Abstract
We studied the molecular basis of transfusion-dependent hemolytic anemia in an infant who rapidly developed the phenotype of beta thalassemia major. DNA sequence of one beta-globin gene of the proband revealed two mutations, one for the moderately unstable hemoglobin (Hb) Köln and another for a novel codon 32 cytosine-thymidine-guanine-->cytosine-adenine-guanine transversion encoding a leucine-->glutamine mutation. A hydrophilic glutamine residue at beta 32 has an uncharged polar side chain that could potentially distort the B helix and provoke further molecular instability. This new hemoglobin was called Hb Medicine Lake. Biosynthesis studies showed a deficit of beta-globin synthesis with early loss of beta-globin chains. An abnormal unstable hemoglobin, globin chain, or tryptic globin peptide was not present, demonstrating the extreme lability of this novel globin. Hb Medicine Lake mRNA was present, but an aberrantly spliced message was not. Absence of an abnormal beta-globin gene in the mother makes it likely that a de novo mutation occurred in the proband. The molecular pathogenesis of Hb Medicine Lake illustrates a mechanism whereby the phenotype of a genetic disorder, like the mild hemolytic anemia associated with a hemoglobinopathy, can be modulated by a coincident mutation in the same gene.
Full text
PDF
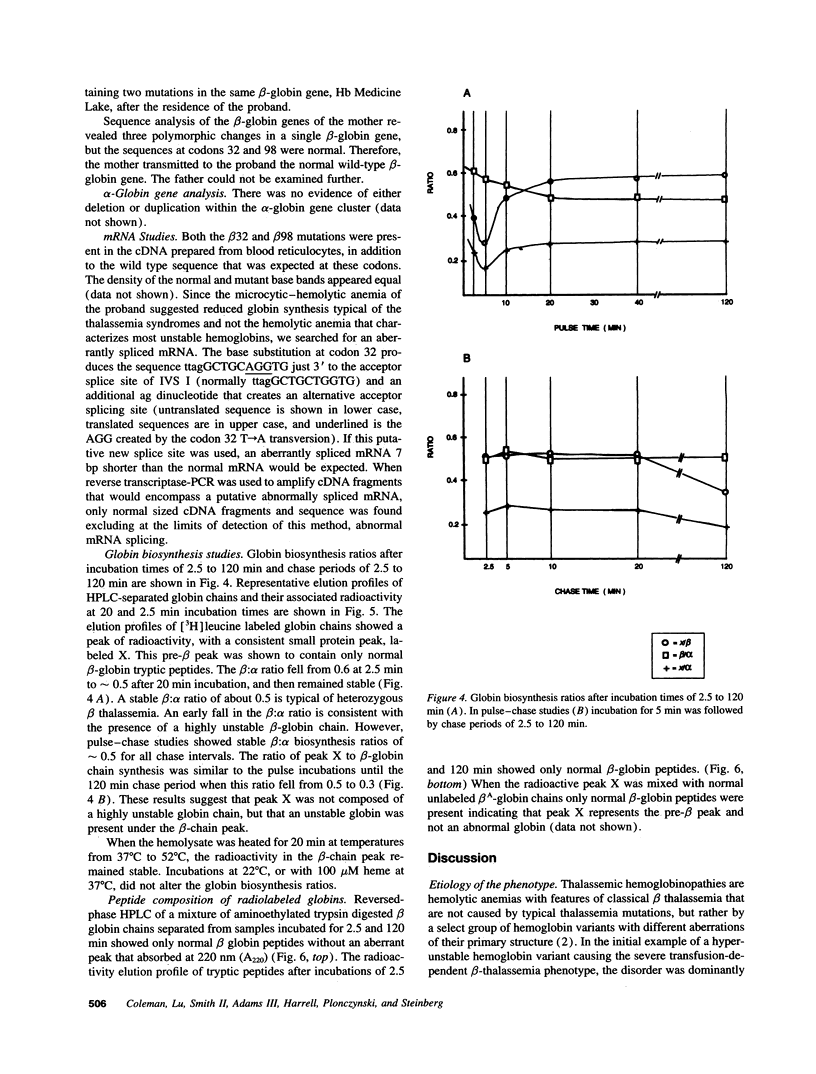

Images in this article
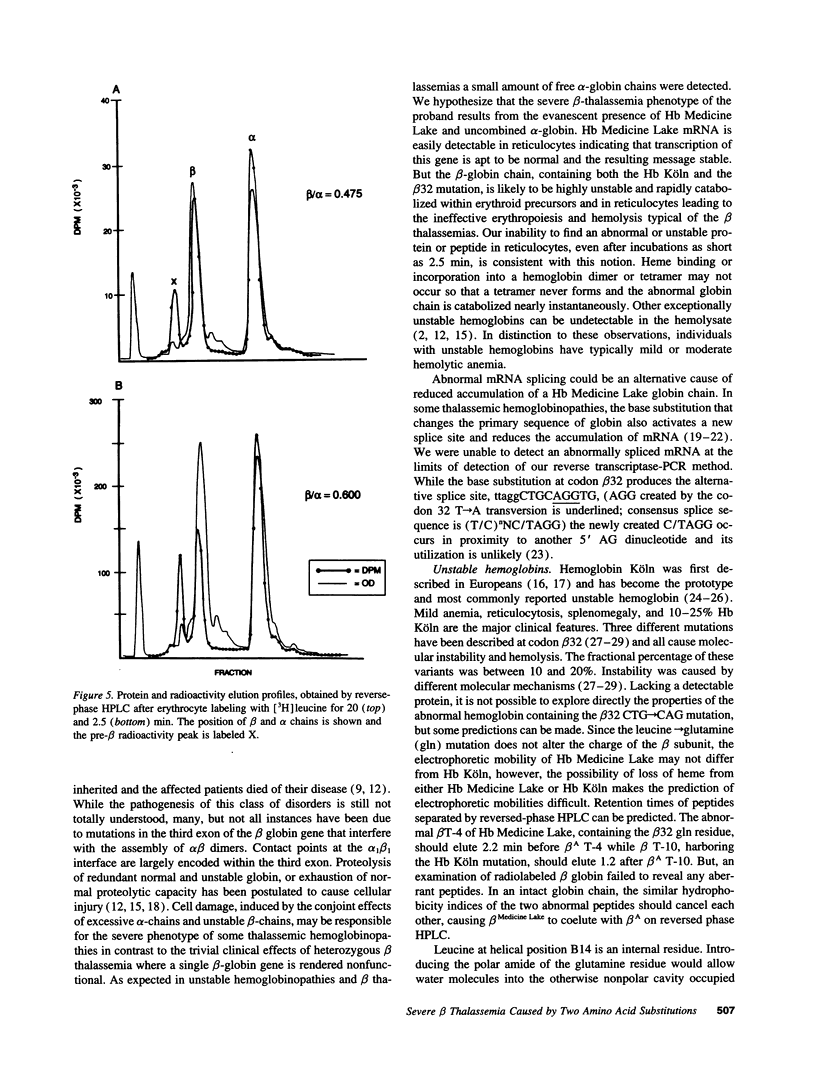
Selected References
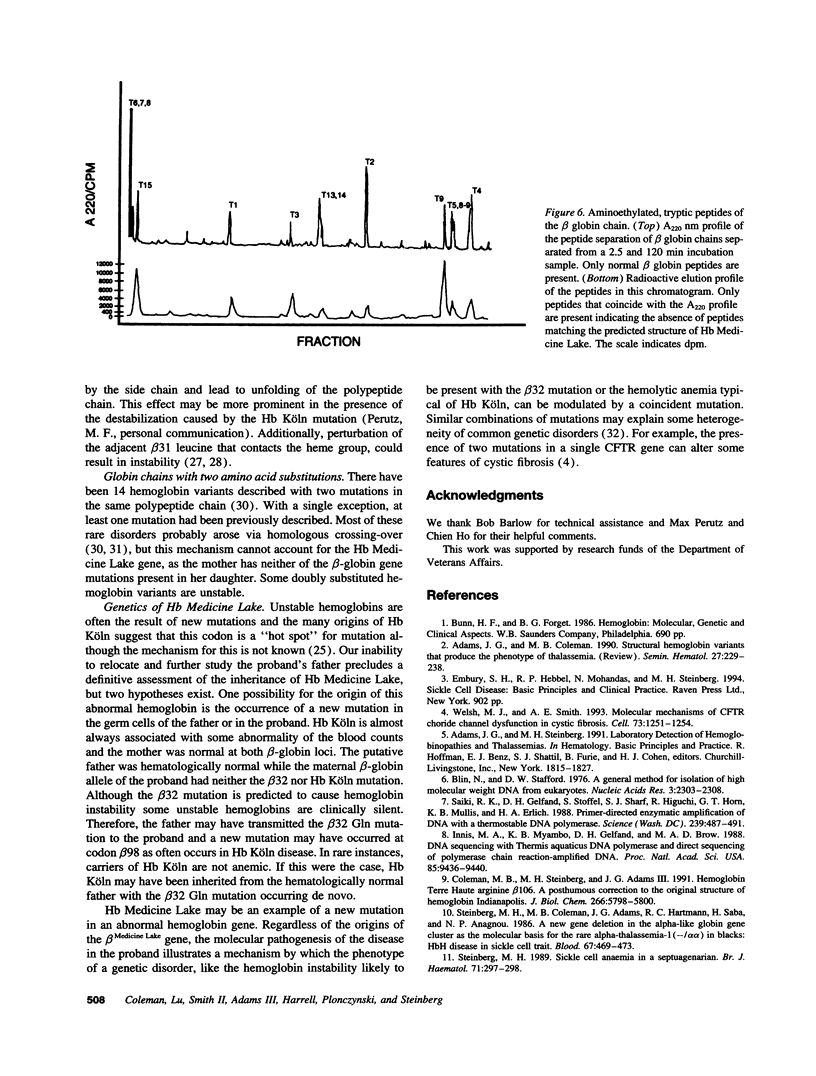
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. G., 3rd, Boxer L. A., Baehner R. L., Forget B. G., Tsistrakis G. A., Steinberg M. H. Hemoglobin Indianapolis (beta 112[G14] arginine). An unstable beta-chain variant producing the phenotype of severe beta-thalassemia. J Clin Invest. 1979 May;63(5):931–938. doi: 10.1172/JCI109393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. G., 3rd, Coleman M. B. Structural hemoglobin variants that produce the phenotype of thalassemia. Semin Hematol. 1990 Jul;27(3):229–238. [PubMed] [Google Scholar]
- Baklouti F., Ouazana R., Gonnet C., Lapillonne A., Delaunay J., Godet J. Beta+-thalassemia in cis of a sickle cell gene: occurrence of a promoter mutation on a beta s chromosome. Blood. 1989 Oct;74(5):1817–1822. [PubMed] [Google Scholar]
- Benz E. J., Jr, Berman B. W., Tonkonow B. L., Coupal E., Coates T., Boxer L. A., Altman A., Adams J. G., 3rd Molecular analysis of the beta-thalassemia phenotype associated with inheritance of hemoglobin E (alpha 2 beta2(26)Glu leads to Lys). J Clin Invest. 1981 Jul;68(1):118–126. doi: 10.1172/JCI110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Gaucher disease as a paradigm of current issues regarding single gene mutations of humans. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5384–5390. doi: 10.1073/pnas.90.12.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H., Hutchison H. E. Haemoglobin Köln (beta-98 valine--methionine): an unstable protein causing inclusion-body anaemia. Nature. 1966 May 28;210(5039):915–916. doi: 10.1038/210915a0. [DOI] [PubMed] [Google Scholar]
- Coleman M. B., Steinberg M. H., Adams J. G., 3rd Hemoglobin Terre Haute arginine beta 106. A posthumous correction to the original structure of hemoglobin Indianapolis. J Biol Chem. 1991 Mar 25;266(9):5798–5800. [PubMed] [Google Scholar]
- Egan E. L., Fairbanks V. F. Postsplenectomy erythrocytosis in hemoglobin Köln disease. N Engl J Med. 1973 May 3;288(18):929–931. doi: 10.1056/NEJM197305032881802. [DOI] [PubMed] [Google Scholar]
- Garel M. C., Blouquit Y., Rosa J., Arous N., Romero Garcia C. Hemoglobin Castilla beta 32 (B14) Leu leads to Arg; a new unstable variant producing severe hemolytic disease. FEBS Lett. 1975 Oct 15;58(1):144–148. doi: 10.1016/0014-5793(75)80245-6. [DOI] [PubMed] [Google Scholar]
- Honig G. R., Green D., Shamsuddin M., Vida L. N., Mason R. G., Gnarra D. J., Maurer H. S. Hemoglobin Abraham Lincoln, beta32 (B14) leucine leads to proline. An unstable variant producing severe hemolytic disease. J Clin Invest. 1973 Jul;52(7):1746–1755. doi: 10.1172/JCI107356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis M. A., Myambo K. B., Gelfand D. H., Brow M. A. DNA sequencing with Thermus aquaticus DNA polymerase and direct sequencing of polymerase chain reaction-amplified DNA. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9436–9440. doi: 10.1073/pnas.85.24.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Hemoglobin Information Center (IHIC) variants list. Hemoglobin. 1993 Apr;17(2):89–198. [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Dowling C. E., Hurwitz R. L., Coleman M., Stopeck A., Adams J. G., 3rd Dominant thalassemia-like phenotypes associated with mutations in exon 3 of the beta-globin gene. Blood. 1992 Jun 1;79(11):3014–3018. [PubMed] [Google Scholar]
- Miller D. R., Weed R. I., Stamatoyannopoulos G., Yoshida A. Hemoglobin Köln disease occurring as a fresh mutation: erythrocyte metabolism and survival. Blood. 1971 Dec;38(6):715–729. [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba Y., Miyaji T., Shibata S. Identical substitution in Hb Ube-1 and Hb Köln. Nat New Biol. 1973 Jun 13;243(128):205–207. doi: 10.1038/newbio243205a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Antonarakis S. E., Loukopoulos D. Abnormal processing of beta Knossos RNA. Blood. 1984 Jul;64(1):311–313. [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Ostrer H., Goff S. C., Sexton J. P. Abnormal RNA processing due to the exon mutation of beta E-globin gene. Nature. 1982 Dec 23;300(5894):768–769. doi: 10.1038/300768a0. [DOI] [PubMed] [Google Scholar]
- Pribilla W., Klesse P., Betke K., Lehmann H., Beale D. Hämoglobin-Köln-Krankheit: Familiäre hypochrome hämolytische Anämie mit Hämoglobinanomalie. Klin Wochenschr. 1965 Oct 1;43(19):1049–1053. doi: 10.1007/BF01746594. [DOI] [PubMed] [Google Scholar]
- Ramachandran M., Gu L. H., Wilson J. B., Kitundu M. N., Adekile A. D., Liu J. C., McKie K. M., Huisman T. H. A new variant, HB Muscat [alpha 2 beta (2)32(B14)Leu----Val] observed in association with HB S in an Arabian family. Hemoglobin. 1992;16(4):259–266. doi: 10.3109/03630269208998866. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Steinberg M. H., Adams J. G., 3rd, Morrison W. T., Pullen D. J., Abney R., Ibrahim A., Rieder R. F. Hemoglobin Mississippi (beta 44ser----cys). Studies of the thalassemic phenotype in a mixed heterozygote with beta +-thalassemia. J Clin Invest. 1987 Mar;79(3):826–832. doi: 10.1172/JCI112890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. H., Coleman M. B., Adams J. G., 3rd, Hartmann R. C., Saba H., Anagnou N. P. A new gene deletion in the alpha-like globin gene cluster as the molecular basis for the rare alpha-thalassemia-1(--/alpha alpha) in blacks: HbH disease in sickle cell trait. Blood. 1986 Feb;67(2):469–473. [PubMed] [Google Scholar]
- Steinberg M. H. Sickle cell anaemia in a septuagenarian. Br J Haematol. 1989 Feb;71(2):297–298. doi: 10.1111/j.1365-2141.1989.tb04274.x. [DOI] [PubMed] [Google Scholar]
- Traeger J., Wood W. G., Clegg J. B., Weatherall D. J. Defective synthesis of HbE is due to reduced levels of beta E mRNA. Nature. 1980 Dec 4;288(5790):497–499. doi: 10.1038/288497a0. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Smith A. E. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell. 1993 Jul 2;73(7):1251–1254. doi: 10.1016/0092-8674(93)90353-r. [DOI] [PubMed] [Google Scholar]
- Wilson J. B., Lam H., Pravatmuang P., Huisman T. H. Separation of tryptic peptides of normal and abnormal alpha, beta, gamma, and delta hemoglobin chains by high-performance liquid chromatography. J Chromatogr. 1979 Nov 21;179(2):271–290. doi: 10.1016/s0021-9673(00)83830-3. [DOI] [PubMed] [Google Scholar]