Abstract
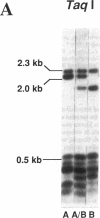
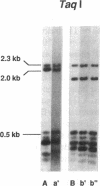
MUC-2, the first described intestinal mucin gene, has become important as a prototype for secreted mucins in several organ systems. However, little is known about its protein backbone structure and hence its role in diseases such as colon cancer, ulcerative colitis, and cystic fibrosis, which are known to have mucin abnormalities. Studies in this manuscript show that MUC-2 contains two distinct regions with a high degree of internal homology, but the two regions bear no significant homology to each other. Region 1 consists mostly of 48-bp repeats which are interrupted in places by 21-24-bp segments. Several of these interrupting sequences show similarity to each other, creating larger composite repeat units. Region 1 has no length polymorphisms. Region 2 is composed of 69-bp tandem repeats arranged in an uninterrupted array of up to 115 individual units. Southern analysis of genomic DNA samples using TaqI and HinfI reveals both length and sequence polymorphisms which occur within region 2. The sequence polymorphisms have different ethnic distributions, while the length polymorphisms are due to variable numbers of tandem repeats.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland C. R., Montgomery C. K., Kim Y. S. Alterations in human colonic mucin occurring with cellular differentiation and malignant transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2051–2055. doi: 10.1073/pnas.79.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand J. P., Andrews S. P., Jr, Cahill E., Conway N. A., Young J. D. Investigation of the requirements for O-glycosylation by bovine submaxillary gland UDP-N-acetylgalactosamine:polypeptide N-acetylgalactosamine transferase using synthetic peptide substrates. J Biol Chem. 1981 Dec 10;256(23):12205–12207. [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Found Symp. 1984;109:157–172. doi: 10.1002/9780470720905.ch11. [DOI] [PubMed] [Google Scholar]
- Gendler S. J., Lancaster C. A., Taylor-Papadimitriou J., Duhig T., Peat N., Burchell J., Pemberton L., Lalani E. N., Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990 Sep 5;265(25):15286–15293. [PubMed] [Google Scholar]
- Gerard C., Eddy R. L., Jr, Shows T. B. The core polypeptide of cystic fibrosis tracheal mucin contains a tandem repeat structure. Evidence for a common mucin in airway and gastrointestinal tissue. J Clin Invest. 1990 Dec;86(6):1921–1927. doi: 10.1172/JCI114925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths B., Matthews D. J., West L., Attwood J., Povey S., Swallow D. M., Gum J. R., Kim Y. S. Assignment of the polymorphic intestinal mucin gene (MUC2) to chromosome 11p15. Ann Hum Genet. 1990 Oct;54(Pt 4):277–285. doi: 10.1111/j.1469-1809.1990.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Byrd J. C., Hicks J. W., Toribara N. W., Lamport D. T., Kim Y. S. Molecular cloning of human intestinal mucin cDNAs. Sequence analysis and evidence for genetic polymorphism. J Biol Chem. 1989 Apr 15;264(11):6480–6487. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Hanover J. A., Lennarz W. J., Young J. D. Synthesis of N- and O-linked glycopeptides in oviduct membrane preparations. J Biol Chem. 1980 Jul 25;255(14):6713–6716. [PubMed] [Google Scholar]
- Itzkowitz S. H., Yuan M., Montgomery C. K., Kjeldsen T., Takahashi H. K., Bigbee W. L., Kim Y. S. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res. 1989 Jan 1;49(1):197–204. [PubMed] [Google Scholar]
- Jany B. H., Gallup M. W., Yan P. S., Gum J. R., Kim Y. S., Basbaum C. B. Human bronchus and intestine express the same mucin gene. J Clin Invest. 1991 Jan;87(1):77–82. doi: 10.1172/JCI115004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern S. E., Fearon E. R., Tersmette K. W., Enterline J. P., Leppert M., Nakamura Y., White R., Vogelstein B., Hamilton S. R. Clinical and pathological associations with allelic loss in colorectal carcinoma [corrected]. JAMA. 1989 Jun 2;261(21):3099–3103. doi: 10.1001/jama.261.21.3099. [DOI] [PubMed] [Google Scholar]
- Ligtenberg M. J., Vos H. L., Gennissen A. M., Hilkens J. Episialin, a carcinoma-associated mucin, is generated by a polymorphic gene encoding splice variants with alternative amino termini. J Biol Chem. 1990 Apr 5;265(10):5573–5578. [PubMed] [Google Scholar]
- Mantle M., Stewart G. Intestinal mucins from normal subjects and patients with cystic fibrosis. Variable contents of the disulphide-bound 118 kDa glycoprotein and different reactivities with an anti-(118 kDa glycoprotein) antibody. Biochem J. 1989 Apr 1;259(1):243–253. doi: 10.1042/bj2590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen V. C., Aubert J. P., Gross M. S., Porchet N., Degand P., Frézal J. Assignment of human tracheobronchial mucin gene(s) to 11p15 and a tracheobronchial mucin-related sequence to chromosome 13. Hum Genet. 1990 Dec;86(2):167–172. doi: 10.1007/BF00197699. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984 Nov;87(5):991–998. [PubMed] [Google Scholar]
- Porchet N., Nguyen V. C., Dufosse J., Audie J. P., Guyonnet-Duperat V., Gross M. S., Denis C., Degand P., Bernheim A., Aubert J. P. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991 Mar 15;175(2):414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui J., Abe M., Hayes D., Shani E., Yunis E., Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2320–2323. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Gendler S., Griffiths B., Corney G., Taylor-Papadimitriou J., Bramwell M. E. The human tumour-associated epithelial mucins are coded by an expressed hypervariable gene locus PUM. Nature. 1987 Jul 2;328(6125):82–84. doi: 10.1038/328082a0. [DOI] [PubMed] [Google Scholar]
- Swallow D. M., Gendler S., Griffiths B., Kearney A., Povey S., Sheer D., Palmer R. W., Taylor-Papadimitriou J. The hypervariable gene locus PUM, which codes for the tumour associated epithelial mucins, is located on chromosome 1, within the region 1q21-24. Ann Hum Genet. 1987 Oct;51(Pt 4):289–294. doi: 10.1111/j.1469-1809.1987.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., Hareuveni M., Tsarfaty I., Smorodinsky N., Horev J., Zaretsky J., Kotkes P., Weiss M., Lathe R., Dion A. Human epithelial tumor antigen cDNA sequences. Differential splicing may generate multiple protein forms. Eur J Biochem. 1990 May 20;189(3):463–473. doi: 10.1111/j.1432-1033.1990.tb15511.x. [DOI] [PubMed] [Google Scholar]