Summary
Hip fractures are a significant cause of morbidity and mortality worldwide and the burden of disability associated with hip fractures globally vindicates the need for high-quality research to advance the care of patients with hip fractures. Historically, large, multi-centre randomized controlled trials have been rare in the orthopaedic trauma literature. Similar to other medical specialties, orthopaedic research is currently undergoing a paradigm shift from single centre initiatives to larger collaborative groups. This is evident with the establishment of several collaborative groups in Canada, in the United States, and in Europe, which has proven that multi-centre trials can be extremely successful in orthopaedic trauma research.
Despite ever increasing literature on the topic of his fractures, the optimal treatment of hip fractures remains unknown and controversial. To resolve this controversy large multi-national collaborative randomized controlled trials are required. In 2005, the International Hip Fracture Research Collaborative was officially established following funding from the Canadian Institute of Health Research International Oppurtunity Program with the mandate of resolving controversies in hip fracture management. This manuscript will describe the need, the information, the organization, and the accomplishments to date of the International Hip Fracture Research Collaborative.
Keywords: hip fracture, multicenter collaboratives
INTRODUCTION
Injuries account for 12% of the global burden of disease and this number is predicted to increase to 20% by the year 2020.1 It is also estimated that by 2020, disability from traffic accidents, the major cause of fractures, will rank in the top 3 of all causes of disability.2 Population rates of death resulting from traffic accidents are 3 times higher in lower income countries than in higher income countries1 and orthopaedic injuries are even more prominent internationally. For instance, in India, a vehicular accident is reported every 3 minutes and a death every 10 minutes.3 In developing countries, much of the death and disability from injury is attributable to absent or inadequate surgical care.1 In addition, the Global Forum for Health Research described the 10 of 90 gap in health research expenditure, whereby 90% of the spending is on diseases affecting 10% of the population.1 In other words, research and research funding are currently directed largely to medicine to treat chronic diseases among the aging population in wealthy countries.1
HIP FRACTURE GLOBAL BURDEN
Hip fractures are a significant cause of morbidity and mortality worldwide. Although hip fractures occur all over the world, they most frequently occur in Western countries, mainly Europe and the United States, but it is expected that there will be a large increase in the number of hip fractures in other countries due to changes in demographics.4 In 1990, there were an estimated 1.31 million new hip fractures globally and the prevalence of hip fracture disability was 4.48 million.5 In the year 2000, an estimated 1.6 million hip fractures occurred and hip fractures accounted for 0.82 million disability-adjusted life years in men and 1.53 million disability-adjusted life years in women.6 It is believed that the global number of hip fractures worldwide will increase to over 6 million by the year 2050.4 There were 740,000 deaths estimated to be associated with hip fractures, and there were 1.75 million disability-adjusted life years lost, which represents 0.1% of the global burden of disease worldwide.5
WHY IS OUR CURRENT EVIDENCE INSUFFICIENT?
Randomized controlled trials have been historically scarce in the orthopaedic literature. Poolman et al7 found that of the articles published in the Journal of Bone and Joint Surgery American Volume from January 2003 to December 2004, only 3.4% were randomized controlled trials. Similarly, a previous review of the same journal from 1988 to 2000 found that only 2.9% of published reports were randomized controlled trials.8
Despite ever increasing literature on the topic of hip fractures, the optimal treatment of hip fractures remains unknown. Limitations of the current study’s methodology include study design (ie, few randomized controlled trials), trials with sample sizes that are too small, and studies that report treatment effects that are largely implausible. Sample sizes of randomized controlled trials evaluating a surgical procedure have ranged from 18 to 552 participants and the average sample size was 113 participants.9 In addition, only 32% of trials performed a priori sample size calculation,9 indicating that these trials are unfortunately likely to be underpowered. These authors also found that 77% of the studies were single-center initiatives.9
Lack of sufficient evidence regarding optimal surgical approaches in the treatment of hip fractures has fueled many debates at international orthopaedic surgical meetings. The American Academy of Orthopaedic Surgeons, the Orthopaedic Trauma Association, Canadian Orthopaedic Association, and the International Society for Fracture Repair have all presented symposia highlighting this issue. In a survey of 298 North American and European orthopaedic surgeons treating patients aged 65–80 years old with displaced hip fractures, the authors identified variability in surgeons’ preferences for management.10 Sample sizes in hip fracture surgery trials have ranged from 20 to 409 patients.11 The small sizes, methodological limitations, and resultant wide confidence intervals around the treatment effect leave the optimal approach to treating hip fractures uncertain. Only large randomized trials will resolve the optimal type of internal fixation, the superior form of arthroplasty, and ultimately whether arthroplasty or internal fixation is the preferred option in this common fracture.
CAN LARGE DEFINITIVE TRIALS BE CONDUCTED?
Other medical areas have been successful in the completion of sufficiently powered multicenter collaborations to evaluate the effectiveness of new treatment options. A recently completed OAISIS-6 randomized controlled trial evaluated the effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction.12 This large collaborative trial included 447 hospitals in 41 countries and enrolled over 12,000 patients.12 Another successful example from the cardiology literature is the POISE trial. This large multicenter trial was initiated to definitively establish the effects of beta-blocker therapy in patients undergoing noncardiac surgery.13,14 Over 8000 patients undergoing noncardiac surgery at 190 hospitals in 23 countries were randomly assigned to receive extended release metoprolol succinate or placebo. The authors also promoted the importance and need for large, randomized, controlled trials in the perioperative setting. We believe that this model can guide us in achieving a similar improvement in management and outcomes of patients with hip fracture.
Large multicenter collaborations are most successful when problems being evaluated are common such as hip fractures, ankle fractures, distal radius fractures, and proximal humerus fractures.15 It is also necessary to ensure that the techniques are currently relevant to the majority of surgeons. The recently completed SPRINT trial is an example of a successful multicenter collaboration in orthopaedic trauma.16 The SPRINT study that began patient enrollment in 2000 evaluated the effects of reamed versus unreamed intra-medullary nailing on patients with tibial shaft fractures. This study included 1339 patients from 29 clinical sites in North America and The Netherlands.16
Orthopaedic research is currently undergoing a paradigm shift from single-center initiatives to larger collaborative groups. This is evident with the establishment of several successful groups in Canada (the Canadian Orthopaedic Trauma Society and the JOINTs study Group), in the United States (the Multicenter Trial Group—Trauma and the South Eastern Fracture Consortium), and in Europe (Multicenter Randomized Controlled Trial Group). The successful establishment of these collaboratives has proven that multicenter trials can be extremely successful in orthopaedic trauma research. In addition, recent symposia have focused on the importance and development of multicenter collaboration in orthopaedic research.17,18
Despite ever increasing literature on the topic of hip fractures, the hip fractures remain unresolved. Determining the optimal treatment of hip fractures will require large, multinational, collaborative, randomized, controlled trials. This article will describe the need, the formation, and the accomplishments to date of the International Hip Fracture Research Collaborative (IHFRC).
THE NEED FOR LARGE COLLABORATIVE TRIALS IN HIP FRACTURES
Clinical research should focus on fractures that represent the greatest burden to society.15 Disease burden may be quantified in many forms, but it is essentially a function of the frequency, secondary disability, and the cost associated with the treatment of the condition.15 Hip fractures account for more hospital days than any other musculoskeletal injury and represent more than two-thirds of all hospital days due to fracture.19 The burden of disability associated with hip fractures globally vindicates the need for high-quality research to advance the care of patients with hip fractures.
When looking at how to resolve the current uncertainties in hip fracture management and ultimately minimize morbidity and mortality, we find the model of cardiology instructive. Twenty years ago, the best form of management of acute coronary syndromes was unclear. Large, relatively simple, international randomized controlled trials have led to an enormous reduction in mortality and morbidity through the definitive demonstration of optimal management approaches.
We therefore proposed to develop an internationally recognized group of clinical researchers with the following aims: (1) to identify key unresolved issues and focus future clinical research in the operative management of patients with hip fractures; (2) to bridge smaller ongoing research networks in North America, Australia, Europe, and Asia into a large single collaborative effort; and (3) to design, plan, and coordinate timely large, randomized, controlled trials to provide definitive answers to the priority research questions identified by the participating investigators.
THE INTERNATIONAL HIP FRACTURE RESEARCH COLLABORATIVE (IHFRC)
Establishment of the IHFRC
The IHFRC was officially established in 2005 after funding from the Canadian Institutes of Health Research (CIHR) International Opportunity Program. The CIHR recognizes the important role that international collaborations play in improving and advancing the health of Canadians and citizens around the world. As a result, CIHR wishes to encourage Canadian researchers to collaborate internationally in CIHR’s priority areas of health research through the International Opportunities Program. In preparation for this grant, researchers at McMaster University contacted and gained the support of leading investigators in Canada, the United States, and Europe to establish an initial working group. By a series of communications, investigators identified other key individuals and trial networks to approach for participation in the research activities.
The initial funding from CIHR provided us with the resources to establish international membership and host an international development meeting in Toronto in November 2005. At the meeting activities focused on the identification of additional investigators with interest and expertise in hip fracture research, a systematic evaluation of the current evidence in hip fracture management, the development of research questions of global importance, and plans to design and conduct relevant clinical trials. In addition, this meeting introduced all members of the initial working group and reviewed documents regarding the mission of the IHFRC, evidence summaries on hip fracture management, and proposed research questions for discussion. Investigators confirmed the group’s mission and discussed the research questions of priority. Specifically, we discussed which comparisons to begin with (ie, internal fixation or arthroplasty) and examined the implications for ethical approval, sample size considerations, and practical aspects of study coordination.
Organization and Membership of the IHFRC
Following the initial meeting, we have developed a growing international collaborative of investigators focused on examining alternative surgical procedures to improve outcomes in patients with hip fractures. An IHFRC Executive Committee was established that is composed of orthopaedic surgeons with expertise in trauma, hip reconstruction, and orthopaedic trials and methodologists and biostatisticians with extensive clinical trial experience.
To expand membership, information regarding IHFRC was sent to all active members of the Orthopaedic Trauma Association, the Canadian Orthopaedic Association, the American Association of Hip and Knee Surgeons, and to International Members of the American Academy of Orthopaedic Surgeons. A series of surveys were sent to each potential member asking about the number of hip fractures they treat, their previous clinical research experience, and their interest and willingness to participate in large multicenter trials.
The IHFRC membership is currently composed of 310 members internationally, with 195 members in North America, 54 members in Europe, 45 members in Asia, 11 members in Australia, 4 members in South America, and 1 member in Africa. The IHFRC membership includes surgeons in developing nations, including India and China. Membership continues to expand through promotion of the trials at national meetings, through the IHFRC website (www.ihfrc.ca), and through personal communication and word of mouth.
Current Trials
The IHFRC is in the process of developing and initiating a series of large, multicenter, randomized clinical trials in patients with femoral neck fractures. Our goal is to identify the most favorable approach to internal fixation and to arthroplasty in 2 separate trials. The FAITH trial will evaluate Fixation Using Alternative Implants for the Treatment of Hip Fractures (FAITH): A Multicenter Randomized Trial Comparing Sliding Hip Screws and Cancellous Screws on Revision Surgery Rates in the Treatment of Femoral Neck Fractures, whereas the HEALTH trial will compare total hip arthroplasty and hemiarthroplasty [Hip Fracture Evaluation with ALternatives of Total Hip Arthroplasty versus Hemi-Arthroplasty (HEALTH): a multicenter randomized trial comparing total hip arthroplasty and hemiarthroplasty on reoperations and quality of life in patients with displaced femoral neck fractures]. The final study after the completion of the FAITH and HEALTH trials will compare the optimal internal fixation versus optimal approach to arthroplasty.
Funding
Well-conducted randomized controlled trials in orthopaedic surgery are as costly as trials in other fields, with costs ranging from 1 million to 10s of millions of dollars.15 Peer reviewed funding for clinical trauma trials is extremely limited, often ranging from $10,000 to $100,000 across most orthopaedic-specific agencies, and rarely greater than a few million dollars across the national funding agencies.15 Obtaining sufficient funding has been one of the challenges the IHFRC has faced. Similar to the strategy used in the SPRINT trial,20 the IHFRC has submitted multiple grant applications to multiple agencies for funding either pilot funding or definitive funding for FAITH and HEALTH.
The IHFRC members have developed trial protocols and have conducted the preliminary studies required to resolve feasibility issues before the submission of grant proposals. The IHFRC members recently completed prospective screening studies to provide accurate patient enrollment estimates for funding applications by applying the inclusion and exclusion criteria to all hip fracture patients at their clinical site for a period of 8 weeks. Electronic communication and conference calls facilitated full participation of the groups’ leaders in development of grant proposals.
After several reiterations, both studies have recently received pilot funding from the CIHR to determine the feasibility of each trial at Canadian and at several international sites. The National Institutes of Health Research has recently awarded funding for a HEALTH pilot study for clinical sites in the United States, and the Physician Services Incorporated Foundation has provided funding for pilot sites in Ontario for the FAITH study.
Pilot studies are an important step in determining if a large clinical trial will be feasible.21 Pilot testing is desirable to confirm or refute our ability to recruit patients and to assess the consistency between site estimates and actual recruitment. Specifically, pilot testing will (1) determine the degree to which site investigators can adhere to trial protocol including novel aspects such as “expertise-based randomization”22 in the HEALTH trial, (2) confirm or refute our anticipated ability to achieve close to 100% follow-up, (3) assess our ability to maintain 100% data quality, (4) field test our case report forms for clarity and appropriateness, and (5) will determine whether we can successfully manage the logistics of coordinating trial activities with sites in Europe, Asia, and Australia.
Several funding agencies did not require formal pilot studies and have provided sufficient funding for a definitive trial for specific jurisdictions. The Netherlands has received full funding for both FAITH and HEALTH for the Dutch sites from Stichting Nuts Ohra. The National Institutes of Health has provided full funding for the American sites for the FAITH study. The IHFRC will continue to prepare grant applications to secure sufficient funding to complete both trials.
Trial Organization
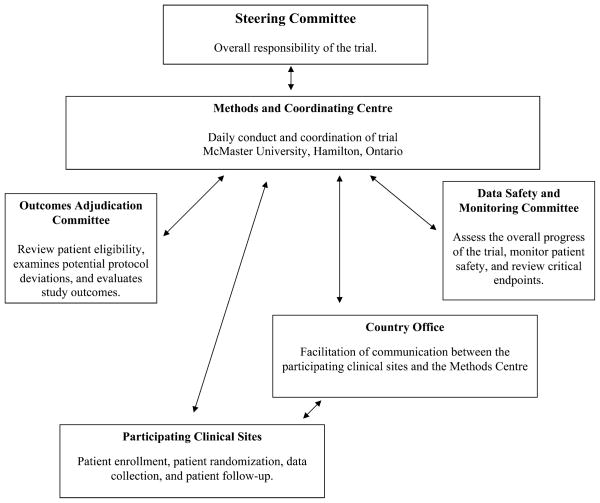
Clinical trials will be successful only with sufficient infrastructure.18 A Methods Center has been established at McMaster University to coordinate all aspects of the day-today activities of the IHFRC and the FAITH and HEALTH trials (Fig. 1). The Methods Center has previous experience in the coordination of large multicenter trials.20 Key activities of the Methods Center include communication with membership, protocol development, designing cases report forms, ensuring concealed randomization by an Internet-based randomization system, data management using DataFax, and data analysis.
FIGURE 1.
Trial Organization of FAITH and HEALTH: a diagram illustrating the day-to-day activities of the FAITH and HEALTH trials.
In addition to the Methods Center, we have established Country Offices who will be responsible for the facilitation of communication between the Methods Center and the clinical sites in countries where language and communication barriers are likely to exist. The Country Offices are responsible for the translation of study documents (ie, protocol, informed consent, and case report forms), training of the clinical sites, ensuring that ethics approval has been obtained, and facilitating communication between the Methods Center and the clinical sites. The Country Offices also help to ensure that the trial protocol is followed and that the data that is submitted to the Methods Center is accurate and complete.
A Call to Action
The recent promotion of evidence-based orthopaedic practice across all major orthopaedic journals and orthopaedic societies has started the next generation of clinical trials in orthoapedics.15 In addition, recent orthopaedic forums have urged musculoskeletal specialty societies to take action in the organization of multicenter randomized controlled trials17 and have provided orthopaedic surgeons with some guidelines on how to participate in orthopaedic randomized controlled trials.18
In the past, international research expenditures have trivialized the burden of disease attributable to injury, especially in developing countries.1 Academic orthopaedic surgeons are urged to partner effectively with their counterparts in developing nations to collaborate in research initiatives.1 To have a successful collaboration, experienced orthopaedic investigators need to partner effectively with their colleagues in developing countries who have the cases and the numbers, but possibly not the expertise and experience to work them into meaningful research.1 For example, a recent report indicates that trauma research output from India is currently insufficient and vague and is therefore unable to inform the practice of medicine and surgery in the country.23 Despite this, a recent survey of orthopaedic surgeons in India found that 85 of 114 surgeons surveyed supported a research collaborative.23 In addition, clinical sites in India have the capacity to potentially recruit thousands of fractures each year.23
We need to learn from our colleagues in other subspecialties who have successfully and efficiently completed large multinational studies including countries all over the world. The burden of disability associated with trauma and fractures vindicates the need for international collaboration to conduct high-quality research to advance the care of fracture patients. Only through the development of large multinational collaborative will controversies in areas such as hip fractures be resolved.
SUMMARY
Randomized controlled trials should play a major role in determining best practices for fracture care. As the number and the quality of randomized controlled trials increase, the outcomes of fracture patients will improve. The IFHRC will focus its activities to identify surgical alternatives to improve patient important outcomes after hip fractures. Our collective expertise and resources will enable an efficiency of trial conduct rare in surgical trials. This multinational group of investigators will strive toward a similar magnitude of impact and success achieved in cardiovascular and cancer trial networks. For additional information on the IHFRC, please visit our website at www.ihfrc.ca.
Acknowledgments
Funding was received from the National Institutes of Health, Canadian Institutes of the Health, Physician Services Incorporated, and Stichting Nuts Ohra.
Dr. Bhandari’s salary was funded in part by a Canada Research Chair, McMaster University. No grant funds were received in preparation of this article.
APPENDIX
IHFRC Membership
IHFRC Executive Committee: Mohit Bhandari (Chair), Thomas Einhorn, Gordon H. Guyatt, George Haidukewych, John Keating, Kenneth Koval, Clifford Rosen, Emil H. Schemitsch, Marc Swiontkowski, Paul Tornetta III, and Stephen D. Walter.
Africa—South Africa: Silas Motsitsi. Asia—China: Fuxing Pei, Tian-fu Yang, and Zong-ke Zhou. India: Shobha Arora, Sushrut Babhulkar, Rakesh Bhargava, Mohan M. Desai, Mandeep S. Dhillon, Harpreet Singh Gill, S. C. Goel, A. V. Gurava Reddy, Anil K. Jain, Niraj V. Kalore, Nitin Kammatkar, Vijay Kumar, Rajesh Malhorta, S. S. K. Marthandam, Amite Pankaj, Gopinathan Patinharayil, B. Sachidanand Rai, Alankar Ambadas Ramteke, Parag K. Sancheti, Navin N. Thakkar, and George S. Thomas. Israel: Dror Robinson and Ely Steinberg. Japan: Fujio Higuchi, Sumito Kawamura, Hirotsugu Ohashi, and Takeshi Sawagu-chi. Korea: Myung-Sik Park and Ho Hyun Yun. Nepal: Murali Poduval. Saudi Arabia: Ahmed Siddiqui. Taiwan: Je-Ken Chang, Gwo-Jaw Wang, Chung-Hwan Chen, Yin-Chih Fu, and Yen-Mou Lu. Turkey: Emre Acaroglu, Mumtaz Alpasian, Bulent Atilla, Mehmet Ayvaz, Omur Caglar, and Mazhar Tokgozoglu. Australia—Australia: Peter Campbell Gray, Ian Harris, Michael O’Sullivan, Richard Samuel Page, David Parker, Martin Richardson, Michael Solomon, Kevin Tets-worth, Richard Walker, and Simon Williams. New Zealand: Peter Devane. EUROPE—Belgium: Paul Broos. Croatia: Dragan Djurdjevic. Denmark: Ole Brink. Finland: Pekka Jalovaara and Jukka Ristiniemi. Germany: Jochen Blum, Felix Bonnaire, Tosten G. Gerich, Volker Herrwerth, Michael Klein, Werner Knopp, Christian Krettek, Holger Mueller-Daniels, Uwe Ochs, Hans-Joerg Oestern, Tim Pohlemann, Peter Schandelmaier, Thomas Schreiber, Andreas Seekamp, Klaus Michael Stuermer, Tim Walde, Hans-Joachim Walde, and Kuno Weise. Ireland: Keith Synnott. Italy: Elena Grosso and Antonio Moroni. The Netherlands: G. H. Robert Albers, Victor de Ridder, J. Carel Goslings, M. C. Haaglanden, Martin J. Heetveld, Gerolt N. Jukema, Rudolf W. Poolman, S. Rhemrev, Michiel J. M. Segers, Roger Simmermacher, Maarten van der Elst, Harm van der Vis, Arie van Vugt, and Michael H. J. Verhofstad. Norway: Wilhelm Bugge, Wender Figved, Frede Frihagen, Jan Erik Madsen, Lars Nordsletten, and Ludvig Fjeld Solheim. Scotland: Peter V. Giannoudis, Nikolaos Kanakaris, John Keating, Martyn Parker, and Keith Willett. Sweden: Carl Ekholm, Sune Larsson, and Cecilia Rogmark. North America—Canada: Fathi Hadi Abuzgaya, Carel W. Ackerman, Jonathan (Rick) Adachi, Anthony Adili, Mohit Bhandari, Earl A. Bogoch, Eric Bohm, Deke John Botsford, Robert B. Bourne, Richard E. Buckley, Chad P. Coles, Timothy Daniels, Justin de Beer, P. J. Devereaux, Paul Duffy, Forough Farrokhyar, Robert J. Feibel, Luis A. Flores, Cyril B. Frank, Mark Glazebrook, Thomas J. Goetz, John A. Gordon, Michael Gross, Gordon Guyatt, Tim Heron, Steven Hoey, Robert G. Josefchak, Richard S. Kaminker, Paul Jack Karanicolas, Richard Kendall, Paul R. Kim, Robert Korbyl, Robert Korley, Hans J. Kreder, Stephen Kwan, Yves Laflamme, Ross K. Leighton, James Leone, Lawrence Lincoln, Kieran MacCon, David C. Martin, Frank Mastogiacomo, George Mathew, Patrick McAllister, Robert G. McCormack, Jeffrey G. McKerrell, Jaydeep K. Moro, John J. Murnaghan, Douglas Naudie, Peter J. O’Brien, Terrance A. O’Farrell, Rick A. Ogilvie, John Oliver, Kostantinos Pan-agiotopoulos, Bertrand Perey, Robert J. R. Perlau, Devin C. Peterson, Brad Petrisor, Ralph Pototschnik, David Pugh, Shannon K. T. Puloski, Bernard E. Rerri, Bryan R. Ritten-house, Cleo C. Rogakou, Leonard Rosen, Robert Russell, Paul T. Salo, David Sanders, Emil H. Schemitsch, Arno Smit, John Young Song, W. Peter Southcott, Apostolos Tountas, Vikram Venugopal, James P. Waddell, Eugene Wai, Stephen Walter, Kevin Willits, Jeff D. Yach, Paul Zalzal, and Zane Zarzour. USA: Animesh Agarwal, Jeffrey O. Anglen, Marc Appel, Michael T. Archdeacon, William Timothy Ballard, Sam Barzideh, Daniel J. Berry, Hari P. Bezwada, Timothy Bhattacharyya, Robert H. Blotter, Mary Larsen Bouxsein, Gregory A. Brown, Thomas E. Brown, Matthew R. Camuso, Peter A. Cole, Joseph M. Conflitti, Brett D. Crist, Maria Guerrero Davila, Gregory Della Rocca, Dennis Scott Devinney, Frank R. DiMaio, Doug Dirschl, Gervan Duffy, Paul Duwelius, Kenneth J. Egol, Thomas Einhorn, Thomas Ellis, Tania Ferguson, Maureen Finnegan, Jonathan P. Garino, Gurdev S. Gill, John Gorczyca, Gary S. Gruen, George John Haidukewych, David J. Hak, Mark Hammerberg, Adam Harris, Mitchel Harris, Richard Iorio, Avinash Jadhav, James E. Jennings, Kyle J. Jeray, William Jiranek, David Karges, Phil Kregor, Mark A. Kwartowitz, Peter Lammens, Theodore Toan Le, Patrick B. Leach, Charles Michael LeCroy, Paul Levin, Courtland Lewis, Frank Liporace, Dean Lorich, Steven Louis, Jeffrey Lozman, John Lyden, William B. Macaulay, Edward Shawn Mansour, Randall Evan Marcus, David Markel, John Masonis, Eric Meinberg, Daniel Meldrum, Russell Meldrum, Charles N. Moon, Thomas Moore, Matthew A. Mormino, David Navid, William Obremskey, Javad Parvizi, Michael J. Patney, Laura S. Phieffer, Michael Prayson, Robert Probe, Laura Prokuski, Robert N. Reddix Jr, Mark C. Reilly, William Ricci, Craig S. Roberts, Clifford Rosen, Andy Sems, Todd Sekundiak, Andrew Schmidt, John Schwappach, Michael Sirkin, Rena L. Stewart, Elton Strauss, Marc Swiontkowski, Julie A. Switzer, Charles J. Taunt, Nirmal Tejwani, Paul Tornetta III, Heather A. Vallier, Barry Waldman, Larry Web, Joseph Williams, Frederic Wilson, George Wright, Bruce Ziran, and Robert D. Zura. South America—Argentina: Roger Torga-Spak. Chile: Mario Reyes. Columbia: Rodrigo Pesan-tez. Uruguay: Antonio Barquet.
References
- 1.Beveridge M, Howard A. The burden of orthopaedic disease in developing countries. J Bone Joint Surg Am. 2004;86-A:1819–1822. doi: 10.2106/00004623-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Dormans JP, Fisher RC, Pill SG. Orthopaedics in the developing world: present and future concerns. J Am Acad Orthop Surg. 2001;9:289–296. doi: 10.5435/00124635-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Joshipura MK. Total trauma care: international perspective. Hosp Today. 1996;11:43–44. [Google Scholar]
- 4.De Laet CEDH, Pols HAP. Fractures of the elderly: epidemiology and demography. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14:171–179. doi: 10.1053/beem.2000.0067. [DOI] [PubMed] [Google Scholar]
- 5.Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004;15:897–902. doi: 10.1007/s00198-004-1627-0. [DOI] [PubMed] [Google Scholar]
- 6.Johnell O, Kannis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic hip fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 7.Poolman RW, Struijs PA, Krips R, et al. Does a “Level I Evidence” rating imply high quality of reporting in orthopaedic randomized controlled trials? BMC Med Res Methodol. 2006;6:44. doi: 10.1186/1471-2288-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhandari M, Richards RR, Sprague S, et al. The quality of reporting randomized trials in the Journal of Bone and Joint Surgery from 1998 to 2000. J Bone Joint Surg Am. 2002;84-A:388–396. doi: 10.2106/00004623-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Chan S, Bhandari M. The quality of reporting of orthopaedic randomized trials with use of a checklist for nonpharmacological therapies. J Bone Joint Surg Am. 2007;89-A:1970–1978. doi: 10.2106/JBJS.F.01591. [DOI] [PubMed] [Google Scholar]
- 10.Chua D, Jaglal SB, Schatzker J. An orthopedic surgeon survey on the treatment of displaced femoral neck fracture: opposing views. Can J Surg. 1997;40:271–277. [PMC free article] [PubMed] [Google Scholar]
- 11.Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85-A:1673–1681. doi: 10.2106/00004623-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Mehta SR, Chrolavicius S, et al. OASIS-6 Trial Group. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA. 2006;295:1519–1530. doi: 10.1001/jama.295.13.joc60038. [DOI] [PubMed] [Google Scholar]
- 13.POISE Trial Investigators. Devereaux PJ, Yang H, et al. Rationale, design, and organization of the PeriOperative ISchemic Evaluation (POISE) trial: a randomized controlled trial of metoprolol versus placebo in patients undergoing noncardiac surgery. Am Heart J. 2006;152:223–230. doi: 10.1016/j.ahj.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 14.POISE Study Group. Devereaux PJ, Yang H, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 15.Morshed S, Bhandari M. Clinical trial design in fracture-healing research: meeting the challenge. J Bone Joint Surg Am. 2008;90-A(1):S55–S61. doi: 10.2106/JBJS.G.01478. [DOI] [PubMed] [Google Scholar]
- 16.S.P.R.I.N.T. Investigators. Randomized trial of reamed versus non-reamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg Am. 2008;90:2567–2578. doi: 10.2106/JBJS.G.01694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright JG, Gebhardt MC. Multicenter clinical trials in orthopaedics: time for musculoskeletal specialty societies to take action. J Bone Joint Surg Am. 2005;87-A:214–217. doi: 10.2106/JBJS.D.02555. [DOI] [PubMed] [Google Scholar]
- 18.Trippel SB, Bosse MJ, Heck DA, et al. Symposium. How to participate in orthopaedic randomized clinical trials. J Bone Joint Surg Am. 2007;89-A:1856–1864. doi: 10.2106/JBJS.F.01596. [DOI] [PubMed] [Google Scholar]
- 19.American Academy of Orthopaedic Surgeons. AAOS urges hip fracture care reform. Am Acad Orthop Surg Bull. 1999;47 Unknown online at: www2.aaos.org/aaos/archives/bulletin/aug99/acdnw11.htm. [Google Scholar]
- 20.S.P.R.I.N.T. Investigators. Study to prospectively evaluate reamed intramedullary nails in patients with tibial fractures (S.P.R.I.N.T.): study rationale and design. BMC Musculoskelet Disord. 2008;9:91. doi: 10.1186/1471-2474-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csimma C, Swiontkowski MF. Large clinical trials in musculoskeletal trauma: are they possible? Lessons learned from the international study of the use of rhBMP-2 in open tibial fractures. J Bone Joint Surg Am. 2005;87-A:218–222. doi: 10.2106/JBJS.D.01938. [DOI] [PubMed] [Google Scholar]
- 22.Devereaux PJ, Bhandari M, Clarke M, et al. Need for expertise based randomised controlled trials. BMJ. 2005;330:88. doi: 10.1136/bmj.330.7482.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthew G, Sanchetti P, Jain A, et al. Multicenter collaborative for orthopaedic research in India: an opportunity for global leadership. Indian J Orthop. 2008;42:165–168. doi: 10.4103/0019-5413.40252. [DOI] [PMC free article] [PubMed] [Google Scholar]