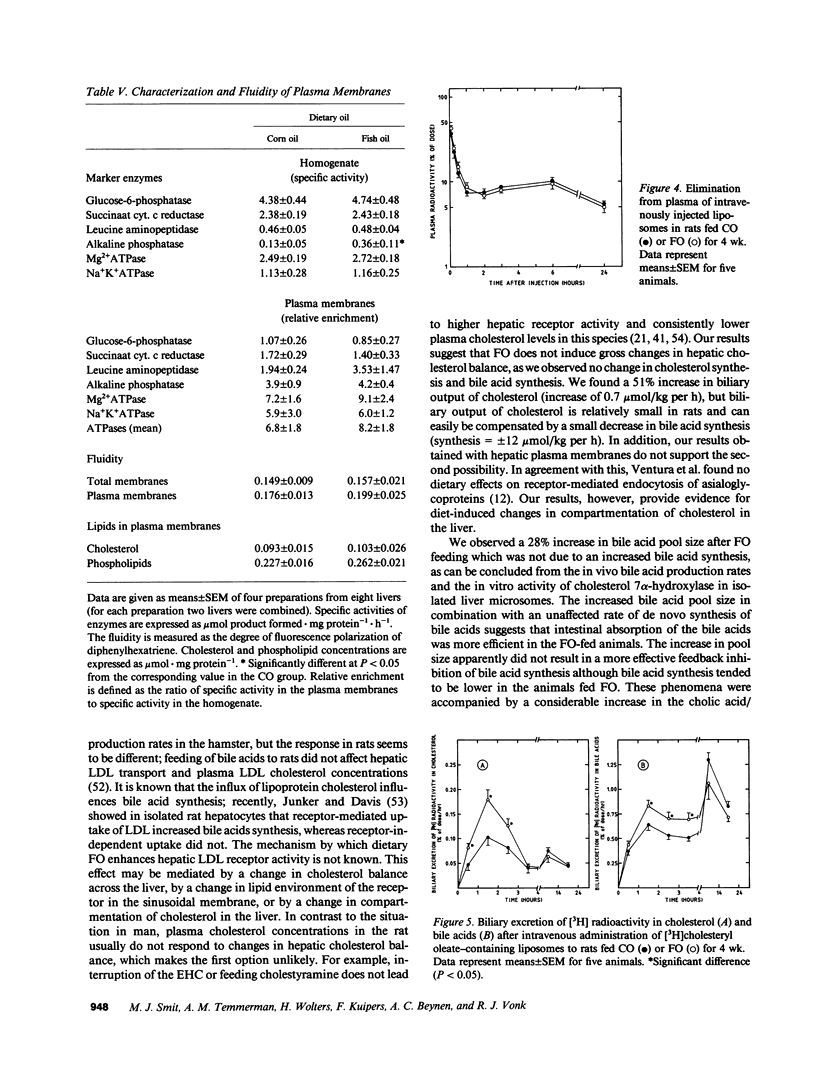
Hepatic cholesterol metabolism was studied in rats fed purified diets supplemented (9% wt/wt) with either fish oil (FO) (n-3 fatty acids) or corn oil (CO) (n-6 fatty acids) for 4 wk. Rats were equipped with permanent catheters in heart, bile duct, and duodenum to allow studies under normal feeding conditions. [3H]-cholesteryl oleate-labeled small unilamellar liposomes, which are rapidly endocytosed by hepatocytes, were intravenously injected to label intrahepatic cholesterol pools, and plasma and bile were collected. FO as compared to CO induced a lowering of plasma cholesterol levels by 38% and of triglyceride levels by 69%. This reduction in plasma lipids in FO rats was accompanied by: (a) an increased bile acid pool size (28%); (b) a fourfold increase in the ratio cholic acid/chenodeoxycholic acid in bile; (c) increased biliary excretion of cholesterol (51%); (d) accelerated excretion of endocytosed free cholesterol into bile; (e) accelerated incorporation of endocytosed cholesterol in bile acids; (f) a significant increase in the bile acid-independent fraction of bile flow; and (g) a threefold increase in hepatic alkaline phosphatase activity. The results show that FO induces changes in transport and metabolic pathways of cholesterol in the rat liver, which result in a more rapid disposition of plasma-derived cholesterol into the bile.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelin B., Carlson L. A. Bile acids and plasma high density lipoproteins: biliary lipid metabolism in fish eye disease. Eur J Clin Invest. 1986 Apr;16(2):157–162. doi: 10.1111/j.1365-2362.1986.tb01323.x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S., Simons L. A., Chang S., Hickie J. B. Reduction in plasma cholesterol and increase in biliary cholesterol by a diet rich in n-3 fatty acids in the rat. J Lipid Res. 1985 Jun;26(6):684–689. [PubMed] [Google Scholar]
- Berthelot P., Erlinger S., Dhumeaux D., Preaux A. M. Mechanism of phenobarbital-induced hypercholeresis in the rat. Am J Physiol. 1970 Sep;219(3):809–813. doi: 10.1152/ajplegacy.1970.219.3.809. [DOI] [PubMed] [Google Scholar]
- Bijleveld C. M., Vonk R. J., Kuipers F., Havinga R., Boverhof R., Koopman B. J., Wolthers B. G., Fernandes J. Benign recurrent intrahepatic cholestasis: altered bile acid metabolism. Gastroenterology. 1989 Aug;97(2):427–432. doi: 10.1016/0016-5085(89)90079-6. [DOI] [PubMed] [Google Scholar]
- Billheimer J. T., Tavani D., Nes W. R. Effect of a dispersion of cholesterol in Triton WR-1339 on acyl CoA: cholesterol acyltransferase in rat liver microsomes. Anal Biochem. 1981 Mar 1;111(2):331–335. doi: 10.1016/0003-2697(81)90570-4. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Danielsson H., Gustafsson J. On the effect of thyroid hormone on 26-hydroxylation of C 27 -steroids in rat liver. FEBS Lett. 1973 Apr 1;31(1):20–22. doi: 10.1016/0014-5793(73)80064-x. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Eriksson M., Einarsson K. Evidence for a lack of regulatory importance of the 12 alpha-hydroxylase in formation of bile acids in man: an in vivo study. J Lipid Res. 1983 Nov;24(11):1451–1456. [PubMed] [Google Scholar]
- Brasitus T. A., Dahiya R., Dudeja P. K., Bissonnette B. M. Cholesterol modulates alkaline phosphatase activity of rat intestinal microvillus membranes. J Biol Chem. 1988 Jun 25;263(18):8592–8597. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson H., Gustafsson J. Biochemistry of bile acids in health and disease. Pathobiol Annu. 1981;11:259–298. [PubMed] [Google Scholar]
- Dietschy J. M. Regulation of cholesterol metabolism in man and in other species. Klin Wochenschr. 1984 Apr 16;62(8):338–345. doi: 10.1007/BF01716251. [DOI] [PubMed] [Google Scholar]
- Dyerberg J., Bang H. O. A hypothesis on the development of acute myocardial infarction in Greenlanders. Scand J Clin Lab Invest Suppl. 1982;161:7–13. [PubMed] [Google Scholar]
- Erlinger S. Does Na+-K+-atpase have any role in bile secretion? Am J Physiol. 1982 Oct;243(4):G243–G247. doi: 10.1152/ajpgi.1982.243.4.G243. [DOI] [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Gamble W., Vaughan M., Kruth H. S., Avigan J. Procedure for determination of free and total cholesterol in micro- or nanogram amounts suitable for studies with cultured cells. J Lipid Res. 1978 Nov;19(8):1068–1070. [PubMed] [Google Scholar]
- Hall R., Kok E., Javitt N. B. Bile acid synthesis: down-regulation by monohydroxy bile acids. FASEB J. 1988 Feb;2(2):152–156. doi: 10.1096/fasebj.2.2.3342968. [DOI] [PubMed] [Google Scholar]
- Harris W. S., Connor W. E., Inkeles S. B., Illingworth D. R. Dietary omega-3 fatty acids prevent carbohydrate-induced hypertriglyceridemia. Metabolism. 1984 Nov;33(11):1016–1019. doi: 10.1016/0026-0495(84)90230-0. [DOI] [PubMed] [Google Scholar]
- Harris W. S., Connor W. E., McMurry M. P. The comparative reductions of the plasma lipids and lipoproteins by dietary polyunsaturated fats: salmon oil versus vegetable oils. Metabolism. 1983 Feb;32(2):179–184. doi: 10.1016/0026-0495(83)90226-3. [DOI] [PubMed] [Google Scholar]
- Harris W. S. Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res. 1989 Jun;30(6):785–807. [PubMed] [Google Scholar]
- Illingworth D. R., Harris W. S., Connor W. E. Inhibition of low density lipoprotein synthesis by dietary omega-3 fatty acids in humans. Arteriosclerosis. 1984 May-Jun;4(3):270–275. doi: 10.1161/01.atv.4.3.270. [DOI] [PubMed] [Google Scholar]
- Junker L. H., Davis R. A. Receptor-mediated uptake of low density lipoprotein stimulates bile acid synthesis by cultured rat hepatocytes. J Lipid Res. 1989 Dec;30(12):1933–1941. [PubMed] [Google Scholar]
- Keefee E. B., Scharschmidt B. F., Blankenship N. M., Ockner R. K. Studies of relationship among bile flow, liver plasma membrane NaK-ATPase, and membrane microviscosity in the rat. J Clin Invest. 1979 Dec;64(6):1590–1598. doi: 10.1172/JCI109620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromann N., Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950-1974. Acta Med Scand. 1980;208(5):401–406. [PubMed] [Google Scholar]
- Kromhout D., Bosschieter E. B., de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985 May 9;312(19):1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Dijkstra T., Havinga R., van Asselt W., Vonk R. J. Acute effects of pentobarbital-anaesthesia on bile secretion. Biochem Pharmacol. 1985 May 15;34(10):1731–1736. doi: 10.1016/0006-2952(85)90642-2. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Havinga R., Bosschieter H., Toorop G. P., Hindriks F. R., Vonk R. J. Enterohepatic circulation in the rat. Gastroenterology. 1985 Feb;88(2):403–411. doi: 10.1016/0016-5085(85)90499-8. [DOI] [PubMed] [Google Scholar]
- Kuipers F., Spanjer H. H., Havinga R., Scherphof G. L., Vonk R. J. Lipoproteins and liposomes as in vivo cholesterol vehicles in the rat: preferential use of cholesterol carried by small unilamellar liposomes for the formation of muricholic acids. Biochim Biophys Acta. 1986 May 21;876(3):559–566. doi: 10.1016/0005-2760(86)90044-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leaf A., Weber P. C. Cardiovascular effects of n-3 fatty acids. N Engl J Med. 1988 Mar 3;318(9):549–557. doi: 10.1056/NEJM198803033180905. [DOI] [PubMed] [Google Scholar]
- Marsh J. B., Topping D. L., Nestel P. J. Comparative effects of dietary fish oil and carbohydrate on plasma lipids and hepatic activities of phosphatidate phosphohydrolase, diacylglycerol acyltransferase and neutral lipase activities in the rat. Biochim Biophys Acta. 1987 Nov 21;922(2):239–243. doi: 10.1016/0005-2760(87)90160-3. [DOI] [PubMed] [Google Scholar]
- Meier P. J., Sztul E. S., Reuben A., Boyer J. L. Structural and functional polarity of canalicular and basolateral plasma membrane vesicles isolated in high yield from rat liver. J Cell Biol. 1984 Mar;98(3):991–1000. doi: 10.1083/jcb.98.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar B. A., Huang Y. S., Manku M. S., Das U. N., Morse N., Horrobin D. F. The influence of dietary manipulation with n-3 and n-6 fatty acids on liver and plasma phospholipid fatty acids in rats. Lipids. 1986 Oct;21(10):652–656. doi: 10.1007/BF02537216. [DOI] [PubMed] [Google Scholar]
- Nestel P. J., Connor W. E., Reardon M. F., Connor S., Wong S., Boston R. Suppression by diets rich in fish oil of very low density lipoprotein production in man. J Clin Invest. 1984 Jul;74(1):82–89. doi: 10.1172/JCI111422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossen J. O., Rustan A. C., Gloppestad S. H., Målbakken S., Drevon C. A. Eicosapentaenoic acid inhibits synthesis and secretion of triacylglycerols by cultured rat hepatocytes. Biochim Biophys Acta. 1986 Oct 24;879(1):56–65. doi: 10.1016/0005-2760(86)90266-3. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Mink J., Hardison W. G., Miyai K. Alkaline phosphatase activity in hepatic tissue and serum correlates with amount and type of bile acid load. Lab Invest. 1990 Jan;62(1):87–95. [PubMed] [Google Scholar]
- Packard C. J., Shepherd J. The hepatobiliary axis and lipoprotein metabolism: effects of bile acid sequestrants and ileal bypass surgery. J Lipid Res. 1982 Nov;23(8):1081–1098. [PubMed] [Google Scholar]
- Philipp B. W., Shapiro D. J. Improved methods for the assay and activation of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Lipid Res. 1979 Jul;20(5):588–593. [PubMed] [Google Scholar]
- Princen H. M., Huijsmans C. M., Kuipers F., Vonk R. J., Kempen H. J. Ketoconazole blocks bile acid synthesis in hepatocyte monolayer cultures and in vivo in rat by inhibiting cholesterol 7 alpha-hydroxylase. J Clin Invest. 1986 Oct;78(4):1064–1071. doi: 10.1172/JCI112662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M., Meijer P. Hydroxylation, conjugation and sulfation of bile acids in primary monolayer cultures of rat hepatocytes. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1114–1121. doi: 10.1016/0006-291x(88)90256-2. [DOI] [PubMed] [Google Scholar]
- Princen H. M., Meijer P., Kwekkeboom J., Kempen H. J. Assay of cholesterol 7 alpha-hydroxylase activity in rat hepatocytes in primary monolayer culture. Anal Biochem. 1988 May 15;171(1):158–165. doi: 10.1016/0003-2697(88)90137-6. [DOI] [PubMed] [Google Scholar]
- Roach P. D., Kambouris A. M., Trimble R. P., Topping D. L., Nestel P. J. The effects of dietary fish oil on hepatic high density and low density lipoprotein receptor activities in the rat. FEBS Lett. 1987 Sep 28;222(1):159–162. doi: 10.1016/0014-5793(87)80211-9. [DOI] [PubMed] [Google Scholar]
- Rothstein T. L., Blum J. J. Lysosomal physiology in Tetrahymena. I. Effect of glucose, acetate, pyruvate, and carmine on intracellular content and extracellular release of three acid hydrolases. J Cell Biol. 1973 Jun;57(3):630–641. doi: 10.1083/jcb.57.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustan A. C., Nossen J. O., Christiansen E. N., Drevon C. A. Eicosapentaenoic acid reduces hepatic synthesis and secretion of triacylglycerol by decreasing the activity of acyl-coenzyme A:1,2-diacylglycerol acyltransferase. J Lipid Res. 1988 Nov;29(11):1417–1426. [PubMed] [Google Scholar]
- Sanders T. A., Sullivan D. R., Reeve J., Thompson G. R. Triglyceride-lowering effect of marine polyunsaturates in patients with hypertriglyceridemia. Arteriosclerosis. 1985 Sep-Oct;5(5):459–465. doi: 10.1161/01.atv.5.5.459. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Keeffe E. B., Blankenship N. M., Ockner R. K. Validation of a recording spectrophotometric method for measurement of membrane-associated Mg- and NaK-ATPase activity. J Lab Clin Med. 1979 May;93(5):790–799. [PubMed] [Google Scholar]
- Scherphof G. L., Spanjer H. H., Derksen J. T., Kuipers F., Vonk R. J., Daemen T., Roerdink F. H. Delivery of liposome-associated drugs to liver cells. Biochem Soc Trans. 1987 Jun;15(3):345–348. doi: 10.1042/bst0150345. [DOI] [PubMed] [Google Scholar]
- Smit M. J., Temmerman A. M., Havinga R., Kuipers F., Vonk R. J. Short- and long-term effects of biliary drainage on hepatic cholesterol metabolism in the rat. Biochem J. 1990 Aug 1;269(3):781–788. doi: 10.1042/bj2690781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Stange E. F., Bilhartz L. E., Dietschy J. M. Bile acids regulate hepatic low density lipoprotein receptor activity in the hamster by altering cholesterol flux across the liver. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1916–1920. doi: 10.1073/pnas.83.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Turley S. D., Dietschy J. M. Rates of low density lipoprotein uptake and cholesterol synthesis are regulated independently in the liver. J Lipid Res. 1985 Apr;26(4):465–472. [PubMed] [Google Scholar]
- Spanjer H. H., Morselt H., Scherphof G. L. Lactosylceramide-induced stimulation of liposome uptake by Kupffer cells in vivo. Biochim Biophys Acta. 1984 Jul 11;774(1):49–55. doi: 10.1016/0005-2736(84)90273-6. [DOI] [PubMed] [Google Scholar]
- Spanjer H. H., Scherphof G. L. Targeting of lactosylceramide-containing liposomes to hepatocytes in vivo. Biochim Biophys Acta. 1983 Sep 21;734(1):40–47. doi: 10.1016/0005-2736(83)90072-x. [DOI] [PubMed] [Google Scholar]
- Spanjer H. H., van Galen M., Roerdink F. H., Regts J., Scherphof G. L. Intrahepatic distribution of small unilamellar liposomes as a function of liposomal lipid composition. Biochim Biophys Acta. 1986 Dec 16;863(2):224–230. doi: 10.1016/0005-2736(86)90262-2. [DOI] [PubMed] [Google Scholar]
- Stange E. F., Scheibner J., Ditschuneit H. Role of primary and secondary bile acids as feedback inhibitors of bile acid synthesis in the rat in vivo. J Clin Invest. 1989 Jul;84(1):173–180. doi: 10.1172/JCI114137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson W. F., Seetharam B., Talkad V., Pickett W., Dudeja P., Brasitus T. A. Effects of dietary fish oil supplementation on membrane fluidity and enzyme activity in rat small intestine. Biochem J. 1989 Oct 1;263(1):41–45. doi: 10.1042/bj2630041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. R., Kruijswijk Z., West C. E., Kohlmeier M., Katan M. B. Determination of serum triglycerides by an accurate enzymatic method not affected by free glycerol. Clin Chem. 1985 Jul;31(7):1227–1228. [PubMed] [Google Scholar]
- Topping D. L., Illman R. J., Roach P. D., Trimble R. P., Kambouris A., Nestel P. J. Modulation of the hypolipidemic effect of fish oils by dietary fiber in rats: studies with rice and wheat bran. J Nutr. 1990 Apr;120(4):325–330. doi: 10.1093/jn/120.4.325. [DOI] [PubMed] [Google Scholar]
- Ventura M. A., Woollett L. A., Spady D. K. Dietary fish oil stimulates hepatic low density lipoprotein transport in the rat. J Clin Invest. 1989 Aug;84(2):528–537. doi: 10.1172/JCI114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters H., Konings A. W. Radiosensitivity of normal and polyunsaturated fatty acid supplemented fibroblasts after depletion of glutathione. Int J Radiat Biol Relat Stud Phys Chem Med. 1984 Aug;46(2):161–168. doi: 10.1080/09553008414551231. [DOI] [PubMed] [Google Scholar]
- Wong S. H., Nestel P. J., Trimble R. P., Storer G. B., Illman R. J., Topping D. L. The adaptive effects of dietary fish and safflower oil on lipid and lipoprotein metabolism in perfused rat liver. Biochim Biophys Acta. 1984 Feb 9;792(2):103–109. doi: 10.1016/0005-2760(84)90209-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki R. K., Shen T., Schade G. B. A diet rich in (n-3) fatty acids increases peroxisomal beta-oxidation activity and lowers plasma triacylglycerols without inhibiting glutathione-dependent detoxication activities in the rat liver. Biochim Biophys Acta. 1987 Jul 13;920(1):62–67. doi: 10.1016/0005-2760(87)90311-0. [DOI] [PubMed] [Google Scholar]
- von Lossonczy T. O., Ruiter A., Bronsgeest-Schoute H. C., van Gent C. M., Hermus R. J. The effect of a fish diet on serum lipids in healthy human subjects. Am J Clin Nutr. 1978 Aug;31(8):1340–1346. doi: 10.1093/ajcn/31.8.1340. [DOI] [PubMed] [Google Scholar]


