Abstract
Background
The Dmbx1 gene is important for the development of the midbrain and hindbrain, and mouse gene targeting experiments reveal that this gene is required for mediating postnatal and adult feeding behaviours. A single Dmbx1 gene exists in terrestrial vertebrate genomes, while teleost genomes have at least two paralogs. We compared the loss of function of the zebrafish dmbx1a and dmbx1b genes in order to gain insight into the molecular mechanism by which dmbx1 regulates neurogenesis, and to begin to understand why these duplicate genes have been retained in the zebrafish genome.
Results
Using gene knockdown experiments we examined the function of the dmbx1 gene paralogs in zebrafish, dmbx1a and dmbx1b in regulating neurogenesis in the developing retina and midbrain. Dose-dependent loss of dmbx1a and dmbx1b function causes a significant reduction in growth of the midbrain and retina that is evident between 48-72 hpf. We show that this phenotype is not due to patterning defects or persistent cell death, but rather a deficit in progenitor cell cycle exit and differentiation. Analyses of the morphant retina or anterior hindbrain indicate that paralogous function is partially diverged since loss of dmbx1a is more severe than loss of dmbx1b. Molecular evolutionary analyses of the Dmbx1 genes suggest that while this gene family is conservative in its evolution, there was a dramatic change in selective constraint after the duplication event that gave rise to the dmbx1a and dmbx1b gene families in teleost fish, suggestive of positive selection. Interestingly, in contrast to zebrafish dmbx1a, over expression of the mouse Dmbx1 gene does not functionally compensate for the zebrafish dmbx1a knockdown phenotype, while over expression of the dmbx1b gene only partially compensates for the dmbx1a knockdown phenotype.
Conclusion
Our data suggest that both zebrafish dmbx1a and dmbx1b genes are retained in the fish genome due to their requirement during midbrain and retinal neurogenesis, although their function is partially diverged. At the cellular level, Dmbx1 regulates cell cycle exit and differentiation of progenitor cells. The unexpected observation of putative post-duplication positive selection of teleost Dmbx1 genes, especially dmbx1a, and the differences in functionality between the mouse and zebrafish genes suggests that the teleost Dmbx1 genes may have evolved a diverged function in the regulation of neurogenesis.
Background
The vertebrate diencephalon/mesencephalon homeobox 1 (dmbx1) gene belongs to the K50 subclass of paired-like homeobox genes (related to Goosecoid) whose expression in the neural plate has been shown to demarcate the presumptive mesencephalic (midbrain) territory in mouse [1-6], chick [2] and zebrafish [7,8]. After neural tube formation, Dmbx1 is predominantly expressed in the hindbrain, posterior forebrain and midbrain. Loss of function studies highlight a role for Dmbx1 (mouse) and dmbx1a (zebrafish) in proper midbrain and hindbrain development and in the case of zebrafish development of the retinotectal pathway [8,9], although in mouse the loss of function embryonic phenotype is comparatively mild. Moreover, early postnatal lethality occurs in the vast majority of Dmbx1 knockout mice. Those that survive to adulthood predominantly display hypophagia and hyperactivity, likely a result of defects in the development of neural circuitry involved in energy homeostasis [9,10].
Genomic sequence analyses indicate that basal metazoans, such as Ctenophores [11], and Poriferans [12], do not have a Dmbx1 gene. However, a putative Dmbx1 ortholog has been identified in the Cnidarian genome [13], suggesting a pre-bilaterian origin of the Dmbx1 homeobox gene family during animal evolution. Non-chordate invertebrate Dmbx1 orthologs have not been clearly identified, but based on overall class homology and function, the Drosophila Pph13/Mu gene is a plausible candidate [14]. A single Dmbx1 gene exists in the genomes of terrestrial vertebrates, while teleost genomes contain at least two paralogs: dmbx1a and dmbx1b (previously annotated as mbx1 and mbx2) [7], coincident with the more recent additional round of gene/genome duplication that is speculated to have occurred in the teleost lineage [15,16]. This has lead us to examine why both copies of the dmbx1 gene duplicate have been retained in the zebrafish genome.
The retention of functional gene duplicates (paralogs) in genomes is often attributed to their role in buffering against loss-of-function mutations in one copy of an essential gene [17,18]. However, the proportion of genes that when deleted are embryonic lethal or lead to infertility in mouse (i.e. essential in a laboratory context) is not significantly different between duplicates and singletons [19,20]. Even though protein sequences of some paralogous genes in vertebrates are functionally interchangeable [21,22], paralogs can evolve distinct expression patterns and functions. The subfunctionalization model suggests that subsequent to gene duplication, degenerative mutations in regulatory or coding regions of the gene result in complementary expression patterns or function, respectively, the composite of which would be representative of the pre-duplicated ancestral gene [23,24]. This appears to be a predominant mechanism in teleosts [22,25-28]. Neofunctionalization has also been proposed to account for the retention of duplicate genes [29]. A recent genome wide study in Xenopus laevis indicated that as many as 6% of duplicate genes exhibit an asymmetric rate of non-synonymous substitution in one of the paralogs, which is consistent with a neofunctionalization model for paralog retention [30]. However, experimental evidence for neofunctionalization in vertebrates is limited.
Our previous gene expression analyses of zebrafish dmbx1a and dmbx1b showed that despite conservation in the regulation of expression within particular regions (e.g. midbrain primordium), there were clear differences in onset, spatial distribution and relative abundance during the first two days of development [7]. These differences in regulation correlated with variation in the extent of genomic sequence conservation between the paralogs in three separate fish species [7]. We also noted that although the amino acid sequence similarity was high (72%) between the paralogs, this was mostly due to the N-terminus and DNA binding domain, whereas much of the C-terminus contained relatively divergent sequence. From these results, we concluded that the zebrafish dmbx1 paralogs might have been subfunctionalized during the course of their evolution.
Here, we examined the functional requirement of zebrafish dmbx1a and dmbx1b during neural development using morpholino oligonucleotide based loss of function to decipher a possible mechanism for the retention of these duplicate genes. We demonstrate that neither functional redundancy nor subfunctionalization is an adequate model to account for the retention of these paralogs in the zebrafish genome. Instead, we show that both genes are required during development. Loss of function of dmbx1a and dmbx1b causes a prolonged progenitor cell cycle in the midbrain and retina, which results in reduced growth, and a significant defect in differentiation. Although these knockdown phenotypes can be rescued by exogenous zebrafish dmbx1 mRNA, they cannot be efficiently rescued by exogenous mouse Dmbx1 mRNA to the same degree. Finally, analyses of the rate of non-synonymous to synonymous substitution in the coding region of Dmbx1 genes provide evidence for post-duplication positive selection in the dmbx1a and dmbx1b gene families of teleost fish. These data suggest that both zebrafish dmbx1 paralogous genes are non-redundantly required for cell cycle exit regulation and differentiation. Although the differences in gene expression domains between the dmbx1 paralogs is subtle at late embryonic and early larval stages, the knockdown data demonstrate that regional functional specialization, especially with respect to dmbx1a, exists. Thus, the function of dmbx1 genes in teleost fish may be evolutionarily diverged.
Results
Discrete variation in the timing and distribution of dmbx1a and dmbx1b gene expression during the early larval stage
Previous analyses suggested that the dmbx1a and dmbx1b paralogs displayed partially divergent spatial and temporal patterns of gene expression in early development [7]. For example, between shield and tailbud stages, dmbx1a has a characteristic annulus expression pattern specifying the midbrain territory, as well as progenitor cells of retinal and diencephalic lineages, whereas dmbx1b expression during these stages is barely detectable and appears later at mid-somitogenesis only in the presumptive midbrain [7,8]. Between 24 - 48 hpf, the expression domains of these two genes partially overlapped in the midbrain and hindbrain, but neither gene is expressed in the retina. We analyzed the expression patterns of the dmbx1 paralogs in the brain and retina during the late embryonic to early larval stages using in situ hybridization in order to clarify where the paralogs exert their function during development.
At 72 hpf, dmbx1a was expressed in the optic tectum (TeO) and very weakly in the tegmentum (T) (Figure 1A, B, F). Scattered cells of the retinal inner nuclear layer (INL) and ganglion cell layer (GCL) also express dmbx1a (Figure 1E). However, there is very weak or no dmbx1a expression in the photoreceptor layer and no expression in the ciliary marginal zone (CMZ), where the post-embryonic retinal stem and progenitor cells undergo continual proliferation [31]. Dmbx1a is also expressed in discrete hindbrain cell populations that appear to demarcate the cerebellar eminentia granularis (EG) anteriorly (Figure 1A; B, white arrow) and the medulla oblongata posteriorly (Figures 1A; B, red arrow). In addition, dmbx1b was expressed throughout the midbrain and within the medulla at 72 hpf (Figure 1C; D, red arrow), but only very weakly in the INL and EG (Figures 1D, G).
Figure 1.

Spatiotemporal expression patterns of dmbx1a and dmbx1b at 72 hpf and 96 hpf. Lateral view, anterior to the left (A, C, I, K); dorsal view, anterior to the top (B, D, J, L). Coronal plastic sections (~1 μm; dorsal to the top) of the retina (E, G, M, O) and midbrain (F, H, N, P) were obtained after whole mount labeling with RNA probes. White dotted line in B and D define the position of the representative coronal plastic section shown in F and H, respectively. White dotted line in J and L define the position of the representative coronal plastic section shown in N and P, respectively. White arrow in B, D, J, L demarcates the rostrolateral region of the hindbrain (where the EG is located), and red arrow demarcates the expression in the medulla oblongata. Black arrows in E, M and O point to the INL (inner nuclear layer). The optic tectum (TeO) is demarcated by dotted lines in F, H, N, P and the region of the tegmentum (T) lies just ventral to the TeO. The position of the lens is denoted in E, G, M, O. hpf, hours post-fertilization.
Expression analyses at 96 hpf continued to reveal discrete variation in the distribution of cells expressing dmbx1a and dmbx1b. For example, dmbx1a is predominantly localized to the medial and lateral compartments of the TeO in the midbrain and weak expression is detected in the tegmentum (Figure 1I,J,N), whereas dmbx1b expression extends more ventrally to encompass more of the tegmentum (Figure 1K,L,P). This difference in dorsoventral expression domains is consistent with our previous observations at 24 - 48 hpf [7]. Furthermore, the predominantly tectal expression of dmbx1a is similar to that of mouse dmbx1 [4]. Expression of dmbx1b in the anterolateral hindbrain is also more substantial at this stage, but not quite comparable to dmbx1a (Figure 1I,K). Finally, both dmbx1a and dmbx1b are expressed throughout the retinal INL, but dmbx1a appears enhanced and localized to the central region of the INL, whereas dmbx1b expression is relatively diffuse and less intense throughout most layers (Figure 1M,O).
Taking into account the relative temporal, spatial, and quantitative expression patterns of the dmbx1 paralogs in the first 4 days of life ([7]; present results), our data suggests that dmbx1 genes would predominantly function in midbrain formation, plus a role in retinal and hindbrain development during later differentiation. Therefore, we sought to compare the functional requirement of Dmbx1a and Dmbx1b.
Gene knockdown using dmbx1 paralog specific antisense morpholino oligonucleotides
Given the high degree of sequence similarity between the dmbx1 paralogs, our strategy for using antisense morpholino oligonucleotide (MO) based knockdown required confirmation that paralog specific MOs could independently block translation of dmbx1a and dmbx1b mRNA. In the absence of commercially available antibodies that are validated to detect zebrafish Dmbx1a or Dmbx1b proteins, we opted for an alternative approach to estimate the level of protein knockdown for each paralog. We constructed in-frame GFP fusion constructs containing the unique paralog specific MO targeting sequences, and co-injected each of the MOs with their corresponding in vitro transcribed fusion mRNAs (Figure 2A,B). When either dmbx1a-GFP (n = 62) or dmbx1b-GFP (n = 73) fusion mRNA was injected, ~75% of the embryos had bright ubiquitous GFP protein expression after 24 hpf (Figure 2C,G). GFP expression was completely suppressed in embryos that were co-injected with MO1a + dmbx1a-GFP (Figure 2D, n = 74) or MO1b + dmbx1b-GFP (Figure 2H, n = 59), indicating that the MOs result in very efficient translation inhibition.
Figure 2.
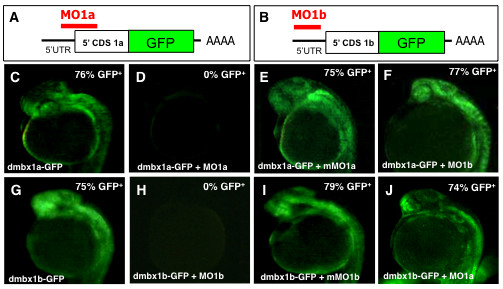
Specificity of morpholino induced dmbx1a and dmbx1b knockdown using fusion protein constructs Dmbx1a-GFP and Dmbx1b-GFP. (A, B) Schematic diagrams showing sequence of fusion constructs: 5' UTR (black horizontal line); 5' end of the CDS for either dmbx1a or dmbx1b (white box), the full-length coding sequence for GFP (green box); site of polyadenylation (AAAA); relative position binding sites (red horizontal line) where the MOs block translation of the fusion proteins. (C-J) dmbx1a- and dmbx1b-GFP mRNA was either injected alone or co-injected with MOs as indicated at the bottom left of each panel, and embryos were examined for the presence of GFP fluorescence at 24 hpf (lateral view with anterior to the left). The percentage of GFP positive embryos is shown on the top right. UTR, untranslated region; CDS, coding sequence; MO, morpholino; mMO, mismatch morpholino; GFP, green fluorescent protein; hpf, hours post-fertilization.
In order to control for MO sequence specificity, we also co-injected the GFP fusion mRNA constructs with a 5-bp mismatched MO (mMO1a or mMO1b; see Materials & Methods for details) and quantified the percentage of injected embryos that were GFP positive after 24 hpf. Of the embryos that were co-injected with mMO1a + dmbx1a-GFP (Figure 2E, n = 53) or mMO1b + dmbx1b-GFP (Figure 2I, n = 64), ~75 - 80% of the embryos demonstrated ubiquitous GFP expression, which was similar to the percentage of fusion construct injected embryos expressing GFP without MO co-injection. These results indicate that the knockdown of either GFP fusion construct depends precisely on the complementary MO sequences.
We further examined the paralog specific knockdown characteristics of the MOs in order to confirm that there were no cross-target effects. Co-injection of MO1b + dmbx1a-GFP (Figure 2F, n = 60) or MO1a + dmbx1b-GFP (Figure 2J, n = 62) resulted in ~75 - 80% of the embryos with ubiquitous GFP expression in injected embryos after 24 hpf. Again, the numbers of GFP positive embryos within the test pool were comparable to the results obtained from injecting the GFP fusion mRNA alone, indicating that it is unlikely that cross targeting of MOs is occurring. These data suggest that the MO knockdown of the Dmbx1-GFP fusion proteins, and by inference the endogenous Dmbx1 proteins, is both efficient and paralog specific.
Midbrain growth defects in embryos with reduced levels of either Dmbx1a or Dmbx1b
The early onset and sustained expression of dmbx1a and dmbx1b within the midbrain suggested that these genes play an important role in the development of this neuroanatomical structure. Morphological analyses were carried out to compare the MO-injected embryos (10 ng/embryo each) with mMO-injected (10 ng/embryo each) as well as un-injected controls. After 24 hpf, we were able to detect subtle differences in the size of the midbrain (data not shown), but this morphological change was more prominent at 48 hpf, where the size of the dorsal tectum of the MO1a-injected embryos was reduced (Figure 3C,D) compared to un-injected (Figure 3A,B) and mMO1a-injected embryos (Figure 3E,F), consistent with previous results using the same morpholino (MO1a) [8]. We observed that the overall cross-sectional area (or thickness) of the tectal hemispheres is diminished in the MO1a morphant, but that the shape of the contours of the tectal hemispheres and the extent of the cerebellar plate (CeP) remains relatively unaffected. We analyzed transverse sections to quantify these differences by measuring the average cross-sectional area of the tectal wall unilaterally [from the lateral sulcus separating the tectum dorsally from the region of the torus semicircularis (TS) ventrally] and observed a reduction of ~ 50% in the MO1a morphants compared to controls at the same position along the anteroposterior axis (Figure 3O). Interestingly, the tectal morphology of MO1b morphant embryos (Figure 3G,H) was also affected compared to mMO1b control injected embryos (Figure 3I,J), and cross-sectional area measurements revealed a ~ 35% reduction in size (Figure 3O).
Figure 3.
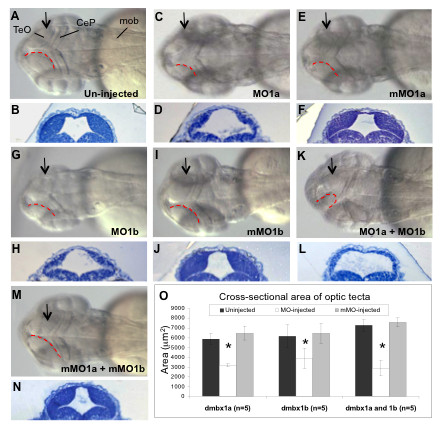
Hypoplasia of the optic tectum in dmbx1 morphants at 48 hpf. Gross morphologies of the optic tecta were compared between morphants (C, G, K) and both the un-injected (A) and mMO-injected (E, I, M) embryos. Dorsal view, anterior to the left. Contour of the medial-posterior ridge of the optic tectum is demarcated by red doted lines. The MOs used in each group are shown on the bottom right of the panel. Arrows in A, C, E, G, I, K, M represent the relative position where ~ 1 μm plastic sections were obtained as depicted in B, D, F, H, J, L, N, respectively. Measurements of the cross-sectional tectal area are summarized in the graph (O). Asterisk indicates significant difference (p < 0.05) between the morphant samples and the controls. TeO, optic tectum; CeP, cerebellar plate; mob, medulla oblongata; MO, morpholino; mMO, mismatch morpholino.
Knockdown of both dmbx1 genes simultaneously (MO1a + MO1b) resulted in an obvious change in the overall morphology of the tectum (Figure 3K,L) compared to the double control injected (mMO1a + mMO1b) embryos (Figure 3M). In order to compare with the single morphant embryos, we co-injected 5 ng of each morpholino. In contrast to the single morphant phenotype, we observed that the shape of the tectal hemispheres in the double morphant often was abnormal and that the extent of the CeP was reduced, which can be observed from a dorsal perspective (compare red dotted line in Figure 3K with red dotted lines in 3A, C, G). Despite the more extensive morphological alterations in the double morphants, the overall cross-sectional area of the tectal hemispheres was reduced to a similar degree (~ 60%) when compared to the differences observed in the single morphant analyses (Figure 3L,O). Overall we observed that the average tectal cross-sectional areas in a transverse section of the un-injected and mMO1 injected embryos ranged between 5800 - 7600 μm2, whereas the morphant embryos had an average area that ranged between 2800-3900 μm2, which were statistically significantly less than their cognate controls, but not significantly different from each other. The overall growth of the morphant embryos was not significantly impaired by 48 hpf [length WT = 2986 ± 51 μm; MO1a+b = 2910 ± 210 μm; mMO1a+b = 3018 ± 65 μm (n = 5 per group)] and we confirmed that after normalizing the tectal cross-sectional area measurements to embryo length, the area in the double morphants was significantly reduced compared to un-injected (t = 9.5, p = 1.2 × 10-5; n = 5 per group) as well as the mMO1a + mMO1b injected (t = 11.2, p = 3.0 × 10-5; n = 5 per group) embryos, whereas the two control groups were not significantly different (t = 0.7, p = 0.5; n = 5 per group).
Midbrain gene expression defects in embryos with reduced levels of either Dmbx1a or Dmbx1b
In order to determine whether the loss of function of dmbx1a and dmbx1b altered neural differentiation, various neural markers were examined using whole-mount in situ hybridization focusing on the combined knockdown of dmbx1 genes. Otx2, foxb1.2, and lim1 were examined at 48 hpf and the expression of these markers in the optic tecta was decreased in the morphants compared to control embryos (Additional file 1). The expression of otx2 in the retina indicated a reduced overall size in the morphant embryos beginning at 48 hpf (Additional file 1A - C). Expression of foxb1.2 in the tectum was virtually eliminated in the double morphants (Additional file 1D - F), but there was no change in foxb1.2 expression in the ventral diencephalic domain or in the MHB. Similarly, expression of lim1 (Additional file 1G - I) was significantly reduced in the posterior tectum adjacent to the MHB, but not altered in the cerebellar primordium on the posterior side of the MHB. Expression of egr2b (krox20) (Additional file 1J - L) and pax2a (Additional file 1M - O) confirmed that early specification and segmentation in the hindbrain region was relatively unaffected in the double morphant embryos at this stage. Nonetheless, judging by the foxb1.2 expression, specific subpopulations of hindbrain precursors may be affected and analyses of transverse sections demonstrate that overall tissue growth of the hindbrain was reduced (data not shown). Interestingly, pax2a expression within the optic stalk region was significantly enhanced (Additional file 1P - R). This is consistent with the fact that dmbx1a expression during gastrula stages partially overlaps with anterior neural plate cells destined for a retinal fate [7,8] and suggests that in the absence of dmbx1a some of these cells are transformed toward an optic stalk identity [32,33] while delaying further retinal development during optic cup formation. This may partly explain the persistent rx1 progenitor cell marker expression in the dmbx1a morphant retina up to 48 hpf [8], even though dmbx1 genes are not apparently expressed in the retina proper before this stage. Finally, we examined in more detail the expression of markers of the MHB (eng3, fgf8, erm, pax2a, wnt1), as well as markers for various telencephalic and diencephalic structures (shh, dlx2a, axial, emx2) between 24 - 48 hpf. In general, we observed no significant differences between the double morphant embryos and controls in the MHB (Additional file 2) or the forebrain (data not shown), although we note that there is an apparent increase in fgf8 expression in rostral telencephalon of the morphant embryos (Additional file 2D-F), which correlates with a loss of dlx2a in the ventral telencephalon (data not shown). This could have implications for telencephalic development.
The specific loss of foxb1.2 expression in midbrain and hindbrain regions in the double morphants suggested that the development of specific sub-regions with dmbx1 gene expression were compromised as a result of gene knock down. However, given that dmbx1a and dmbx1b have partially non-overlapping expression domains, we also investigated foxb1.2 expression in single morphant embryos. Knockdown of either Dmbx1a or Dmbx1b caused a significant reduction in foxb1.2 expression in the midbrain, which was enhanced further in the double morphants (Figure 4A,C,E,G, black arrow head). However, in the hindbrain, foxb1.2 expression in the anterolateral domain (presumptive EG) was eliminated only in the presence of MO1a and not MO1b (black arrow in Figure 4B,D,F,H), which is consistent with the expression of dmbx1a and absence of dmbx1b in this region at 48 hpf (Figure 1). Although the trigeminal ganglion is also present in this anterior-lateral domain, it does not appear to be defective in the MO1a morphant as indicated by the relatively normal expression of GFP in the isl2b:GFP transgenic embryos (see Additional file 4B,D,F,H). The posterior-medial domain of foxb1.2 expression was reduced in the MO1a + MO1b injected embryos (red arrow in Figure 4B,D,F,H). We performed a dose-response analysis for morpholino efficacy using foxb1.2 gene expression as a reliable correlated readout for the midbrain development defects observed at 48 hpf. When 5 ng of either MO1a or MO1b was used, there was no discernable change in foxb1.2 expression in the midbrain (or hindbrain) compared to un-injected controls (Additional file 3). However, when 10 ng of either MO1a or MO1b was injected separately, a similar reduction in foxb1.2 expression and size of the midbrain was observed (Figure 3; Additional file 3). Combined injection of 5 ng MO1a and 5 ng MO1b caused a significant reduction in foxb1.2 expression (Additional file 3). Furthermore, we confirmed that the ventral diencephalic/hypothalamic (white arrowhead Figure 4A,C,E,G) and MHB (black arrow Figure 4A,C,E,G) expression of foxb1.2 were relatively less affected in the morphants. Thus, dmbx1a and dmbx1b appear to be independently required for region-specific midbrain and hindbrain development perhaps through a synergistic mechanism.
Figure 4.
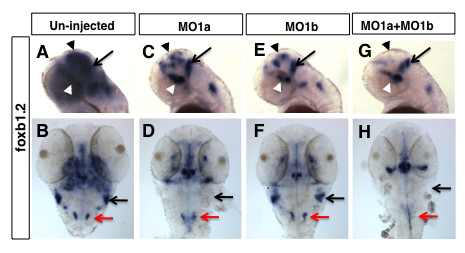
Distinct patterns of hindbrain foxb1.2 expression in dmbx1a and dmbx1b morphant embryos. Lateral view anterior to the left (A, C, E, G) and dorsal view anterior to the top (B, D, F, H) of 48 hpf embryos un-injected (A, B), or injected with MO1a (C, D), MO1b (E, F) or MO1a + MO1b (G, H). The mismatch control injected morpholinos resembled the un-injected controls and are not shown. (A, C, E, G) Black arrow indicates the position of the MHB, white arrowhead demarcates the position of the ventral diencephalic/hypothalamic region of the forebrain, and black arrowhead demarcate expression domain in the region of optic tectum. Black arrow in B, D, F, H demarcates the region of the rostrolateral hindbrain (where EG is located), and red arrow demarcates the expression in the medulla oblongata. MO, morpholino; MHB, midbrain-hindbrain boundary; hpf, hours post-fertilization
Retinal growth defects in embryos with reduced levels of either Dmbx1a or Dmbx1b
Comparing our previous expression analyses [7] with the present results (Figure 1), the onset of expression of dmbx1a within the retina (after the optic cup is formed) occurs ~48 hpf, whereas retinal expression of dmbx1b appears to be similar but slightly delayed. Thus, we reasoned that a requirement for both dmbx1 paralogs in the retina proper would be manifest most prominently between 72 - 96 hpf. Indeed, we did not observe any significant gross morphological defects in the retina in combined MO1a + MO1b injected embryos compared to controls at 24 hpf (data not shown).
In order to determine the relative requirement of dmbx1a and dmbx1b in the development of the retina, we examined transverse sections of the retina at 72 hpf. Single MO1a injected embryos had severely compromised differentiation and lamination (Figure 5B) compared to the un-injected (Figure 5A) or mMO1a injected (Figure 5C) embryos. Furthermore, there was a significant decrease in the average overall area of a mid-transverse section (thickness) of the retina in the dmbx1a morphants compared to controls (Figure 5H). In contrast, the MO1b injected embryos displayed a relatively mild retinal differentiation phenotype (most prominently in the dorsal regions) (Figure 5D) compared to the un-injected (Figure 5A) and mMO1b injected (Figure 5E) controls. However, similar to the dmbx1a morphants, the dmbx1b morphants demonstrated an overall reduction in the average mid-transverse area of the retina (Figure 5H). From these results we predicted that the combined MO1a + MO1b injected embryos would resemble the MO1a injected embryos. Indeed, the severe defect in differentiation in the double morphant (Figure 5F) compared to controls (Figure 5A,G) appeared identical to that of the single MO1a morphant. Interestingly, the reduction in the average mid-transverse area in the double morphants was not significantly different from that observed in the single morphant embryos, ranging from 40 - 60% less than controls (Figure 5H). These observations indicate that retinal growth is primarily dependent on the dmbx1a paralog, which can partially compensate for the lack of dmbx1b, but that dmbx1b is also required for the proper continued growth of the retina (from approximately 48 - 72 hpf).
Figure 5.
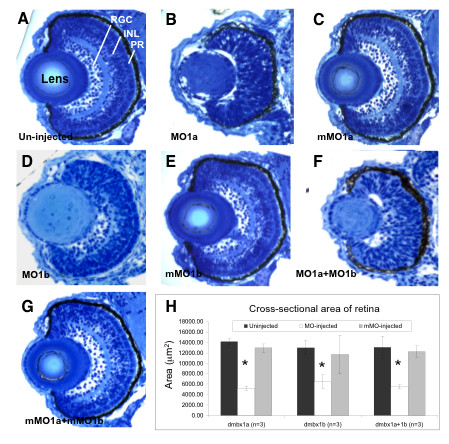
Retinal hypoplasia in dmbx1 morphants at 72 hpf. Coronal plastic sections (~ 1 μm) of retina from un-injected (A), MO-injected (B, D, F), and mMO-injected (C, E, G) embryos. Layers in the retina can be clearly distinguishable at this stage: retinal ganglion cell layer (RGC), inner nuclear layer (INL), and photoreceptor layer (PR). Measurements of the retinal cross-sectional area summarized in the graph (H). Asterisk indicates significant difference (p < 0.05) between the morphant samples and the controls. MO, morpholino; mMO, mismatch morpholino.
The overall growth by 72 hpf [length WT = 3302 ± 54 μm; MO1a+b = 2996 ± 159 μm; mMO1a+b = 3278 ± 65 μm (n = 5 per group)] of the double morphant embryos was significantly less than un-injected (t = 3.36, p = 0.01; n = 5 per group) and mMO1a + mMO1b injected (t = 3.36, p = 0.01; n = 5 per group) embryos. However, despite this difference, we still confirmed that after normalizing the retinal area measurements to embryo length, the area in the double morphants was significantly reduced compared to un-injected (t = 7.7, p = 2.4 × 10-4; n = 5 per group) as well as the mMO1a + mMO1b injected (t = 10.0, p = 2.1 × 10-5; n = 5 per group) embryos, whereas the two control groups were not significantly different (t = 0.08, p = 0.94; n = 5 per group). The overall length difference in the morphants is directly related to the reduced midbrain, retina and hindbrain since other regions of the embryo appeared normal in size (data not shown). We also observed that the lens was smaller in MO1a and MO1a+MO1b morphants. Although the peripherally localized epithelial cells are present, in all cases there appears to be a defect in the differentiation of fiber cells forming the characteristic darkly stained concentric rings. This defect may be due to a secondary disruption in the normal lens-retina interactions during development. However, we did not explore this possibility and further experiments are required to characterize this lens defect in more detail.
Retinal differentiation defects in embryos with reduced levels of either Dmbx1a or Dmbx1b
In order to gain more insight into retinal differentiation changes due to reduced levels of the dmbx1 genes, we examined a panel of markers on transverse sections using in situ hybridization and immunohistochemistry in double morphant embryos at 72 hpf. In control embryos, otx2 was mostly absent from the RGC layer, ONL and the CMZ, but expressed in central regions of the INL at 72 hpf (Figure 6A). In the double morphant embryos, otx2 expression appeared expanded and relatively uniform throughout the central retina, but not in the CMZ (Figure 6B). Consistent with the notion that differentiation of at least some of the retinal cell types is reduced in the morphant embryos, we observed a significant expansion of vsx2-expressing stem and/or progenitor cells in the CMZ [34] compared to controls (Figure 6C,D). There was also an increase in neurod-expressing cells (Figure 6E,F), a marker of photoreceptor progenitor cells [35]. Together these data reveal that reduced levels of Dmbx1 results in a persistent progenitor identity in cells throughout the retina, which correlates with our histological analyses.
Figure 6.
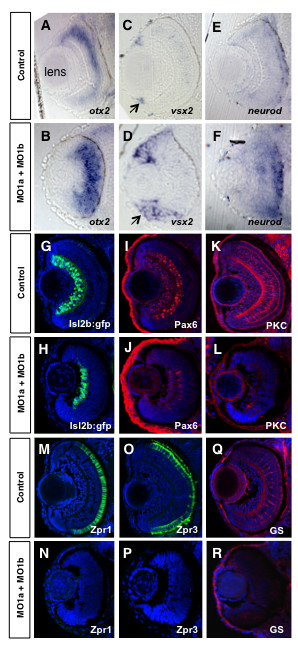
Altered retinal gene expression in morphant embryos. Coronal plastic sections (~ 1 μm) of retina at 72 hpf after whole mount in situ hybridization labeling for progenitor markers from control mMO1a + mMO1b injected (A, C, E), or MO1a + MO1b injected (B, D, F) embryos. Position of the lens (shown in A) is similar for all panels. Arrows in C and D demarcate the ciliary marginal zone (CMZ). Cryosections (~15 μm) of control (G, I, K, M, O, Q) and MO1a + MO1b (H, J, L, N, P, R) embryos immunolabelled with antibodies for indicated differentiation markers (red or green) and counter-stained with DAPI (blue). MO, morpholino.
To gain greater insight into the differentiation defects of the morphant embryos, we analyzed several retinal cell-type specific markers using immunohistochemistry and confocal microscopy. Using an isl2b:gfp transgene to mark retinal ganglion cells [36], we observed a reduction, but not a complete loss, of retinal ganglion cells (GFP+) in the double morphant embryos compared to controls (Figure 6G,H). The expression of Pax6, which marks most amacrine cells and a subpopulation of ganglion cells [34], was also reduced (Figure 6I,J). Consistent with these observations, expression of PKC, which marks bipolar neurons, general markers for cone photoreceptors (Zpr1), rod photoreceptors (Zpr3), and Müller glia (glutamine synthetase, GS) were almost completely abolished in the double morphant embryos (Figure 6K-R). Thus, although there are some differentiated ganglion cells and amacrine cells, most cells in the dmbx1 double morphant retina have not differentiated by 72 hpf, and this correlates with the relative increase in progenitor cell marker expression and morphology.
Based on our histological analyses, we predicted that the effects of gene knockdown on retinal cell differentiation might be paralog-specific. Thus, we carefully examined the expression of rhodopsin (rho) in single morphant embryos compared to the double morphants as a marker for photoreceptor differentiation. Consistent with our histological and immunolabeling data, MO1a injected embryos (Additional file 4C) showed a significant loss of rho expression that mimicked the double morphant phenotype (Additional file 4G). The MO1b injected embryos, in contrast, had a less attenuated rho expression phenotype (Additional file 4E), confirming that loss of dmbx1b in the retina results in a less severe differentiation phenotype. Previous analyses demonstrated that knockdown of dmbx1a alone caused defects in retinotectal projections and reduced terminal fields within the TeO [8]. We confirmed this observation in MO1a injected embryos using a Tg(isl2b:GFP)zc7 transgenic zebrafish line that robustly marks the RGCs in the retina, their axonal trajectories along the retinotectal pathway, and the contralateral terminal fields in the TeO [36]. Approximately 75% of MO1a injected embryos (n = 25; Additional file 4D) showed a defasciculated optic nerve prior to the chiasma compared to control (n = 30) (white arrowhead, Additional file 4B) as well as reduced terminal fields in the TeO (white arrow, Additional file 4D). In contrast, the retinotectal projection in MO1b injected embryos (n = 25) appeared normal (Additional file 4F), which could be due to the presence of normal levels of dmbx1a. If so, then a functional role of dmbx1b in RGC development may be absent. Thus, we predicted that the retinotectal projection in the double morphants would resemble that of the MO1a injected embryos. Again in approximately 75% of embryos, we observed a significant defect in the retinotectal projections when both paralogs are knocked down (n = 35), with significantly reduced terminal fields in the TeO and optic nerve defasciculation (Additional file 4H). These data suggest that dmbx1a has a predominant role in the development of the retinotectal projection, compared to dmbx1b, which was due to a defect in RGC differentiation, a defect in the development of the TeO, or both.
We examined the relative requirement of each paralog in retinal differentiation by performing a similar dose response analysis as mentioned above using rho gene expression as a reliable correlated readout for the retinal defects observed at 72 hpf. In contrast to the observations made for foxb1.2 expression in the midbrain/hindbrain, 5 ng of MO1a resulted in a substantial reduction (~50%) in the extent of rho expression in the retina, whereas 5 ng of MO1b resulted in a negligible difference in rho expression compared to un-injected controls (Additional file 3). However, when 5 ng of MO1a and MO1b was combined for a total of 10 ng injected, we observed a significant reduction in rho expression (Additional file 3). When 10 ng of MO1a was injected individually, a similar reduction in rho expression and size of the retina is observed when compared to the double morphants derived from injections of 5 ng of each morpholino combined (Additional file 3). Unexpectedly, when 10 ng of MO1b was injected individually, a relatively mild reduction in rho expression was observed compared to controls (Additional file 3). These data suggest that dmbx1a has a predominant functional role in retinal differentiation and that dmbx1b may only have a minor, additive role.
Defects in tissue size and cell differentiation in the midbrain and retina are not due to persistent cell death
Our data comparing the dmbx1 single and double morphants demonstrate that the size of the midbrain and retina are reduced and that neural differentiation is significantly attenuated. One possible mechanism to account for this phenotype is increased cell death in progenitor cells within these regions, resulting in diminished growth and differentiation potential of the tissue. We tested this hypothesis by examining the level of cell death at 24, 48 and 72 hpf using the chromatin binding fluorescent marker acridine orange (AO) to detect apoptotic cells in live embryos [37]. An increased number of AO+ cells was observed in the midbrain and hindbrain at 24 hpf in MO1a + MO1b injected embryos compared to un-injected and mMO1a + mMO1b injected embryos (Additional file 5A, B). However, the growth defects in morphant embryos were not apparent until after this stage. Thus, to test whether persistent apoptotic cell death was the cause of the growth defects, we analyzed AO labelling at 48 and 72 hpf. Little or no apoptosis was detected in any of the groups at 48 hpf (Additional file 5C, D; data not shown). At 72 hpf, there is substantial remodelling occurring within the teleostean retinotectal pathway, which results in apoptotic cell death in both retinal and tectal tissue [38]. This was detected in un-injected embryos and similar levels of cell death were observed in embryos injected with a combination of mMO1a + mMO1b (Additional file 5E, K). In contrast, combined MO1a + MO1b injected embryos showed less AO labelling in both the midbrain and the retina compared to controls (Additional file 5F, L). We confirmed these observations by staining for activated Caspase3 and by performing TUNEL labelling at 72 hpf to detect apoptotic cells. The number of Caspase3+ or TUNEL+ nuclei in transverse sections of the midbrain (Additional file 5G-J) or retina (Additional file 5M-P) at this stage were not different between the controls and double morphants. Furthermore, examination of our semi-thin plastic sections from morphant tissues did not reveal any evidence for enhanced pyknosis, cellular debris or large autophagic vacuoles relative to controls at any time point between 48 - 96 hpf, suggesting that neither necrosis nor autophagy appears to account for the morphant phenotype. Thus, these data indicate that persistent cell death likely does not account for the defects in size and differentiation of the midbrain and retina in dmbx1 morphants.
Dmbx1a and Dmbx1b are required for normal progenitor cell cycle regulation
An alternative mechanism that can account for the reduced size and attenuated differentiation of midbrain and retinal tissue may entail changes in the capacity for progenitor cell proliferation. We analyzed nuclear PCNA protein expression by immunohistochemistry to label cells that are actively in cell cycle [39]. By 72 hpf cell proliferation becomes substantially restricted to periventricular locations within the brain [39], and the CMZ in the retina [40] under normal conditions. Thus, we predicted that a defect in cell cycle regulation would be most evident by 72 hpf when neurogenic compartments are normally relatively small and very well circumscribed. PCNA labelling in the brain at 72 hpf revealed that cell proliferation in the optic tectum is maintained throughout the entire perimeter bilaterally in the double morphants (Figure 7B), whereas at this stage in control embryos the tectal neurogenic zones are more restricted to the lateral (bordering with the torus semicircularis anlagen) and most prominently the medial (bordering with the torus longitudinalis anlagen) compartments (Figure 7A). These observations reveal that the reduction of Dmbx1a and Dmbx1b expression caused an increase in the number of actively cycling cells in the midbrain.
Figure 7.
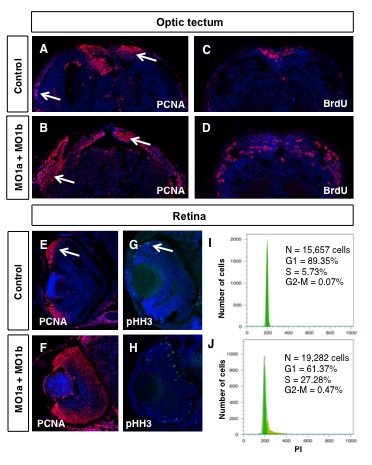
Proliferation defects in dmbx1 morphant embryos. Embryos at 72 hpf were examined for the presence of proliferating cells using immunohistochemistry with antibodies to PCNA, pHH3 or BrdU (30 min survival). Coronal (~15 μm) sections of midbrain (A-D, dorsal to the top) and retina (E-H, dorsal to the top, lens to the left). Arrow in A and B demarcates the proliferative zones of the dorsal and lateral TeO. Arrow in E and G demarcates the CMZ. (I, J) Flow cytometric analysis of retinal cells derived from un-injected or MO1a + MO1b injected embryos at 72 hpf using propidium iodide labeling. N, total number of cells analyzed; G1, gap-1 phase of the cell cycle; S, synthesis phase of the cell cycle; G2, gap-2 phase of the cell cycle; MO, morpholino; mMO, mismatch morpholino; hpf, hours post-fertilization.
To confirm these results with another cell cycle marker, we tested whether cells can incorporate 5-bromo-2'-deoxyuridine (BrdU), a thymidine analog, during S phase of the cell cycle. Embryos at 72 hpf received a single intracerebroventricular bolus of 5 mM BrdU dissolved in embryo media, and subsequently processed for immunohistochemistry after a 30 min survival period. The combined knockdown of Dmbx1a and Dmbx1b caused a significant increase in BrdU labelled cells (Figure 7D), compared to controls (Figure 7C).
An analysis of cell proliferation in the retina at 72 hpf yielded similar results. PCNA expression in the double morphants was greatly expanded toward the central retina from the CMZ (Figure 7F), compared to the relatively few PCNA+ cells in the CMZ of control retinas (Figure 7E) and this was similarly confirmed with BrdU labelling (Figure 8, discussed below). Given that the retina is smaller in the morphants, an increase in PCNA+ and BrdU+ cells suggests that these cells might be delayed or stalled in the G1/S transition of the cell cycle. We examined the expression of phospho-histone H3 (pHH3), which labels cells in M-phase of the cell cycle, and found that there was approximately a two-fold increase in the number of pHH3+ cells [Control (n = 3): 15.3 ± 4.7 vs. MO1a + MO1b (n = 3): 28.0 ± 3.4; t = 3.7, p < 0.05] in transverse sections of the double morphant retinas primarily localized to the apical domain if the CMZ and central retina (Figure 7H) compared to controls (Figure 7G). Thus, cells are able to progress beyond S-phase of the cell cycle. We were also able to rule out that progenitor cells were undergoing endoreduplication of their DNA by using propidium iodide (PI) labelling and flow cytometry to quantify the average DNA content among a population of retinal cells from 72 hpf embryos injected with MO1a + MO1b and compared to cells from un-injected control embryos of equivalent age. We pooled dissected retinal tissue from 120 embryos in both groups. The vast majority of un-injected retinal cells at this stage (89.3%) were within G1 of the cell cycle, whereas only 5.7% were in S phase (Figure 7I). In contrast, 61.4% of the morpholino injected cells were in G1 phase of the cell cycle and 27.3% were in S phase (Figure 7J). The proportion of cells in G2-M of the cell cycle in either group was < 1% and this is due to the fact that the overall fraction of the cell population captured in these short phases of the cell cycle is rather low using this method. Nonetheless, there is a ~6.7 fold increase (from 0.07 to 0.47) in the proportion of cells in G2-M in the morphant retinas. Importantly, there was no evidence of polyploidy in either the control samples or the morphant samples. Therefore, these data indicate that retinal progenitor cells (and by inference midbrain cells) in morphant embryos are not stalled in any particular phase of the cell cycle and that they complete mitosis. Taken together, the smaller retinal size and increased proportion of cells that remain in cycle at 72 hpf suggests that progenitor cell cycle length is significantly increased.
Figure 8.
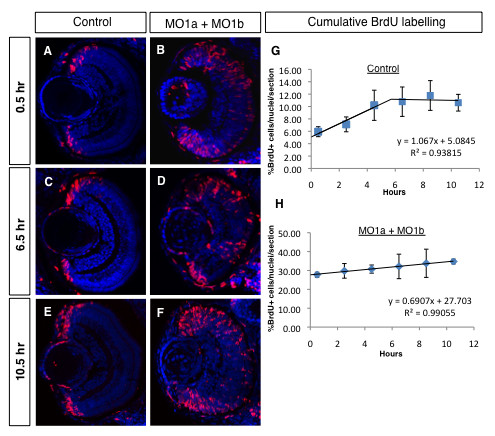
Cumulative BrdU labelling reveals an increase in the morphant progenitor cell cycle in the 72 hpf retina. (A-F) Immunolabelled coronal sections (dorsal to the top, lens to the left) from embryos repeatedly injected with BrdU (red) after different survival times (indicated on the left). Sections were counter-stained with DAPI (blue). (G, H) Regression analyses from cumulative labelling data. MO, morpholino; hpf, hours post-fertilization, hr, hours.
To precisely quantify potential changes in cell cycle length in the dmbx1 double morphants at 72 hpf, we performed a BrdU cumulative labelling experiment (see Materials and Methods section for details) focusing on the retina. A 5 mM bolus of BrdU was given by intracerebroventricular (ICV) injection with survival times ranging from 0.5 hours to 10.5 hours at 2-hour intervals. For example, embryos in the 0.5 hour group would receive only a single BrdU injection and then processed for immunolabeling after 30 min. In contrast, embryos in the 10.5 hour group would have received a total of 6 separate injections (2 hours apart) and then processed for immunolabeling 30 min after the last injection. In preliminary experiments we determined that embryos receiving 6 repeated ICV injections remained viable and healthy (data not shown). The central assumption in this analysis is that an asynchronously dividing population of cells exhibits single population kinetics (i.e. all cells in the population have the same cell cycle time). Representative confocal images of BrdU labelled cells in control retinas at 0.5 hr, 6.5 hr, and 10.5 hr are shown in Figure 8A,C,E where proliferating cells are exclusively confined to the region of the CMZ. In contrast, in dmbx1 double morphant retinas, BrdU+ cells appear scattered through the peripheral and central retina (Figure 8B,D,F). Proliferating cells entering S-phase over time will incorporate BrdU and become labelled until they re-enter S-phase, at which point they can still incorporate BrdU, but they will not be marked as newly positive cells. In controls, the data during this interval (~0 - 5.5 hr) were fit to a linear regression model (R2 = 0.93815), which allowed us to estimate when the maximal number of BrdU+ cells in the population were labelled (the first time point when the plateau is reached) (Figure 8G). Thus, by ~ 5.5 hours of cumulative BrdU labelling, all of the cells that are cycling in the population (the growth fraction) are labelled and further incorporation of BrdU at later time points does not increase this value. This allowed us to estimate the growth fraction in the controls to be ~11%. Using these values obtained from the plotted data (Figure 8G), we were able to estimate the progenitor cell cycle to be ~ 10.5 hours in the control retina (see Material and Methods for calculation).
The same analysis for dmbx1 double morphant retinas resulted in a significantly different cell cycle estimate. First, the fraction of cells incorporating BrdU over time continued to increase over the entire labelling interval (R2 = 0.99055; Figure 8H). Therefore, were not able to accurately determine the growth fraction for the 72 hpf morphant retina, which would have required continuing the cumulative BrdU labelling well beyond 10 hours. However, we reasoned that the last time point assayed (10.5 hours) could be used as a minimum estimate for the time at which the growth fraction (i.e. ~35%) is reached (Figure 8H). Therefore, a minimal estimate for the cell cycle in these morphant progenitor cells is ~50.6 hours, which is approximately 5-fold longer than in the control retina. This increase in cell cycle length could account for the fact that the size of the retina at 72 hpf is significantly smaller since on average progenitor cells in the morphant retinas would not have completed one cell division between 48 hpf and 72 hpf. Our data indicate that a reduction in Dmbx1 proteins causes a significant increase in the cell cycle time of progenitor cells in the retina (and by inference the optic tectum) resulting is fewer differentiated cells.
Rescue of the zebrafish morphant phenotype
In order to confirm the specificity of our knockdown phenotype, we co-injected either MO1a or MO1b morpholino with the corresponding zebrafish full-length mRNA (lacking the morpholino binding sequence). Microinjection of either dmbx1a mRNA or dmbx1b mRNA resulted in a significant dose-dependent dorsalization phenotype that was evident in embryos as early as tailbud stage and confirmed by 24 hpf (Additional file 6A; unpublished observations). We therefore, titrated the mRNA and evaluated the percentage of embryos co-injected with optimal amounts of mRNA and morpholino that demonstrated a rescued morphant phenotype. The highest concentration of either dmbx1a or dmbx1b mRNA (250 pg) caused mild to severe dorsalization in ~70 - 75% of the embryos injected and that ≥ 50% of these were in the severe category (Additional file 6B). Because some of these embryos showed signs of necrosis at 24 hpf, in particular in the tail region, we opted for a lower concentration of mRNA (150 pg for dmbx1a and 188 pg for dmbx1b, yielding similar phenotypic results) in order to test whether the paralog specific mRNA could rescue the morphant phenotype (see below). Using this lower concentration, ~ 50-70% fewer embryos were severely dorsalized (Additional file 6B).
In a separate set of experiments we tested whether zebrafish dmbx1a mRNA could rescue the zebrafish MO1a morphant phenotype more by analyzing foxb1.2 and rho gene expression. The vast majority of embryos injected with MO1a resulted in a reduction of foxb1.2 expression in the midbrain and a loss of foxb1.2 expression in the rostrolateral hindbrain compared to the un-injected controls (Figure 9A,B). Furthermore, similarly treated embryos had dramatically reduced rho expression in the retina compared to un-injected controls (Figure 9E,F). By co-injecting zebrafish dmbx1a mRNA with MO1a, we were able to rescue the expression of foxb1.2 in the midbrain and rostrolateral hindbrain in ~50% of the injected embryos (when compared to the MO1a only group), as well as rescue the expression of rho in the retina by over 70% of the injected embryos (when compared to the MO1a only group) (Figure 9C,D). We also noticed that in embryos injected with zebrafish dmbx1a mRNA (especially without co-injection of MO1a) that there was a marked increase in foxb1.2 expression in the midbrain and rostromedial hindbrain (e.g. asterisk in Figure 9C; data not shown). Although we cannot completely rule-out subtle morpholino off-target effects, our data strongly suggest that the morphant phenotype is specifically due to the reduction in dmbx1 gene expression.
Figure 9.
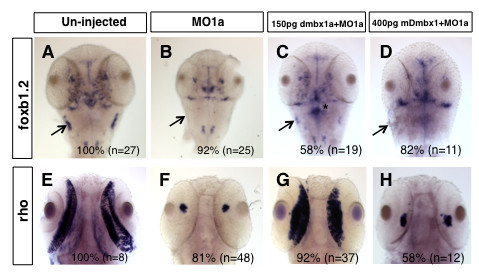
Rescue of the midbrain and retinal phenotype in morphants is species-specific. The expression domain of foxb1.2 in un-injected 48 hpf embryos (A) and rho in un-injected 72 hpf embryos (E) is significantly reduced in the MO1a injected embryos (B, F). Co-injection of zebrafish dmbx1a significantly rescues these phenotypes (C, G), whereas co-injection of mouse Dmbx1 mRNA does not (D, H). Arrow in A-D demarcates the region of the rostrolateral hindbrain (where the EG is located) at 48 hpf. For A-D, images represent dorsal views, anterior to the top. For E-H, images represent ventral views, anterior to the top. MO, morpholino; hpf, hours post-fertilization. Asterisk in (C) demonstrates ectopic foxb1.2 expression, which was commonly observed in embryos over-expressed with zebrafish dmbx1a, but not mouse Dmbx1 mRNA.
We also tested if the dmbx1b gene can rescue the dmbx1a morphant phenotype by examining foxb1.2 expression in the brain. As shown above, foxb1.2 is expressed in the dorsal midbrain and in the anterolateral hindbrain (in the region of the EG) and this pattern is similar to dmbx1a. In contrast, dmbx1b is not expressed in this anterolateral hindbrain domain, although it is expressed in the dorsal midbrain. Knockdown of dmbx1a, but not dmbx1b, results in the loss of foxb1.2 expression in the anterolateral hindbrain (Figure 4B,D,F). Therefore, we reasoned that if dmbx1b was capable of rescuing the dmbx1a morphant phenotype, then the expression of foxb1.2 in the anterolateral hindbrain would be restored. The results showed that while dmbx1a mRNA could rescue the midbrain and anterolateral hindbrain foxb1.2 expression in ~75% of the embryos co injected with MO1a (Additional file 7C), dmbx1b mRNA co-injected with MO1a was completely ineffective at restoring foxb1.2 expression in the anterolateral hindbrain, although midbrain expression was enhanced to control levels in ~50% of the embryos (Additional file 7D). We conclude that the functionality of the dmbx1 paralogs, in this experimental context, is not completely interchangeable.
Given the coding-sequence conservation between the mammalian Dmbx1 genes and the teleost dmbx1 genes, we also examined whether the full-length mouse Dmbx1 mRNA (lacking the morpholino binding site) could rescue the MO1a phenotype. The mouse Dmbx1 mRNA yielded a comparable dorsalization phenotype only after a 2-3 fold relative increase in mRNA was injected (Additional file 6C). However, the mouse Dmbx1 mRNA cannot rescue the foxb1.2 morphant expression in the midbrain and anterolateral hindbrain (Figure 9D), or the rho morphant expression in the retina (Figure 9H), as efficiently as the zebrafish mRNA. There were ~20% of the embryos co-injected with mouse mRNA and MO1a that had a slight increase in rho expression (compared to the MO1a only group), but this was substantially less than the control levels of expression (data not shown). We observed similar results when comparing the MO1b rescue with zebrafish dmbx1b compared to mouse Dmbx1 mRNA (data not shown).
It is possible that the mouse Dmbx1 mRNA is less stable in a zebrafish embryo accounting for the reduced potency of mouse Dmbx1 to induce a dorsalized phenotype. Unfortunately, currently available commercial antibodies for Dmbx1 do not recognize the zebrafish proteins (data not shown). Therefore, in order to address whether the mouse mRNA was less stable, we generated c-myc epitope-tagged mouse Dmbx1 and zebrafish dmbx1a constructs and monitored protein levels by immunohistochemistry using an anti-c-myc antibody at tailbud after injecting into 1-2 cell stage embryos. Compared to un-injected controls (Additional file 8A), embryos injected with either mouse myc-Dmbx1 (Additional file 8B) or zebrafish myc-dmbx1a (Additional file 8C) resulted in protein expression. Similar observations were made with injected 24 hpf embryos (data not shown). There was some variability in the overall intensity of staining between embryos regardless of the construct used, but in all cases injected embryos had discernable nuclear staining compared to un-injected controls (Additional file 8D-F). Thus, although subtle differences in mRNA stability may not be detectable in this assay, these data suggest that there is no overt difference in the mRNA stability of zebrafish and mouse mRNA that would account for the inability of the mouse Dmbx1 to rescue midbrain and retinal morphant gene expression phenotypes.
A limitation in our morphant rescue analyses relates to the dorsalization phenotype. The rescue of the brain and retinal morphant phenotypes with zebrafish dmbx1 mRNAs is consistent with the fact that there is a significant loss of function phenotype in these specific tissues. However, the rescue of the overall mRNA-induced dorsalization phenotype with co-injection of morpholino is more difficult to interpret. The onset of dmbx1 expression occurs as early as 9 hpf and the expression domain is strongest on the dorsal side close to the blastoderm margin [7,8]. It is possible that in addition to affecting the development of the midbrain territory, changes in the relative levels of dmbx1 expression at this early time might also indirectly affect the morphogenesis of tissues adjacent to the dmbx1 domain (e.g., convergence-extension defects) resulting in dorsalized embryos with high concentrations of mRNA. The co-injection of morpholino and zebrafish mRNA is presumably sufficient to mitigate these early morphogenetic defects. However, this hypothesis remains to be tested. In contrast, the co-injection of morpholino and mouse mRNA may not mitigate these early defects if the downstream effects of ectopic mouse mRNA are different. In other words, the dorsalization phenotypes from zebrafish and mouse mRNA injections might be caused by distinct molecular mechanisms. Further experiments are required to better understand the underlying mechanisms that might account for the dorsalization defects. Nonetheless, we also know that lowering the concentration of injected dmbx1a mRNA tends to significantly reduce the number of dorsalized embryos (Additional file 6), yet we are still able to observe a rescue of the foxb1.2 expression in the brain of MO1a morphants (preliminary data not shown). Thus, overall these data suggest that we can achieve a reliable rescue of the zebrafish MO1a and MO1b morphant phenotypes with corresponding zebrafish dmbx1 mRNAs.
Molecular evolutionary analyses of vertebrate Dmbx1 genes
The morphant rescue results prompted us to examine whether there are any changes in the protein coding sequences among the vertebrate Dmbx1 genes that might correlate with the putative functional differences that we observed between the zebrafish and mouse genes. The results of a variety of phylogenetic analyses, including neighbour-joining, maximum likelihood and Bayesian methods, were largely congruent with current understanding of phylogenetic relationships among vertebrates [41-44]. These analyses strongly support a fish-specific Dmbx1 duplication event (Additional file 9), which we previously proposed [7] and may be associated with a postulated ancient genome duplication event early in the teleost lineage of fishes [45].
Determining the proportion of non-synonymous to synonymous (dN/dS) changes in the coding sequences of Dmbx1 genes allowed us to estimate the rate of amino acid evolution as well as make inferences about any changes in the selective constraints during the evolution of this gene family in vertebrates [46]. The results of our molecular evolutionary analyses of Dmbx1 genes suggest that although this family is generally quite conservative in its evolution, with an overall dN/dS of only 0.036, there was a dramatic change in selective constraint after the duplication event which gave rise to the dmbx1a and dmbx1b families in fish (Additional file 10). The elevation in dN/dS after this gene duplication is particularly marked in the lineage leading to dmbx1a (dN/dS = 46.88), suggestive of positive selection; whereas estimates along the dmbx1b lineage (dN/dS = 0.37), which are much lower, would be consistent with either weak positive selection or relaxed purifying selection. However, these results are based on analyses of a fairly small data set, which is particularly deficient in basal fish Dmbx1 genes; the inclusion of additional sequence data will improve the ability of these statistical methods to detect changes in the form and strength of selection across the Dmbx1 gene family. Additionally, in vivo assays of protein function, as we have done, are necessary to confirm functional divergence following the gene duplication event.
Discussion
The role of Dmbx1 in regulating brain and retinal neurogenesis
Our present results expand upon previous zebrafish dmbx1a knockdown experiments [8] to demonstrate that the dmbx1 paralogs have evolved both cooperative and divergent functions in brain neurogenesis. In the midbrain, particularly in the TeO, there is an independent, non-redundant requirement for both dmbx1a and dmbx1b functions, even though there is substantial overlap in progenitor cell expression of these genes. In contrast, divergent expression of the dmbx1 paralogs, particularly in the anterior hindbrain, correlate with divergent, paralog-specific (i.e. dmbx1a) function in neurogenesis. We reveal for the first time that the zebrafish dmbx1 genes have a fundamental role in regulating either the normal pace of progenitor cell cycle progression and/or the ability to exit the cell cycle and differentiate at the appropriate time. Further investigations will be required to ascertain the precise mechanism by which Dmbx1 proteins influence cell cycle timing.
A recent study demonstrated that zic2a and zic5 paralogous genes are required for TeO neurogenesis as early as 15 hpf, and that transcription of these genes is directly regulated by canonical Wnt signalling [47]. The earlier effect of zic gene expression on tectal proliferation suggests that dmbx1 function may be downstream of zic. One plausible model is that zic genes promote cell cycle entry from G0 in cells with a normal cell cycle, while dmbx1 genes promote cell cycle progression or exit. Given that these genes encode transcription factors, this role in cell cycle regulation could be indirect through the expression of cell cycle genes, such as cyclind1 [48] or cdc16/26 of the APC complex [49], but direct regulation of cell cycle proteins is also possible. Our preliminary yeast-2-hybrid analysis recovered Zic2b as a putative interacting partner with Dmbx1a, among other proteins with known functions in cell cycle regulation (L.W. and V.T., unpublished observations). Thus, it is possible that dmbx1 genes, zic genes and other cell cycle regulators physically interact in a protein complex to regulate transcription and/or other functions. Our data is consistent with the function of other vertebrate paired-like homeodomain transcription factors, such as Chx10 (Vsx2) and Prox1, which have been shown to play an important role in retinal progenitor cell cycle and differentiation [50]. Interestingly, a putative Drosophila ortholog Pph13/Mu, which is expressed in photoreceptor cells, has been shown to be required for differentiation and function, but not retinal progenitor cell specification [14]. One appealing model is that canonical cell cycle inhibitors interact with Dmbx1 and other factors to activate transcription of genes that promote cell differentiation and/or to repress genes that maintain a progenitor cell state.
Patterning and early morphogenesis of the midbrain, prior to neurogenesis, requires the establishment of organizing boundaries at the rostral extent (the diencephalic-midbrain boundary, DMB) and caudal extent (MHB) of the midbrain territory. Otx2 and gbx2 are two early expressing genes that pattern the neural plate rostrocaudally [51,52] and the interface of the two expression domains gives rise to the MHB, an organizer that activates a cascade of downstream transcriptional regulators, such as pou2, pax2a, pax5/8, and eng2/3, through Fgf8 signalling [53-55]. The DMB is established by the mutual repression of the forebrain marker pax6 and midbrain marker eng2 [56,57]. Consistent with previous loss of function data in both zebrafish [8] and mouse [9], neither dmbx1a nor dmbx1b is essential for MHB or DMB formation. Indeed, although the overall size of the midbrain territory is reduced, the expression of dmbx1a, dmbx1b, otx2 and wnt1 are normally regionalized in the double morphant embryos, indicating that Dmbx1 is not essential for brain patterning, but is instead an important regulator for subsequent neurogenesis.
Zebrafish dmbx1a and dmbx1b are both required for foxb1.2 expression in the midbrain and hindbrain. Although we have not determined whether this interaction is direct via transcriptional regulation of the foxb1.2 gene, this data suggests that the function of Dmbx1 may be mediated through foxb1.2. Dmbx1 has previously been shown to act as a transcriptional repressor [58], therefore it is possible that the mechanism for regulating foxb1.2 expression may involve an indirect de-repression mechanism. Interestingly, the relationship between the Dmbx1 and Foxb1 orthologs in mouse is different. In mouse, a null mutation in the orthologous Foxb1 gene affects midbrain neurogenesis, particularly within the inferior colliculus, which is most notable in the postnatal period with little or no effect on the development of the superior colliculus (the homolog of the fish TeO) [59,60]. In contrast, there is no discernable embryonic or postnatal defect in the Dmbx1 null midbrain and there is no change in Foxb1 gene expression in the Dmbx1 null embryos [9]. This is strikingly different from our observations that single knockdowns of either dmbx1a or dmbx1b causes a significant growth and differentiation defect in the midbrain, which is strongly correlated with a significant reduction in foxb1.2 gene expression. These observations lead us to speculate that there is post-duplication evolutionary divergence of function in teleosts in which dmbx1 genes have a more prominent role in regulating neurogenesis compared to other vertebrate classes that harbour a single Dmbx1 gene, possibly through the novel regulation of foxb1 genes. However, we cannot yet rule out that other components regulating neurogenesis in mice may have been functionally compensating in the Dmbx1knockout, and hence masking a phenotype that would otherwise resemble the zebrafish dmbx1 knockdown phenotype. Post-duplication functional divergence has also been proposed for the zebrafish midkine genes [61], and so it would be interesting to compare their functional evolution with that of the dmbx1 genes to determine if similar underlying mechanisms have evolved.
The Dmbx1 loss of function phenotype in the retina (extended cell cycle length and reduced differentiation) resembles the disarrayed [62] and caf-1b [63] mutants, where both cell cycle progression and/or exit, and differentiation, are significantly delayed during retinal development. However, in both of these mutants there is a substantial increase in apoptosis between 42 hpf and 65 hpf (for disarrayed), or 48 hpf and 72 hpf (for caf-1b). Interestingly, rescue of apoptosis with a p53 morpholino did not rescue the differentiation defect in the caf-1b mutants [63], consistent with the possibility that a delayed cell cycle defect (independent from the cell survival defect) prevents differentiation, similar to what we observe in dmbx1 morphants. In contrast, we did not observe persistent cell death in the brain or retina after 24 hpf in the dmbx1 morphants. Nonetheless, it would be interesting to further characterize the network of genes that regulate the transition from a proliferating progenitor cell to a post-mitotic progenitor, for which disarrayed, caf-1b and dmbx1 genes may be required.
Dmbx1 appears to have an essential role in the differentiation of the rod and cone photoreceptor lineages. Interestingly, double morphant embryos had enhanced expression of the basic helix-loop-helix transcription factor gene neurod. It has been recently shown that neurod expression marks early progenitor cells within the INL that predominately give rise to rod (and cone) precursor cells that express crx and traverse the ONL to eventually differentiate into mature photoreceptors [35]. Both Dmbx1 paralogs are expressed in the INL during photoreceptor lineage development, like Neurod1 and Crx [35,64]. However, dmbx1 expression does not appear to overlap with neurod1 and crx in the ONL. Thus, we speculate that Dmbx1 functions in parallel (or upstream) of neurod and crx in regulating photoreceptor progenitor cell cycle exit and/or the onset of photoreceptor differentiation.
The evolution of vertebrate Dmbx1 genes
Studies in zebrafish are increasingly playing an important role in deciphering the functional consequences of gene duplication. In this regard, significant insight into the fundamental changes in brain development that are due to the retention of duplicate genes in zebrafish comes from studies of the Dlx gene family [65,66], Hoxb genes [67,68], the Zic gene family [47,69] and Pax6 [28]. In most cases, redundancy or subfunctionalization is reported to play an important role in the retention of duplicate genes. However, these mechanisms may not result in substantial genomic novelty to account for the developmental specializations during vertebrate neural evolution [70]. Additional mechanisms for duplicate gene retention, such as neofunctionalization [63] or function shuffling [26] may have evolved, and may or may not be operating on different gene duplicates within a single genome [30]. Therefore, it seems reasonable to assume that the function of individual genes of a duplicate pair will have subtle and complex roles in brain development.
The Dmbx1 gene is also conserved in the Amphioxus and ascidian genomes, but is either not expressed in the nervous system during development (as in Amphioxus) or is expressed in a domain that is posterior to the developing sensory vesicle and neck (pax2/5/8a-expressing domain) region of the neural tube starting at approximately tailbud stage in ascidians [71-73]. These observations indicate that the Dmbx1 neural expression domain in invertebrate chordates is likely to be homologous to part of the vertebrate hindbrain domain, consistent with the notion that a true midbrain region is absent in these species [72]. Thus, Dmbx1 expression in a region that is anterior to the midbrain-hindbrain boundary (MHB) demarcates a distinct midbrain territory hypothesized to be a derived anatomical trait that evolved specifically in vertebrates.
Our findings suggest zebrafish Dmbx1 is required for proper cell cycle progression or exit and cell differentiation of progenitor cells in the brain and retina. Furthermore, several observations, including: (1) the differences in gene expression pattern, (2) loss of function phenotypes, (3) lack of complete interchangeable function in rescue experiments, and (4) the changes in selective constraints in protein sequence evolution, suggest that the post-duplication zebrafish dmbx1 genes may have evolved a diverged function during neural development.
Since our present study primarily focused at the level of cell/tissue/development for our analyses, further studies are required to resolve whether changes at the amino acid level have direct consequences for protein function. A recent analysis of the statistical methods used to determine positive selection in protein coding sequences questions the reliability of some models, such as the branch-site model not used in our analyses, in their ability to predict positively selected sites and suggests that experimental confirmation would be necessary in such analyses [74]. Our evidence of positive selection in the teleost dmbx1 coding sequences is consistent with the differences in functionality between zebrafish and mouse Dmbx1 that we observed in our morphant rescue experiments. Does this evidence support the possibility for neofunctionalization [75]? It is tempting, but premature to answer this question in the affirmative. In order to substantiate such a model we need to understand the function of the Dmbx1 proteins in various vertebrate species in much more detail in order to deduce which evolutionary mechanism could plausibly explain the retention of the Dmbx1 paralogs in the teleost genome. Nonetheless, our data provide a reasonable basis to further investigate this problem experimentally.
Conclusion
There are four main observations from our study of the functional role of dmbx1 duplicate genes in zebrafish development. First, both dmbx1a and dmbx1b are independently required, and hence cooperate in regulating neurogenesis in the midbrain. Second, dmbx1a has a predominant role in regulating neurogenesis in the retina and anterior hindbrain, and is therefore partially functionally diverged from dmbx1b. Third, the cellular mechanism of zebrafish dmbx1 function is to control cell cycle exit and/or differentiation in progenitor cells. Finally, we provide evidence for post-duplication positive selection in teleost dmbx1 genes that correlate with differences in over expression/rescue phenotypes between the zebrafish and mouse Dmbx1 genes. Therefore, zebrafish dmbx1 duplicate genes may be functionally diverged and appear to have an important role in regulating the transition from a proliferating progenitor cell to a post-mitotic differentiated neural cell.
Methods
Zebrafish husbandry
Adult zebrafish (Danio rerio) used in this study were maintained at 28°C on a 14-hour light/10-hour dark cycle and housed in an automated re-circulating system (Aquaneering). Animals were treated in accordance with the regulations on animal experimentation established by the Canadian Council on Animal Care. The experimental procedures were approved by the University of Toronto Animal Care Committee. Embryos were staged as described in Kimmel et al. [76] and reared according to standard procedures [77]. The wildtype strain used was AB (Zebrafish International Resource Center) and the Tg(isl2b:GFP)zc7 transgenic strain was a kind gift from Dr. Chi-Bin Chien.
GFP fusion proteins
Primers were designed to flank part of the 5'-UTR and N-terminal domains of dmbx1a and dmbx1b that are complementary to the morpholino sequences. The previously described dmbx1a-MO (MO1a) was complementary to the sequences surrounding the ATG start codon [8] and the corresponding fusion protein was generated by cloning 70 bp of the 5'-UTR and the first 16 amino acids of Dmbx1a in frame with the EGFP gene (Dmbx1a-FP primers: F:5'-CGAGCTAGAAGCAAGAAAATATCA-3' and R:5'-GAGTTCATGGCGTGGAGAGAGTA-3'. The dmbx1b-MO (MO1b) targeted the 5'-UTR sequences just upstream of the start codon. The fusion protein consisted of the 99 bp of the 5'-UTR plus amino acids 1-16 of Dmbx1b, followed by the EGFP gene sequences (Dmbx1b-FP primers: F:5'-TGGGAAAAATCACTCGTGTTC-3' and R: 5'-GAGTTCATGGCGTGCAAA-3'). The PCR fragments of dmbx1a-FP and dmbx1b-FP were cloned upstream and in frame with EGFP in pCS2+. Plasmids were linearlized with BstX1 and in vitro transcribed with the SP6 messenger kit (Ambion). For each fusion construct, 500 pg of mRNA was injected at the 1-cell stage embryo in the presence or absence of morpholinos.
BrdU labelling
To label cells that were in S-phase, we injected 5 mM of 5'-bromo-2'-deoxyuridine (BrdU) into the tectal brain ventricle of 72 hpf embryos and fixed the animals (n = 10) with 4% paraformaldehyde half and hour later. For cumulative cell cycle analysis using BrdU incorporation, embryos (n = 12) were injected with 5 mM BrdU every two hours up to 10 hours followed by 4% paraformaldehyde fixation 30 minutes post-BrdU injection. Cyrosectioning procedures were performed as mentioned above. For BrdU-immunostaining, slides were treated with 20U/mL DNase I at room temperature for 30 minutes followed by extensive washes with PBS+1% DMSO+0.1% Tween-20 (PBDT). Sections were blocked for two hours and incubated in rat anti-BrdU (1:100, Cedarlane) primary antibody overnight at 4°C, which was then detected with Cy3 secondary antibody (1:500, Jackson ImmunoResearch Laboratories, Inc.). Images were obtained from mounted slides using Leica TCS SP5 II Confocal Microscope and analyzed with Leica LAS AF software. For the cumulative BrdU assay, we counted the number of BrdU positive cells per section (averaged over at least 3 separate retinas) and we used cell density (number of DAPI positive nuclei/area of section) to estimate the number of total nuclei from each section and calculated the labelling index (BrdU positive cells/total nuclei) at all six time points. Cell cycle kinetics in control and morphant embryos was determined as described in [78,79] assuming this was a single population model. Briefly, hours of BrdU injection (T) was plotted against the labelling index (LI). Growth fraction (maximum LI on the y-axis, LIm) can be determined from where the curve begins to plateau. The time when the maximum amount of BrdU positive cells was labelled is equal to total cell cycle time (Tc) minus S-phase time (Ts). By extrapolating the curve back to time = 0, we can also find out the labelling index at Ts (LI0). With this information, the total cell cycle time can be estimated using the equation LI0/LIm = Ts/Tc.
Whole-mount in situ hybridization
Embryos treated with 0.003% of 1-phenyl-2-thiourea (Sigma) were fixed in 4% paraformaldehyde and processed as previously described [7]. The following antisense RNA probes were used: eng2b [80], fgf8 [81], foxb1.2 [82], egr2b (krox20) [83], islet1 [84], pax2a [85], otx2 [86] (kind gifts from Dr. Ashley Bruce); dmbx1a, dmbx1b and wnt1 (cloned from cDNA), and erm, pea3 [87] (kind gift from Dr. Herbert Steinbeisser); neurod, opn1sw2, pax6a, rho, and vsx2 (Open Biosystems). Embryos were cleared in glycerol before images were captured with a Leica MZ16F dissecting microscope (whole mounted samples) or a Leica DM4500B compound microscope (flat-mounted samples) with a QIMAGING digital camera and OpenLab software.
Anitsense morpholino and RNA injection
Antisense morpholinos (MOs) were obtained from Gene Tools, Inc. Dmbx1a-MO was complementary to the sequences that flanked the ATG start cordon as previously described [8], whereas dmbx1b-MO was targeted to sequences upstream of the start cordon in the 5' UTR. The sequences of the MOs are as follows, dmbx1a MO (MO1a): 5'-ACTCCGTAGTGCTGCATGATTCACA-3' and dmbx1b MO (MO1b): 5'-TCGAGCTTCTCTCTGGGAAGTTTTG-3'. A 5-mismatched nucleotides MO was also synthesized for both dmbx1a (mMO1a): 5'-ACTgCGTAcTGCTcCATcATTgACA-3' and dmbx1b (mMO1b): 5'-TCcAGCTTgTCTgTGcGAAcTTTTG-3' as controls. Unless otherwise noted, embryos were injected with 10 ng of a single MO, or 5 ng each of the combined MOs into the yolk at 1- to 2-cell stages.
Ectopic gene expression
Dmbx1aCDS primers (F:5'-ATGCAGCACTACGGAGTGAA-3' and R:5'-TCAGTTGGGCAGTGTGTCC-3') and Dmbx1bCDS primers (F:5'-ATGCAGCACTACGGGGTGA-3' and R: 5'-TTAGTTTGGTAGCGTGTCCAGG-3') amplified the full coding sequences of dmbx1a and dmbx1b, respectively, but which lack the 5'-UTR to avoid binding of the corresponding morpholinos. Both PCR fragments were cloned into pCS2+ and linearized with SacII for in vitro RNA transcriptions using the mMESSAGE mMACHINE SP6 kit (Ambion). Mouse Dmbx1 mRNA was synthesized from pCMV6-Kan/Neo plasmid containing the cloned full length mouse Dmbx1 cDNA (OriGene), but lacked 5'-UTR sequences corresponding to morpholino binding sites. The template was linearized with SacII and transcribed using the mMESSAGE mMACHINE T7 kit (Ambion). N-terminal Myc-tagged mouse Dmbx1 was subcloned using EcoRI and XbaI from pCMB6Kan/Neo-Dmbx1 into the pCS2+-MT plasmid, whereas zebrafish N-terminal Myc-dmbx1a was generated by cloning klenow-treated BamHI+XhoI pCS2+-dmbx1aCDS into CIAP-treated (Invitrogen) XbaI digested pCS2+-MT plasmid. RNA for Myc-Dmbx1 and Myc-dmbx1a were synthesized by linearlizing both plasmids with NotI, and then transcribed with the mMESSAGE mMACHINE SP6 kit (Ambion). RNA or RNA+MO were injected into the yolk of 1-cell stage embryos at the concentrations indicated.
Histology
Embryos were fixed in 4% paraformaldehyde and rinsed in phosphate-buffered saline solution after. Embryos were first dehydrated using increasing concentrations of ethanol, followed by embedding with increasing concentrations of Spurr's resin in ethanol. Embryos were then left to polymerization at 65°C in 100% Spurr's resin. Semithin coronal sections (approximately 1 μm thick) were cut with a glass knife using an ultramicrotome and dried onto glass slides. This procedure was followed by counterstaining with toluidine blue to visualize morphology. Whole-mount in situ hybridization embryos in 100% glycerol were washed with PBT and followed by the same embedding and sectioning steps as above. Sections were 1.5 micrometers thick without counterstaining to maximize visualization. To measure the area of the retina and optic tecta, five plastic sections with similar focal plane were chosen to represent each embryo, and images were taken on a Leica DM4500B compound microscope with a QIMAGING digital camera and OpenLab software. The areas of interest were outlined and measured using the program ImageJ (http://rsb.info.nih.gov/ij/) [88]. Results represent the average obtained from at least 5 embryos from each group. Statistical analyses between injected and un-injected groups were performed using student's t-test. Differences were regarded as significant for p < 0.05.
Immunohistochemistry
Two-three dpf embryos from each group (n = 10) were fixed with 4% paraformaldehyde overnight at 4°C and washed in sucrose series (from 5% to 30% sucrose in PBS) for cryoprotection. For PCNA labelling, embryos were fixed in 37% formaldehyde:95% ethanol (1:9 ratio) solution. Samples were left in 30% sucrose:OCT (2:1 ratio) at -20°C before cutting into 10-15 μm sections with a cryostat. Sections were re-hydrated with 1×PBS and blocked for 2 hours in 0.2% Triton X-100 + 2% goat serum in PBS at room temperature. Primary antibody in block solution was applied on sections overnight at 4°C. Slides were washed with PBS + 0.1% Tween-20 and incubated with secondary antibody (1:100 Cy2 or 1:500 Cy3, Jackson ImmunoResearch Laboratories, Inc.) for 2 hours at 4°C. Nuclei were counterstained with DAPI before mounting the slides. Tg(isl2b:GFP) embryos were used to examine retinal ganglion cells and no staining was performed after cryosectioning. Phospho-histone H3 antibody was conjugated to Alexa Fluor® 488 so no secondary antibody was required. The following primary antibodies were used: mouse anti-PCNA (1:100, ZYMED Laboratories), rabbit anti-Phospho-histone H3 (Ser10) (1:100, Cell Signaling), rabbit anti-Pax 6 (1:100, Covance), rabbit anti-PKC (1:100, Santa Cruz Biotechnology, Inc.), mouse anit-GS (1:500, Chemicon), mouse anti-Zpr1 (1:200, ZIRC), and mouse anti-Zpr3 (1:200, ZIRC). Images were taken from Leica TCS SP5 II Confocal Microscope and analyzed with Leica LAS AF software. For phospho-histone H3 positive cell count, we obtained three sections from different embryos of each group and took the average number for comparison.
Whole-mount antibody staining
Ten embryos were fixed in 4% paraformaldehyde at 4°C overnight and washed with PBS. Samples were incubated with block solution (PBS+1%BSA+1%DMSO+0.8% TritonX-100) for 1 hour at room temperature and overnight in anti-c-myc (9E10; Developmental Studies Hybridoma Bank) antibody (1:20) at 4°C. Embryos were then washed with PBS+Triton X-100 and incubated in goat anti-mouse HRP antibody (1:500; Jackson ImmunoResearch Laboratories, Inc.) overnight. Before DAB staining, samples were washed with PBS+Trition X-100 followed by PBS only. Peroxidase activity was detected with DAB and 3% hydrogen peroxide in the dark. Images of the stained embryos were taken with a Leica MZ16F dissecting microscope or a Leica DM4500B compound microscope.
Cell death analyses
Embryos from 24 - 72 hpf (n = 10) were bathed in embryo media that contained 5 μg/mL acridine orange (AO; Sigma) for 15 min at room temperature, and immediately followed by 4×10 min washes using regular media. AO-positive cells were imaged using a Leica DM4500B compound microscope with a QIMAGING digital camera and OpenLab software. For TUNEL labelling, embryos at 72 hpf (n = 10) were fixed in 4% paraformaldehyde at 4°C overnight followed by cryoprotection with sucrose and cryosectioning (same as immunohistochemistry). Sections were rehydrated and TUNEL assay was carried out according to manufacturer's instruction (Apo-Direct TUNEL Assay Kit, Millipore) and images were captured using Leica TCS SP5 II Confocal Microscope and analyzed with Leica LAS AF software.
Retinotectal projections
Tg(isl2b:GFP) larvae treated with 0.003% of 1-phenyl-2-thiourea (PTU) at 4dpf were immobilized on long cover slip and sandwiched with another smaller elevated cover slip in order to observe their retinotectal projections dorsally. Images were collected using Zeiss LSM 510 inverted confocal microscope.
Flow cytometry
Retinas from 60-80 embryos (~2 × 106 cells/mL) were dissected out from sample and control groups and left in Hank's Buffered Salt Solution (HBSS) with trypsin for 30 minutes. Cells were centrifuged, supernatant was discarded, and pellet was re-suspended in 50 μL of HBSS with 2% FBS. We next added 1 mL of ice-cold 80% ethanol and kept samples in -20°C for at least 30 minutes. Cells were collected by centrifugation and washed twice with HBSS + 2% FBS. Removed supernatant and added 500 μL of 0.1 mg/mL propidium iodide (Sigma) in HBSS with 0.6% NP-40, together with 500 μL of 2 mg/mL RNaseA and incubated in the dark for 30 minutes. Samples were filtered through 85 μm Nitex mesh filter and analyzed with BD FACSAria™ cell sorter. Flow cytometry data were analyzed using FlowJo software.
Phylogenetic analyses
An alignment of vertebrate Dmbx1 genes was assembled using BLAST searches of Ensembl (v.52) and NCBI (including both annotated genes and whole-genome shotgun reads). Amino acid-translated sequences were aligned using ProbCons [89], and trimmed using Gblocks (v0.91b; [90]) following the 'relaxed' trim procedure of [91]. This data set was subjected to a variety of phylogenetic analyses, including maximum likelihood, Bayesian inference, and neighbour-joining distance methods [92-94]. Maximum likelihood phylogenetic methods were implemented in the program PHYML v2.2.4 [93,95]. A WAG substitution model was assumed [96], including additional parameters for among-site rate heterogeneity (+G; [97]) and invariant sites (+I; [98]). A neighbour-joining tree [92]) was constructed in the MEGA4 program, with a Poisson distance correction [99]. For both the likelihood and neighbour-joining analyses, bootstrap analysis was used to assess the degree of confidence in nodes of the phylogeny [100]. 100 replicates were performed for the likelihood analysis, and 1000 replicates for the neighbour-joining analysis. Finally, Bayesian inference was performed in MrBayes 3.1.2 [94]. As with the likelihood methods, a WAG+I+G substitution model was assumed. For all parameters in the Bayesian analysis, uniform priors were used, except for branch lengths, for which exponential priors were implemented. Two independent analyses were performed, each composed of 4 Markov chains with default heating values. Markov chains were run until the average standard deviation of split frequencies, a measure of stationarity, dropped below 0.01, sampling trees (and parameters) every 1000 generations. Convergence was assessed using a number of methods. A convergence diagnostic for branch length posterior probabilities, the potential scale reduction factor (PSRF), roughly approached 1 as the runs converged [101]. Convergence to stationarity was also assessed by plotting log-likelihood scores and other parameter values in the program Tracer 1.4.1 to ensure that there were no trends in the data post burn-in [102]. Finally, adequacy of mixing was assessed by examining acceptance rates for parameters in MrBayes, and by calculating in Tracer effective sample sizes (ESS), the number of independent samples from the marginal posterior distribution for each parameter; higher values being indicative of better sampling from the posterior distribution. These values were all well above 100. By these measures convergence was achieved within the first 25% of trees sampled, which were discarded as burn-in, and remaining trees were taken as representative of the posterior probability distribution.
Molecular evolutionary analyses
Maximum likelihood phylogenetic methods were used to estimate the ratio of non-synonymous to synonymous rates (dN/dS) along lineages [46,103] in a pruned Dmbx1 phylogeny consisting of a subset of the sequences used for the phylogenetic analysis. This phylogeny was not only consistent with our analyses of the larger Dmbx1 data set, it is also congruent with current understanding of relationships among vertebrate taxa, assuming a teleost-specific genome duplication event [45]. dN/dS ratios can be used to estimate the form and strength of selection operating in the Dmbx1 gene family. Assuming no selection pressure, coding sequences will evolve neutrally, and nonsynonymous and synonymous substitutions should accumulate at equal rates, resulting in a dN/dS value equal to one [104,105]. Positive (or diversifying) selection is indicated by dN/dS values greater than one, while negative (or purifying) selection is indicated by dN/dS values near zero. Codon models that allow for variation in dN/dS along branches were implemented in the PAML package (v4.2a; [106]). Likelihood ratio tests were used to determine which among nested models provided a statistically significantly better fit to the data at hand [97,107].
Abbreviations
PBS: phosphate-buffered saline; PBT: PBS with Triton-X100; HCl: hydrochloric acid; dNTP: deoxy-nucleotide-triphosphates, where the nucleotide is an equal mixture of adenine, guanine, cytosine, and thymine; FBS: fetal bovine serum.
Authors' contributions
LW contributed to the design of the experiments, performed all of the molecular experiments, immunolabeling, and microinjections and participated in the data analysis and drafting the manuscript. CK performed the semi-thin plastic sectioning. CJW and BSWC carried out the phylogenetic and molecular evolution analyses and analyzed the data. VT conceived of the study, and participated in its design, coordination and data analysis, drafted and edited the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Altered midbrain gene expression in morphant embryos. Lateral (A-F, J-L) and dorsal (G-I, M-O) views anterior to the left, or anterior views (P-R) dorsal to the top of embryos either un-injected (A, D, G, J, M, P) or injected with MO1a + MO1b (B, E, H, K, N, Q) or control mMO1a + mMO1b (C, F, I, L, O, R). All embryos are at 48 hpf, except for J-L, which are at 24 hpf. Arrowhead in B and E indicates the reduced expression in the dorsal midbrain and arrow in E indicates reduced expression in the hindbrain. Arrowhead in G, H and M, N demarcates the position of the MHB. Arrowhead in P, Q indicates the optic stalk region. MO, morpholino; mMO, mismatch morpholino; MHB, midbrain-hindbrain boundary; hpf, hours post-fertilization.
Midbrain-hindbrain boundary is unaffected in dmbx1 morphants. Lateral view (anterior to the left) of embryos at 24 hpf (A-F, M-O) or 48 hpf (G-L). Analysis of genes normally expressed in the midbrain-hindbrain boundary region in un-injected (A, D, G, J, M), MO1a + MO1b injected (B, E, H, K, N), and mMO1a + mMO1b injected (C, F, I, L, O) embryos. Arrowheads demarcate the position of the midbrain-hindbrain boundary. MO, morpholino; mMO, mismatch morpholino; hpf, hours post-fertilization.
Dose-dependent changes in foxb1.2 and rhodopsin gene expression in dmbx1 morphants. Analysis of gene expression at 72 hpf in un-injected, MO1a injected, MO1b injected or MO1a + MO1b injected embryos using the MO concentrations listed. For foxb1.2 expression, embryos are shown in lateral view with anterior to the left. For rhodopsin expression, embryos are shown in ventral view, anterior to the left. Control embryos injected with mismatch MOs at similar concentrations showed no change in expression and are not shown. MO, morpholino; hpf, hours post-fertilization
Distinct patterns of rhodopsin expression and retinotectal projections in dmbx1a and dmbx1b morphant embryos. Dorsal view anterior to the top of un-injected (A, B), MO1a injected (C, D), MO1b injected (E, F), and MO1a + MO1b injected (G, H) embryos demonstrating expression of rhodopsin (rho) (A, C, E, G) or the retinotectal projection pattern (green fluorescence) as defined in isl2b:GFP transgenic embryos (B, D, F, H). The mismatch control injected morpholinos resembled the un-injected controls and are not shown. In B, D, F, H, white arrow demarcates the terminal field of the retinal ganglion cells in the optic tectum and the white arrowhead demarcates axonal fibers of the optic nerve. MO, morpholino. Asterisk demarcates the region of trigeminal ganglion.
Cell death does not persist in dmbx1 morphant embryos. Live embryos at 24 hpf (A, B, lateral view anterior to the left), 48 hpf (C, D, dorsal view anterior to the left) and 72 hpf (E, F, dorsal view anterior to the left; K, L, close up of retina lateral view dorsal to the top) were examined for the presence of apoptotic cells using AO. Arrows point to AO+ cells in the midbrain (bright spots). TUNEL+ cells (green) or Caspase3+ cells (red) on cryosectioned tissue of 72 hpf midbrains (G-J) and retina (M-P, arrow pointing to labelled cell) counter-stained with DAPI (blue). (MO, morpholino; mMO, mismatch morpholino; hpf, hours post-fertilization; AO, acridine orange.
Zebrafish, but not mouse, Dmbx1 mRNA counteracts the zebrafish knockdown phenotype. (A) Representative images of single embryos, lateral view anterior to the left from the different groups analyzed for a dorsalization phenotype. Embryos were injected with the mRNA concentrations shown, co-injected with 10 ng of either MO1a or MO1b as indicated, and scored for a dorsalized phenotype at 24 hpf. Tabulated results for all groups from 2-5 separate experiments using (B) zebrafish mRNA, or (C) mouse mRNA. MO, morpholino; hpf, hours post-fertilization.
Partial rescue of dmbx1a morphant with dmbx1b mRNA. (A) Un-injected and (B) MO1a injected embryos showing foxb1.2 expression at 48 hpf. Note, the data in these two panels are identical to panels (A) and (B) shown in Figure 9; they were duplicated here for ease of reference. Representative images of embryos co-injected with MO1a and either dmbx1a mRNA (C) or dmbx1b mRNA (D). Dorsal view of embryos, anterior to the top. Black arrows in all panels point to the anterolateral hindbrain region where foxb1.2 is normally expressed. White arrows in all panels point to the dorsal midbrain. MO, morpholino.
Monitoring mouse Myc-Dmbx1 and zebrafish Myc-dmbx1a levels in vivo. Representative images of an (A) un-injected, (B) mouse myc-Dmbx1 mRNA injected (400 pg), and (C) zebrafish myc-dmbx1a mRNA injected (150 pg) tailbud stage embryo (lateral view) processed for whole-mount immunolabeling using an anti-Myc antibody (n = 10 per group). Panels D-F are representative high magnification images of embryos in each of the three treatment groups; black arrows indicate nuclei that are DAB+ in the injected groups, but lack DAB staining in the un-injected group.
Phylogenetic analysis of vertebrate Dmbx1 genes. Phylogram showing the neighbour-joining tree topology, with support values indicated for each node in the tree, for the three different phylogenetic methods of analysis employed. Above the nodes are neighbour-joining (1000 replicates), followed by likelihood bootstrap percentages (100 replicates); below the nodes are the Bayesian posterior probabilities. Bootstrap percentages below 50% are indicated by an asterisk.
Molecular evolutionary analysis of vertebrate Dmbx1 genes. Proportions of non-synonymous to synonymous rates (dN/dS) along lineages in the Dmbx1 phylogeny were estimated using codon-based maximum likelihood phylogenetic methods. Results of a branch model in which the post-duplication branches (PDBs) leading to the dmbx1a and dmbx1b clades were each allowed to have independently estimated dN/dS values are shown. Both PDBs display elevated dN/dS estimates compared to the background estimate applied to the remainder of the phylogeny. This model fit the data significantly better than a model with a single dN/dS parameter (M0 model; p < 0.01, d.f. = 2). Increases in dN/dS along each of the PDBs were also confirmed through simpler branch models in which only a single PDB, either for dmbx1a or dmbx1b, received separately estimated dN/dS values; in both cases the increases in dN/dS was statistically significant (M0; p < 0.05, d.f. = 1). Branch lengths shown in this figure were estimated under the 2 PDB branch model under which the dN/dS estimates were derived, and are proportional to the number of substitutions per codon.
Contributor Information
Loksum Wong, Email: loksum.wong@utoronto.ca.
Cameron J Weadick, Email: cameron.weadick@utoronto.ca.
Claire Kuo, Email: claire.kuo@utoronto.ca.
Belinda SW Chang, Email: belinda.chang@utoronto.ca.
Vincent Tropepe, Email: v.tropepe@utoronto.ca.
Acknowledgements
We would like thank Dr. Ashley Bruce and Dr. Herbert Steinbeisser for probe constructs, Dr. Chi-Bin Chien for the isl2b-GFP transgenic fish, Henry Hong for assisting us with our histological analyses, Dr. Brian Link for advice on BrdU labelling, and the Zebrafish International Resource Center for reagents (P40 RR012546 from the NIH-NCRR). We are grateful to members of the Tropepe and Bruce labs for helpful discussions and suggestions throughout the course of this work, and to Dr. Ashley Bruce and Dr. Tony Harris for their helpful comments on an earlier version of this manuscript. Funding for this research was supported by a University of Toronto Vision Science Research Program Scholarship (L.W.), and by grants from NSERC, Canada Foundation for Innovation, and Ontario Innovations Trust, and the Foundation Fighting Blindness - Canada (V.T.).
References
- Broccoli V, Colombo E, Cossu G. Dmbx1 is a paired-box containing gene specifically expressed in the caudal most brain structures. Mech Dev. 2002;114:219–223. doi: 10.1016/S0925-4773(02)00078-3. [DOI] [PubMed] [Google Scholar]
- Gogoi RN, Schubert FR, J-P Martinez-Barbera, Acampora D, Simeone A, Lumsden A. The paired-type homeobox gene Dmbx1 marks the midbrain and pretectum. Mech Dev. 2002;114:213–217. doi: 10.1016/S0925-4773(02)00067-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Kawahara A, Teufel A, Mukhopadhyay M, Zhao Y, Dawid IB, Westphal H. Mbx, a novel mouse homeobox gene. Dev Genes Evol. 2002;212:104–106. doi: 10.1007/s00427-002-0217-4. [DOI] [PubMed] [Google Scholar]
- Ohtoshi A, Nishijima I, Justice MJ, Behringer RR. Dmbx1, a novel evolutionarily conserved paired-like homeobox gene expressed in the brain of mouse embryos. Mech Dev. 2002;110:241–4. doi: 10.1016/S0925-4773(01)00587-1. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Holland PWH, Cohn MJ, Shimizu K, Kurokawa M, Hirai H. An orphan PRD class homeobox gene expressed in mouse brain and limb development. Dev Genes Evol. 2002;212:293–297. doi: 10.1007/s00427-002-0244-1. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Miki T, Iwanaga T, Koseki Y, Okuno M, Sunaga Y, Ozaki N, Yano H, Koseki H, Seino S. Identification, tissue expression, and functional characterization of Otx3, a novel member of the Otx family. J Biol Chem. 2002;277:28065–28069. doi: 10.1074/jbc.C100767200. [DOI] [PubMed] [Google Scholar]
- Chang L, Khoo B, Wong L, Tropepe V. Genomic sequence and spatiotemporal expression comparison of zebrafish mbx1 and its paralog, mbx2. Dev Genes Evol. 2006;216:647–654. doi: 10.1007/s00427-006-0082-7. [DOI] [PubMed] [Google Scholar]
- Kawahara A, C-B Chien, Dawid IB. The homeobox gene mbx is involved in eye and tectum development. Dev Biol. 2002;248:107–117. doi: 10.1006/dbio.2002.0709. [DOI] [PubMed] [Google Scholar]
- Ohtoshi A, Behringer RR. Neonatal lethality, dwarfism, and abnormal brain development in Dmbx1 mutant mice. Mol Cell Biol. 2004;24:7548–7558. doi: 10.1128/MCB.24.17.7548-7558.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto W, Shiuchi T, Miki T, Minokoshi Y, Takahashi Y, Takeuchi A, Kimura K, Saito M, Iwanaga T, Seino S. Dmbx1 is essential in agouti-related protein action. Proc Natl Acad Sci USA. 2007;104:15514–15519. doi: 10.1073/pnas.0707328104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang K, Martindale MQ. Developmental expression of homeobox genes in the ctenophore Mnemiopsis leidyi. Dev Genes Evol. 2008;218:307–319. doi: 10.1007/s00427-008-0222-3. [DOI] [PubMed] [Google Scholar]
- Larroux C, Luke GN, Koopman P, Rokhsar DS, Shimeld SM, Degnan BM. Genesis and expansion of metazoan transcription factor gene classes. Mol Biol Evol. 2008;25:980–996. doi: 10.1093/molbev/msn047. [DOI] [PubMed] [Google Scholar]
- Ryan JF, Burton PM, Mazza ME, Kwong GK, Mullikin JC, Finnerty JR. The cnidarian-bilaterian ancestor possessed at least 56 homeoboxes: evidence from the starlet sea anemone, Nematostella vectensis. Genome Biol. 2006;7(7):R64. doi: 10.1186/gb-2006-7-7-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelhof AC, Koundakjian E, Scully AL, Hardy RW, Pounds L. Mutation of the photoreceptor specific homeodomain gene Pph13 results in defects in phototransduction and rhabdomere morphogenesis. Development. 2003;130:4383–4392. doi: 10.1242/dev.00651. [DOI] [PubMed] [Google Scholar]
- Friedman R, Hughes AL. Pattern and timing of gene duplication in animal genomes. Genome Res. 2001;11:1842–1847. doi: 10.1101/gr.155801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M, Conery JS. The evolutionary fate and consequences of duplicate genes. Science. 2000;290:1151–1155. doi: 10.1126/science.290.5494.1151. [DOI] [PubMed] [Google Scholar]
- Gu Z, Steinmetz LM, Gu X, Scharfe C, Davis RW, Li W-H. Role of duplicate genes in genetic robustness against null mutations. Nature. 2003;421:63–66. doi: 10.1038/nature01198. [DOI] [PubMed] [Google Scholar]
- Conant GC, Wagner A. Duplicate genes and robustness to transient gene knock-downs in Caenorhabditis elegans. Proc Biol Sci. 2004;271:89–96. doi: 10.1098/rspb.2003.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Li W-H. Gene essentiality, gene duplicability and protein connectivity in human and mouse. Trends Genet. 2007;23:375–378. doi: 10.1016/j.tig.2007.04.005. [DOI] [PubMed] [Google Scholar]
- B-Y Liao, Zhang J. Mouse duplicate genes are as essential as singletons. Trends Genet. 2007;23:378–381. doi: 10.1016/j.tig.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Hanks M, Wurst W, Anson-Cartwright L, Auerbach AB, Joyner AL. Rescue of the En-1 mutant phenotype by replacement of En-1 with En-2. Science. 1995;269:679–682. doi: 10.1126/science.7624797. [DOI] [PubMed] [Google Scholar]
- Tvrdik P, Capecchi MR. Reversal of Hox1 gene subfunctionalization in the mouse. Dev Cell. 2006;11:239–250. doi: 10.1016/j.devcel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Hughes AL. The evolution of functionally novel proteins after gene duplication. Proc Biol Sci. 1994;256:119–24. doi: 10.1098/rspb.1994.0058. [DOI] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AE, Oates AC, Prince VE, Ho RK. Additional hox clusters in the zebrafish: divergent expression patterns belie equivalent activities of duplicate hoxB5 genes. Evol Dev. 2001;3:127–144. doi: 10.1046/j.1525-142x.2001.003003127.x. [DOI] [PubMed] [Google Scholar]
- McClintock JM, Kheirbek MA, Prince VE. Knockdown of duplicated zebrafish hoxb1 genes reveals distinct roles in hindbrain patterning and a novel mechanism of duplicate gene retention. Development. 2002;129:2339–2354. doi: 10.1242/dev.129.10.2339. [DOI] [PubMed] [Google Scholar]
- Liu R-Z, Sharma MK, Sun Q, Thisse C, Thisse B, Denovan-Wright EM, Wright JM. Retention of the duplicated cellular retinoic acid-binding protein 1 genes (crabp1a and crabp1b) in the zebrafish genome by subfunctionalization of tissue-specific expression. FEBS J. 2005;272:3561–3571. doi: 10.1111/j.1742-4658.2005.04775.x. [DOI] [PubMed] [Google Scholar]
- Kleinjan DA, Bancewicz RM, Gautier P, Dahm R, Schonthaler HB, Damante G, Seawright A, Hever AM, Yeyati PL, van Heyningen V. et al. Subfunctionalization of duplicated zebrafish pax6 genes by cis-regulatory divergence. PLoS Genet. 2008;4(2):e29. doi: 10.1371/journal.pgen.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. Evolution by gene duplication. Berlin, New York,: Springer-Verlag; 1970. [Google Scholar]
- Chain FJJ, Evans BJ. Multiple mechanisms promote the retained expression of gene duplicates in the tetraploid frog Xenopus laevis. PLoS Genet. 2006;2(4):e56. doi: 10.1371/journal.pgen.0020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock PF, Raymond PA. The teleost retina as a model for developmental and regeneration biology. Zebrafish. 2004;1:257–71. doi: 10.1089/zeb.2004.1.257. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955. doi: 10.1016/S0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Macdonald R, Barth KA, Xu Q, Holder N, Mikkola I, Wilson SW. Midline signalling is required for Pax gene regulation and patterning of the eyes. Development. 1995;121:3267–3278. doi: 10.1242/dev.121.10.3267. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochocinska MJ, Hitchcock PF. Dynamic expression of the basic helix-loop-helix transcription factor neuroD in the rod and cone photoreceptor lineages in the retina of the embryonic and larval zebrafish. J Comp Neurol. 2007;501:1–12. doi: 10.1002/cne.21150. [DOI] [PubMed] [Google Scholar]
- Pittman AJ, Law MY, Chien CB. Pathfinding in a large vertebrate axon tract: isotypic interactions guide retinotectal axons at multiple choice points. Development. 2008;135:2865–71. doi: 10.1242/dev.025049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arama E, Steller H. Detection of apoptosis by terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling and acridine orange in Drosophila embryos and adult male gonads. Nat Protoc. 2006;1:1725–31. doi: 10.1038/nprot.2006.235. [DOI] [PubMed] [Google Scholar]
- Candal E, Anadon R, DeGrip WJ, Rodriguez-Moldes I. Patterns of cell proliferation and cell death in the developing retina and optic tectum of the brown trout. Brain Res Dev Brain Res. 2005;154:101–19. doi: 10.1016/j.devbrainres.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, Knipp S. Proliferation pattern changes in the zebrafish brain from embryonic through early postembryonic stages. Anat Embryol (Berl) 2000;202:385–400. doi: 10.1007/s004290000115. [DOI] [PubMed] [Google Scholar]
- Marcus RC, Delaney CL, Easter SS. Neurogenesis in the visual system of embryonic and adult zebrafish (Danio rerio). off. Vis Neurosci. 1999;16:417–424. doi: 10.1017/S095252389916303X. [DOI] [PubMed] [Google Scholar]
- Cotton JA, Page RD. Going nuclear: gene family evolution and vertebrate phylogeny reconciled. Proc Biol Sci. 2002;269:1555–61. doi: 10.1098/rspb.2002.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettai A, di Prisco G, Lecointre G, Parisi E, Verde C. Inferring evolution of fish proteins: the globin case study. Methods Enzymol. 2008;436:539–570. doi: 10.1016/S0076-6879(08)36030-3. full_text. [DOI] [PubMed] [Google Scholar]
- Setiamarga DHE, Miya M, Yamanoue Y, Mabuchi K, Satoh TP, Inoue JG, Nishida M. Interrelationships of Atherinomorpha (medakas, flyingfishes, killifishes, silversides, and their relatives): The first evidence based on whole mitogenome sequences. Mol Phylogenet Evol. 2008;49:598–605. doi: 10.1016/j.ympev.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Springer MS, Stanhope MJ, Madsen O, de Jong WW. Molecules consolidate the placental mammal tree. Trends Ecol Evol. 2004;19:430–8. doi: 10.1016/j.tree.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Taylor JS, Van de Peer Y, Braasch I, Meyer A. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos Trans R Soc Lond B Biol Sci. 2001;356:1661–1679. doi: 10.1098/rstb.2001.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Nielsen R. Synonymous and nonsynonymous rate variation in nuclear genes of mammals. J Mol Evol. 1998;46:409–418. doi: 10.1007/PL00006320. [DOI] [PubMed] [Google Scholar]
- Nyholm MK, Wu S-F, Dorsky RI, Grinblat Y. The zebrafish zic2a-zic5 gene pair acts downstream of canonical Wnt signaling to control cell proliferation in the developing tectum. Development. 2007;134:735–746. doi: 10.1242/dev.02756. [DOI] [PubMed] [Google Scholar]
- Yarden A, Salomon D, Geiger B. Zebrafish cyclin D1 is differentially expressed during early embryogenesis. Biochim Biophys Acta. 1995;1264:257–260. doi: 10.1016/0167-4781(95)00175-1. [DOI] [PubMed] [Google Scholar]
- Wehman AM, Staub W, Baier H. The anaphase-promoting complex is required in both dividing and quiescent cells during zebrafish development. Dev Biol. 2007;303:144–156. doi: 10.1016/j.ydbio.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Levine EM, Green ES. Cell-intrinsic regulators of proliferation in vertebrate retinal progenitors. Sem Cell Develop Biol. 2004;15:63–74. doi: 10.1016/j.semcdb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Rhinn M, Brand M. The midbrain--hindbrain boundary organizer. Curr Opin Neurobiol. 2001;11:34–42. doi: 10.1016/S0959-4388(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Raible F, Brand M. Divide et Impera--the midbrain-hindbrain boundary and its organizer. Trends Neurosci. 2004;27:727–734. doi: 10.1016/j.tins.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Lun K, Brand M. A series of no isthmus (noi) alleles of the zebrafish pax2.1 gene reveals multiple signaling events in development of the midbrain-hindbrain boundary. Development. 1998;125:3049–62. doi: 10.1242/dev.125.16.3049. [DOI] [PubMed] [Google Scholar]
- Pfeffer PL, Gerster T, Lun K, Brand M, Busslinger M. Characterization of three novel members of the zebrafish Pax2/5/8 family: dependency of Pax5 and Pax8 expression on the Pax2.1 (noi) function. Development. 1998;125:3063–3074. doi: 10.1242/dev.125.16.3063. [DOI] [PubMed] [Google Scholar]
- Belting HG, Hauptmann G, Meyer D, Abdelilah-Seyfried S, Chitnis A, Eschbach C, Soll I, Thisse C, Thisse B, Artinger KB. et al. spiel ohne grenzen/pou2 is required during establishment of the zebrafish midbrain-hindbrain boundary organizer. Development. 2001;128:4165–4176. doi: 10.1242/dev.128.21.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E, Araki I, Nakamura H. Pax6 defines the di-mesencephalic boundary by repressing En1 and Pax2. Development. 2000;127:2357–2365. doi: 10.1242/dev.127.11.2357. [DOI] [PubMed] [Google Scholar]
- Nakamura H. Regionalisation and acquisition of polarity in the optic tectum. Prog Neurobiol. 2001;65:473–488. doi: 10.1016/S0301-0082(01)00015-6. [DOI] [PubMed] [Google Scholar]
- Kimura K, Miki T, Shibasaki T, Zhang Y, Ogawa M, Saisho H, Okuno M, Iwanaga T, Seino S. Functional analysis of transcriptional repressor Otx3/Dmbx1. FEBS Lett. 2005;579:2926–32. doi: 10.1016/j.febslet.2005.04.042. [DOI] [PubMed] [Google Scholar]
- Labosky PA, Winnier GE, Jetton TL, Hargett L, Ryan AK, Rosenfeld MG, Parlow AF, Hogan BL. The winged helix gene, Mf3, is required for normal development of the diencephalon and midbrain, postnatal growth and the milk-ejection reflex. Development. 1997;124:1263–1274. doi: 10.1242/dev.124.7.1263. [DOI] [PubMed] [Google Scholar]
- Wehr R, Mansouri A, de Maeyer T, Gruss P. Fkh5-deficient mice show dysgenesis in the caudal midbrain and hypothalamic mammillary body. Development. 1997;124:4447–4456. doi: 10.1242/dev.124.22.4447. [DOI] [PubMed] [Google Scholar]
- Winkler C, Schafer M, Duschl J, Schartl M, Volff JN. Functional divergence of two zebrafish midkine growth factors following fish-specific gene duplication. Genome Res. 2003;13:1067–1081. doi: 10.1101/gr.1097503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baye LM, Link BA. The disarrayed mutation results in cell cycle and neurogenesis defects during retinal development in zebrafish. BMC Dev Biol. 2007;7:28–28. doi: 10.1186/1471-213X-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Prykhozhij S, Rau MJ, Neumann CJ. Mutation of zebrafish caf-1b results in S phase arrest, defective differentiation and p53-mediated apoptosis during organogenesis. Cell Cycle. 2007;6:2963–2969. doi: 10.4161/cc.6.23.4950. [DOI] [PubMed] [Google Scholar]
- Shen Y-c, Raymond PA. Zebrafish cone-rod (crx) homeobox gene promotes retinogenesis. Dev Biol. 2004;269:237–251. doi: 10.1016/j.ydbio.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Ghanem N, Jarinova O, Amores A, Long Q, Hatch G, Park BK, Rubenstein JLR, Ekker M. Regulatory roles of conserved intergenic domains in vertebrate Dlx bigene clusters. Genome Res. 2003;13:533–543. doi: 10.1101/gr.716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem N, Yu M, Long J, Hatch G, Rubenstein JLR, Ekker M. Distinct cis-regulatory elements from the Dlx1/Dlx2 locus mark different progenitor cell populations in the ganglionic eminences and different subtypes of adult cortical interneurons. J Neurosci. 2007;27:5012–5022. doi: 10.1523/JNEUROSCI.4725-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock JM, Carlson R, Mann DM, Prince VE. Consequences of Hox gene duplication in the vertebrates: an investigation of the zebrafish Hox paralogue group 1 genes. Development. 2001;128:2471–2484. doi: 10.1242/dev.128.13.2471. [DOI] [PubMed] [Google Scholar]
- Hurley IA, Scemama J-L, Prince VE. Consequences of hoxb1 duplication in teleost fish. Evol Dev. 2007;9:540–554. doi: 10.1111/j.1525-142X.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- Elsen GE, Choi LY, Millen KJ, Grinblat Y, Prince VE. Zic1 and Zic4 regulate zebrafish roof plate specification and hindbrain ventricle morphogenesis. Dev Biol. 2008;314:376–392. doi: 10.1016/j.ydbio.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PW, Takahashi T. The evolution of homeobox genes: Implications for the study of brain development. Brain Res Bull. 2005;66:484–90. doi: 10.1016/j.brainresbull.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Holland PWH. Amphioxus and ascidian Dmbx homeobox genes give clues to the vertebrate origins of midbrain development. Development. 2004;131:3285–3294. doi: 10.1242/dev.01201. [DOI] [PubMed] [Google Scholar]
- Takahashi T. The evolutionary origins of vertebrate midbrain and MHB: insights from mouse, amphioxus and ascidian Dmbx homeobox genes. Brain Res Bull. 2005;66:510–517. doi: 10.1016/j.brainresbull.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Ikuta T, Saiga H. Dynamic change in the expression of developmental genes in the ascidian central nervous system: revisit to the tripartite model and the origin of the midbrain-hindbrain boundary region. Dev Biol. 2007;312:631–643. doi: 10.1016/j.ydbio.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Nozawa M, Suzuki Y, Nei M. Reliabilities of identifying positive selection by the branch-site and site-prediction methods. Proc Natl Acad Sci USA. 2009;106:6700–6705. doi: 10.1073/pnas.0901855106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conant GC, Wolfe KH. Turning a hobby into a job: how duplicated genes find new functions. Nature Rev Genet. 2008;9:938–950. doi: 10.1038/nrg2482. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Westerfield M. THE ZEBRAFISH BOOK - A guide for the laboratory use of zebrafish (Danio rerio) 5. Eugene: University of Oregon Press; 2007. [Google Scholar]
- Morshead CM, van der Kooy D. Postmitotic death is the fate of constitutively proliferating cells in the subependymal layer of the adult mouse brain. J Neurosci. 1992;12:249–256. doi: 10.1523/JNEUROSCI.12-01-00249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Shen J. Presenilins are required for maintenance of neural stem cells in the developing brain. Mol Neurodegener. 2008;3:2. doi: 10.1186/1750-1326-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker M, Wegner J, Akimenko MA, Westerfield M. Coordinate embryonic expression of three zebrafish engrailed genes. Development. 1992;116:1001–1010. doi: 10.1242/dev.116.4.1001. [DOI] [PubMed] [Google Scholar]
- Reifers F, Böhli H, Walsh EC, Crossley PH, Stainier DYR, Brand M. Fgf8 is mutated in zebrafish acerebellar mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Moens CB, Yan YL, Appel B, Force AG, Kimmel CB. Valentino: a zebrafish gene required for normal hindbrain segmentation. Development. 1996;122:3981–3990. doi: 10.1242/dev.122.12.3981. [DOI] [PubMed] [Google Scholar]
- Oxtoby E, Jowett T. Cloning of the zebrafish krox-20 gene (krx-20) and its expression during hindbrain development. Nucleic Acids Res. 1993;21:1087–1095. doi: 10.1093/nar/21.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Takahashi M, Hatta K, Hotta Y, Okamoto H. Developmental regulation of islet-1 mRNA expression during neuronal differentiation in embryonic zebrafish. Dev Dyn. 1994;199:1–11. doi: 10.1002/aja.1001990102. [DOI] [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Fjose A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development. 1991;113:1193–1206. doi: 10.1242/dev.113.4.1193. [DOI] [PubMed] [Google Scholar]
- Mercier P, Simeone A, Cotelli F, Boncinelli E. Expression pattern of two otx genes suggests a role in specifying anterior body structures in zebrafish. Int J Dev Biol. 1995;39:559–573. [PubMed] [Google Scholar]
- Munchberg SR, Ober EA, Steinbeisser H. Expression of the Ets transcription factors erm and pea3 in early zebrafish development. Mech Dev. 1999;88(2):233–6. doi: 10.1016/S0925-4773(99)00179-3. [DOI] [PubMed] [Google Scholar]
- Abramoff M, Magelhaes P, Ram S. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Do CB, Mahabhashyam MSP, Brudno M, Batzoglou S. ProbCons: Probabilistic consistency-based multiple sequence alignment. Genome Res. 2005;15:330–340. doi: 10.1101/gr.2821705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Guindon S, Lethiec F, Duroux P, Gascuel O. PHYML Online--a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res. 2005;33:557–559. doi: 10.1093/nar/gki352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol. 2001;18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- Yang Z. Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J Mol Evol. 1994;39:306–314. doi: 10.1007/BF00160154. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- Gelman A, Rubin DB. Inference from iterative simulation using multiple sequences. Statistical Science. 1992;7:457–511. doi: 10.1214/ss/1177011136. [DOI] [Google Scholar]
- Rambaut A, Drummond AJ. Tracer v1.4. Book Tracer v1.4. 2007. City.
- Yang Z. Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol Biol Evol. 1998;15:568–73. doi: 10.1093/oxfordjournals.molbev.a025957. [DOI] [PubMed] [Google Scholar]
- Kimura M. The neutral theory of molecular evolution. Cambridge [Cambridgeshire]; New York: Cambridge University Press; 1983. [Google Scholar]
- Yang Z, Bielawski JP. Statistical methods for detecting molecular adaptation. Trends Ecol Evol. 2000;15:496–503. doi: 10.1016/S0169-5347(00)01994-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–91. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- Navidi WC, Churchill GA, von Haeseler A. Methods for inferring phylogenies from nucleic acid sequence data by using maximum likelihood and linear invariants. Mol Biol Evol. 1991;8:128–143. doi: 10.1093/oxfordjournals.molbev.a040633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Altered midbrain gene expression in morphant embryos. Lateral (A-F, J-L) and dorsal (G-I, M-O) views anterior to the left, or anterior views (P-R) dorsal to the top of embryos either un-injected (A, D, G, J, M, P) or injected with MO1a + MO1b (B, E, H, K, N, Q) or control mMO1a + mMO1b (C, F, I, L, O, R). All embryos are at 48 hpf, except for J-L, which are at 24 hpf. Arrowhead in B and E indicates the reduced expression in the dorsal midbrain and arrow in E indicates reduced expression in the hindbrain. Arrowhead in G, H and M, N demarcates the position of the MHB. Arrowhead in P, Q indicates the optic stalk region. MO, morpholino; mMO, mismatch morpholino; MHB, midbrain-hindbrain boundary; hpf, hours post-fertilization.
Midbrain-hindbrain boundary is unaffected in dmbx1 morphants. Lateral view (anterior to the left) of embryos at 24 hpf (A-F, M-O) or 48 hpf (G-L). Analysis of genes normally expressed in the midbrain-hindbrain boundary region in un-injected (A, D, G, J, M), MO1a + MO1b injected (B, E, H, K, N), and mMO1a + mMO1b injected (C, F, I, L, O) embryos. Arrowheads demarcate the position of the midbrain-hindbrain boundary. MO, morpholino; mMO, mismatch morpholino; hpf, hours post-fertilization.
Dose-dependent changes in foxb1.2 and rhodopsin gene expression in dmbx1 morphants. Analysis of gene expression at 72 hpf in un-injected, MO1a injected, MO1b injected or MO1a + MO1b injected embryos using the MO concentrations listed. For foxb1.2 expression, embryos are shown in lateral view with anterior to the left. For rhodopsin expression, embryos are shown in ventral view, anterior to the left. Control embryos injected with mismatch MOs at similar concentrations showed no change in expression and are not shown. MO, morpholino; hpf, hours post-fertilization
Distinct patterns of rhodopsin expression and retinotectal projections in dmbx1a and dmbx1b morphant embryos. Dorsal view anterior to the top of un-injected (A, B), MO1a injected (C, D), MO1b injected (E, F), and MO1a + MO1b injected (G, H) embryos demonstrating expression of rhodopsin (rho) (A, C, E, G) or the retinotectal projection pattern (green fluorescence) as defined in isl2b:GFP transgenic embryos (B, D, F, H). The mismatch control injected morpholinos resembled the un-injected controls and are not shown. In B, D, F, H, white arrow demarcates the terminal field of the retinal ganglion cells in the optic tectum and the white arrowhead demarcates axonal fibers of the optic nerve. MO, morpholino. Asterisk demarcates the region of trigeminal ganglion.
Cell death does not persist in dmbx1 morphant embryos. Live embryos at 24 hpf (A, B, lateral view anterior to the left), 48 hpf (C, D, dorsal view anterior to the left) and 72 hpf (E, F, dorsal view anterior to the left; K, L, close up of retina lateral view dorsal to the top) were examined for the presence of apoptotic cells using AO. Arrows point to AO+ cells in the midbrain (bright spots). TUNEL+ cells (green) or Caspase3+ cells (red) on cryosectioned tissue of 72 hpf midbrains (G-J) and retina (M-P, arrow pointing to labelled cell) counter-stained with DAPI (blue). (MO, morpholino; mMO, mismatch morpholino; hpf, hours post-fertilization; AO, acridine orange.
Zebrafish, but not mouse, Dmbx1 mRNA counteracts the zebrafish knockdown phenotype. (A) Representative images of single embryos, lateral view anterior to the left from the different groups analyzed for a dorsalization phenotype. Embryos were injected with the mRNA concentrations shown, co-injected with 10 ng of either MO1a or MO1b as indicated, and scored for a dorsalized phenotype at 24 hpf. Tabulated results for all groups from 2-5 separate experiments using (B) zebrafish mRNA, or (C) mouse mRNA. MO, morpholino; hpf, hours post-fertilization.
Partial rescue of dmbx1a morphant with dmbx1b mRNA. (A) Un-injected and (B) MO1a injected embryos showing foxb1.2 expression at 48 hpf. Note, the data in these two panels are identical to panels (A) and (B) shown in Figure 9; they were duplicated here for ease of reference. Representative images of embryos co-injected with MO1a and either dmbx1a mRNA (C) or dmbx1b mRNA (D). Dorsal view of embryos, anterior to the top. Black arrows in all panels point to the anterolateral hindbrain region where foxb1.2 is normally expressed. White arrows in all panels point to the dorsal midbrain. MO, morpholino.
Monitoring mouse Myc-Dmbx1 and zebrafish Myc-dmbx1a levels in vivo. Representative images of an (A) un-injected, (B) mouse myc-Dmbx1 mRNA injected (400 pg), and (C) zebrafish myc-dmbx1a mRNA injected (150 pg) tailbud stage embryo (lateral view) processed for whole-mount immunolabeling using an anti-Myc antibody (n = 10 per group). Panels D-F are representative high magnification images of embryos in each of the three treatment groups; black arrows indicate nuclei that are DAB+ in the injected groups, but lack DAB staining in the un-injected group.
Phylogenetic analysis of vertebrate Dmbx1 genes. Phylogram showing the neighbour-joining tree topology, with support values indicated for each node in the tree, for the three different phylogenetic methods of analysis employed. Above the nodes are neighbour-joining (1000 replicates), followed by likelihood bootstrap percentages (100 replicates); below the nodes are the Bayesian posterior probabilities. Bootstrap percentages below 50% are indicated by an asterisk.
Molecular evolutionary analysis of vertebrate Dmbx1 genes. Proportions of non-synonymous to synonymous rates (dN/dS) along lineages in the Dmbx1 phylogeny were estimated using codon-based maximum likelihood phylogenetic methods. Results of a branch model in which the post-duplication branches (PDBs) leading to the dmbx1a and dmbx1b clades were each allowed to have independently estimated dN/dS values are shown. Both PDBs display elevated dN/dS estimates compared to the background estimate applied to the remainder of the phylogeny. This model fit the data significantly better than a model with a single dN/dS parameter (M0 model; p < 0.01, d.f. = 2). Increases in dN/dS along each of the PDBs were also confirmed through simpler branch models in which only a single PDB, either for dmbx1a or dmbx1b, received separately estimated dN/dS values; in both cases the increases in dN/dS was statistically significant (M0; p < 0.05, d.f. = 1). Branch lengths shown in this figure were estimated under the 2 PDB branch model under which the dN/dS estimates were derived, and are proportional to the number of substitutions per codon.


