Abstract
Identification of odors of compounds introduced into changeable olfactory environments is the essence of olfactory coding, which focuses perception on the latest stimulus with the greatest salience. Effects of stimulus intensity and adapting time on mixture component identification after adapting with one component were each studied in 10 human subjects. Odors of 1 and 5 mM vanillin (vanilla) and phenethyl alcohol (rose) were identified, with adapting time varied by sniffing naturally once or twice, or sniffing 5 times, once every 2 s. Odors of water-adapted single compounds were identified nearly perfectly (94%), self-adapted to 51% but did not cross-adapt (94%), showing the 2 compounds had quickly adapting independent odors. Identifications of the vanilla and rose odors in water-adapted mixtures were reduced to 59% and 79%, respectively. Following single-component adaptation, the average 33% identification of odors of adapted (ambient) mixture components contrasted with the greater average 86% identification of new unadapted (extra) mixture components. Identifications were lower for 1 than 5 mM components when concentrations were not matched, and ambient component identifications were lower after 10-s adaptation than after 1 or 2 sniffs. Rapid selective adaptation and mixture component suppression manipulate effective intensity to promote emergence of characteristic odor qualities in dynamic natural settings.
Keywords: binary mixtures, dynamic olfactory coding, mixture suppression, selective adaptation, inhibition
Introduction
In everyday situations, many natural and synthetic materials contribute to an ambient complex olfactory stimulus mixture, in which most components are undetected by humans. Odorous objects move in and out of range and odors of more volatile compounds quickly drift away—an odor dynamism in which new extra odors appear and, after a short time, fade. Coding of odor quality in natural situations can be difficult to study. However, natural conditions can be simulated in a laboratory setting by selectively adapting components, which reveal the emergence of characteristic odors of new compounds (Goyert et al. 2007). This means that humans may dynamically filter complex natural olfactory environments as rats do with rapid sniffing (Verhagen et al. 2007).
In humans, identification of an odor component in a binary mixture depends on the relative salience of the 2 components (Olsson 1994, 1998). Identification of odor components in higher order mixtures is difficult. More than 2 component compounds are seldom consistently recognized (Livermore and Laing 1996, 1998a, 1998b) even when they are equally intense. This “mixture suppression” (Bartoshuk 1975; Laing et al. 1994) may explain why most odorous compounds contained in natural materials are imperceptible and characteristic odors can be approximated by dominant single compounds across mammalian species (Bell et al. 1987). Single-sniff partial adaptation (Moncrieff 1956; Laing 1983, 1986), which may make waning odors quickly undetectable in natural situations, is also important to consider. Components need not be completely adapted (Moncrieff 1967; Berglund et al. 1978; Dalton 2000) to unbalance mutual suppression. When there are multiple compounds in the odor environment, small intensity changes could make odors reappear or disappear.
We propose that the dependence of identification on relative component intensity and mixture complexity reflects a dual chemosensory coding strategy that focuses multiple receptor–linked signals for characteristic odors (or tastes) on 1 or 2 salient stimuli at a time (Frank 2008; Frank et al. 2008). Mammalian dynamic olfactory coding, the cooperative effect of mixture suppression and rapid adaptation, and recovery from adaptation, are likely dependent on inhibition within and among the hundreds of odor receptors (ORs), each normally segregated (Axel 2005; Buck 2005) in independent subsets of olfactory sensory neurons (OSNs). Disruption of OR segregation (Fleischmann et al. 2008) demonstrates the importance of organized inhibition among independent inputs for odor coding.
We had human subjects identify 2 water-soluble compounds with distinct characteristic odors, vanillin and phenethyl alcohol (PEA), to determine whether dynamic odor coding is affected by variation in stimulus concentration and adapting time. Failure of the vanilla and rose odors to cross-adapt confirmed that the 2 compounds likely activated distinct ORs and few-sniff, self-adaptation confirmed that vanilla and rose odors each rapidly adapted. By having the subjects identify vanilla and rose odors in binary mixtures, we show here how binary mixtures of 2 odorants follow basic rules that determine odor recognition and identification. The rules are consistent with mixture suppression and rapid adaptation guaranteeing that the most salient and newest components are recognized and others are ignored. Namely, of the many chemical compounds present in natural odors, only the most dominant components contribute to the overall perception.
Materials and methods
Subjects
Ten subjects, 7 women and 3 men of mean age 25 (standard deviation [SD] 4) years, volunteered for Experiment 1; and 10 subjects, 8 women and 2 men of mean age 24 (SD 4) years, volunteered for Experiment 2. All were healthy, nonsmokers, reported normal senses of taste and smell, and were able to recognize and reliably identify the 2 odors. Subjects asked to refrain from eating or drinking anything besides water and to refrain from using scented products such as perfume or cologne immediately prior to the appointment, were compensated for participation in two 1-h sessions.
Stimuli and odor labels
The compounds (odor labels) 1 and 5 mM vanillin (vanilla) and (PEA) (rose), at concentrations judged to have about equal lower and higher perceptual intensity, were used. An adapt–test pair of stimuli was presented on each trial. Test stimuli were deionized water, a single compound, or the binary mixture of the 2 compounds. When the test stimulus was the mixture, a component was named “ambient” if it was the same as the adapting stimulus and “extra” if it was different from the adapting stimulus (Goyert et al. 2007). The terms “ambient” and “extra” define the components in the particular adapt–test paradigm, are purely descriptive and meant to avoid confusion over the actual extent of adaptation. The compounds, obtained from Sigma Chemical Co., are water soluble (Moncrieff 1967), nontoxic, nontrigeminal (Doty et al. 1978), pleasant, familiar, and commonly used in clinical testing of the olfactory system (Cowart 1989; Koskinen et al. 2004; Philpott et al. 2009). They have overlapping and distinct molecular features (functional groups, molecular shapes) that have been associated with odors (Moncrieff 1967; Mori et al. 2006).
Stimuli were diluted from stronger stock solutions by adding deionized water so that corresponding concentrations were identical in component and mixture stimuli. Stimuli were delivered to subjects in 250-mL polyethylene squeeze bottles containing 50 mL of solution and fitted with caps having flip-up spouts.
Two duplicate sets of odor stimuli were used in each experiment, alternated across subjects and sessions, and refreshed weekly; stock solutions were replaced every 3–4 weeks. Caps were rinsed with hot water and dried between subjects. Hidden solution labels on each bottle and cap and a blind between the subject and the stimuli prevented the subjects from seeing which stimuli were offered or responses recorded by the experimenter.
Nine different solutions were prepared for Experiment 1, which addressed the effect of stimulus concentration on identification of vanilla (Vv) and rose (Rr) odors with a total of 36 stimulus pairs. Upper case letters are used to represent 5 mM and lower case letters 1 mM concentrations. Besides water (0), stimuli included in 2 replicate, 1-h sessions were 2 concentrations eliciting vanilla and rose odors as single compounds (v, V, r, R), the 2 vanilla–rose mixtures at the same concentration (vr, VR) or different concentrations (Vr, vR).
For Experiment 2, 7 different solutions were prepared to address adapting time by comparing the effect of natural sniffing (once or twice) to timed sniffing (5 times, 1 every 2 s) on identification of vanilla and rose odors elicited by 1 or 5 mM stimuli. Besides water, 1 concentration of each single compound (v, r or V, R) and the 2 binary mixtures at that concentration (vr or VR) were used in each session to construct 32 stimulus pairs for each 1-h session.
Procedure
Two 1-h sessions, scheduled on separate days for each subject, took place in a well-ventilated dental clinic room. The experimenter (H.F.G.), who wore latex gloves throughout, demonstrated how the subject should squeeze the bottle gently beneath the nose, 1 or 2 times with the flip-cap up, and then sniff the vapor in order to obtain the full aroma. Then, subjects were instructed how to sniff stimulus pairs, which differed for the 2 experiments, and rehearsed with water in the bottles. At a pace of 1 pair per minute, subjects were directed to sniff the first bottle (naturally once or twice in Experiment 1; either naturally or 5 times once every 2 s, with timing guided by the experimenter in Experiment 2); and then, within 5 s, exchange and sniff the second bottle once or twice. Subjects were instructed to identify the odor in the second bottle immediately after returning it to the experimenter, who recorded the results, and sniff water afterward until previous scents were no longer detected.
After practicing the sampling sequence with water trials, subjects were presented with water followed by each single compound with its label to engage odor recognition memory (Savic 2001); order was alternated across subjects and sessions. Then, a randomized set of stimulus pairs consisting of water followed by either single odor or water (test stimuli) were presented with corrective feedback. High- and low-concentration test stimuli were used for training in each replicate session of Experiment 1 and at appropriate concentration for training in high- or low-concentration sessions in Experiment 2. Subjects were required to correctly identify the odor of the 3 test stimuli twice in a row before proceeding to experimental trials.
During a 2-min break, subjects were instructed as follows: “As in the training, I will present items to you in pairs. In addition to each single odor and water, you may or may not smell a combination of the 2 odors. In other words, respond to the best of your knowledge as to whether you smell vanilla, rose, both, or water in the second bottle.”
Subjects referred to the list of 4 odors (vanilla, rose, both, and water), chose 1, and did not receive corrective feedback. Throughout the experiment, subjects generally sniffed water immediately following the stimulus and then again halfway through the minute interval. The experimenter tracked identification of the 3 individual stimuli and tabulated data as a vanilla response and a rose response when a subject chose “both.”
Experimental design
One reason for presenting many single-component and blank test stimuli in each of the 2 experiments was to provide a complex balanced testing context to minimize subjects’ chances of guessing correctly. With inclusion of self- and cross-adapt trials, it was just as likely to have vanilla follow rose odor as to have rose follow vanilla odor.
Experiment 1 explored stimulus concentration by using low (1 mM) and high (5 mM) concentrations in adapt–test pairs. Stimuli in adapt–test pairs were either matched high–high or low–low concentrations or unmatched high–low and low–high concentrations. Not to complicate selective adaptation in unmatched trials, adapt stimuli and subsequent ambient mixture components were presented at the same concentration, but unadapted extra mixture component test stimuli were presented at either high or low concentration. The testing protocol consisted of randomized presentation of 36 stimulus pairs, adapt stimulus followed by test stimulus in each of 2 replicate sessions (Figure 1). There were 4 sets of adapt–test stimulus pairs, color coded in Figure 1. Four water test stimuli (0, blue) were blanks; 20 single components (yellow color) tested self-adapted and cross-adapted vanilla odor (V, v) or rose odor (R, r); and 12 binary mixtures tested mixture suppression (tan color at center) by offering mixtures after water or selective adaptation by offering the mixture after one or the other component (rose color).
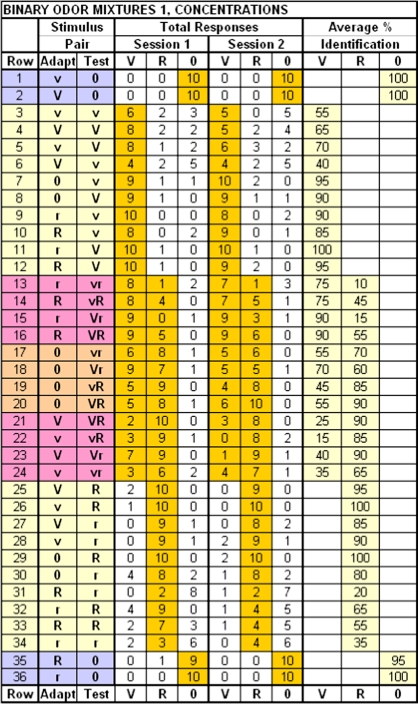
Figure 1.
Data matrix for experiment 1—stimulus concentration: total responses and average percentages identified by 10 subjects. V = 5 mM vanillin, v = 1 mM vanillin, R = 5 mM PEA, r = 1 mM PEA, 0 = water. Gold background highlights correct responses of 10 subjects for the 2 sessions, averaged for % identification. The 36 adapt–test pairs were presented to each subject in each session. Each row represents a separate trial.
Duplicate solutions were used to separate adapt from test stimuli, to maximize head space recuperation of the single odorants, and to prevent the repeated use of the same stimulus bottle for the self-adapt condition. There were a total of 15 bottles: one for the subject to sniff water between stimulus pairs, 5 adapt bottles, and 9 test bottles.
Estimated time from the beginning of the first sniff in the adapt stimulus to the beginning of the first sniff in the test stimulus was about 4 s. All test pairs were presented at a pace of one pair per minute, leaving at least a 45-s rest period, during which subjects were encouraged to sniff water.
Experiment 2 explored adapt stimulus duration as well as any interaction the adapting time may have with test stimulus concentration. To accommodate the adaptation–duration variable in a 1-h session, concentration matched adapt–test stimulus pairs were offered at 1 mM in one session and at 5 mM in the second session (order alternated, Figure 2). The testing protocol consisted of randomized presentation of 32 adapt–test stimulus pairs (16 for each adapting time) in each of 2 sessions. As in Experiment 1, there were 4 sets of stimulus pairs, which are color coded in Figure 2. Eight water test stimuli (0, blue) were blanks. Self-adapted, mixture-adapted, water-adapted, and cross-adapted identification were tested with 16 single components (yellow). Eight binary mixtures tested mixture suppression by offering the mixture after water, mixture adaptation by offering the mixture after the mixture (tan), or selective adaptation by offering the binary mixture after one or the other component (rose color).
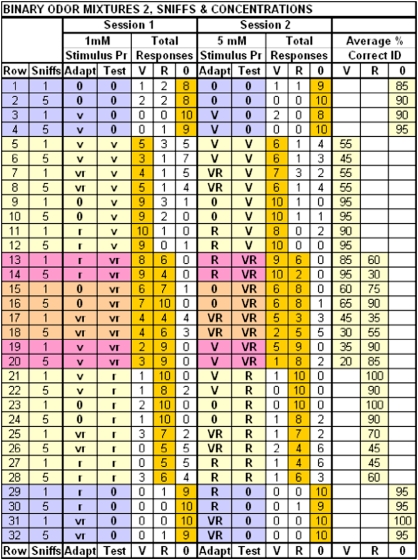
Figure 2.
Data matrix for experiment 2—adapt stimulus time: total responses and average percentages identified by 10 subjects. V = 5 mM vanillin, v = 1 mM vanillin, R = 5 mM PEA, r = 1 mM PEA, 0 = water. 1 sniffs = 1–2 natural sniffs of adapting stimulus, 5 sniffs = 5 timed sniffs, 2 s apart of adapting stimulus. Gold background highlights correct responses of 10 subjects for the 2 sessions, averaged for % identification. Thirty-two adapt–test pairs were presented at 1 or 5 mM concentrations in separate sessions. Each row represents a separate trial.
Duplicate solutions were used to separate “adapting” stimuli from identical test stimuli, to maximize headspace recuperation, and to prevent repeated use of the same stimulus bottle for self-adapt. There were a total of 9 bottles: one for the subject to sniff water between stimulus pairs, 4 adapting bottles, and 4 test bottles.
The time from the beginning of the first sniff of the adapt stimulus to the beginning of the first sniff of the test stimulus was either ∼4 or ∼12 s depending on the number of times the adapting stimulus was sniffed. Average human natural sniff duration is 1.6 s (Laing 1983), and distinct characteristic odors can be correctly identified with 1 sniff (Laing 1986). Stimuli were presented each minute, leaving at least a 40-s rest period, during which subjects sniffed water. This is sufficient time to recover from adaptation (Goyert et al. 2007), which likely has multiple determinants in the ascending olfactory pathways (Zufall and Leinders-Zufall 2000; Song et al. 2008).
In short, a subject, familiarized with single components before the experiment, was presented with pairs of stimuli to sample and identify from a list of labels. The adapting (first) and test (second) stimulus contained either 0 (water) or 1 or 2 odorous components. Olfactory solutions were presented in 250-mL polyethylene squeeze bottles. Each bottle contained 50 mL of solution. Subjects flipped up the spout, and, while squeezing the bottle, slowly sniffed once or twice (or a timed 5 times) during an adaptation period. Immediately following, a second stimulus bottle was sampled with 1 or 2 natural sniffs. The task was to identify all detectable odor components in the test (second) stimulus. The correct labels were vanilla for vanillin, rose for PEA, and water for distilled water. The adapt–test pairs were presented in random order. Each subject received 32–36 pairs in a single session and a second session was run on a separate day.
Data analysis
Aggregate frequency data (the number of times 10 subjects chose vanilla or rose) are provided in Figures 1 and 2. Total responses may exceed 10 for single stimuli if at least 1 subject chose both vanilla and rose. For example, in row 3 of Figure 1, total responses for session 1 sum to 11 because 1 subject mistakenly identified vanillin as the vanilla–rose mixture; in this case, 1 other subject mistakenly identified vanillin as rose. Total responses for the mixture stimulus would sum 20 if all 10 subjects detected both components of the vanilla–rose mixture. Row 16 of Figure 2 gives data for the trial that most closely approached 20 total responses; total responses sum to 17 because 7 subjects chose the mixture and 3 chose rose alone(The number of vanilla-rose mixture responses on a trial equals the sum of the total responses (tabulated in Figures 1 and 2 under V and R) minus 10 (number of subjects) plus the number of water responses (tabulated under 0). The number of subjects who chose vanilla or rose alone equals the total responses (tabulated under V or R) minus the calculated number of mixture responses.). Statistical evaluation used analysis of variance (ANOVA) of the aggregate identification proportions; post hoc tests were Neuman–Keuls; t-tests for dependent samples were also used, α = 0.05. For Experiment 1, main analyses were two 3-way ANOVAs, with 2 stimulus compounds, 2 test stimulus concentrations, and adapting condition as factors. In the first ANOVA, stimuli were single components identified after the conditions of self- or cross-adaptation. In the second ANOVA, the identified test stimulus was a component in a binary mixture, preceded by itself or by the other component; the ambient or extra stimulus, respectively. For Experiment 2, main analyses were two 2-way ANOVAs. In both ANOVAs, 1 factor was the 2 stimulus compounds and the other factor was adapting condition. In 1 ANOVA, test stimuli were single compounds identified after self, mixture, cross or water adaptation. In the second ANOVA, the identified stimulus was a component of a binary mixture (ambient, extra, mixture-adapted or water-adapted). In each main analysis, data for the 2 adapt times and 2 concentrations were considered replicates.
Results
The results of Experiment 1 are in Figure 1 and the results of Experiment 2 are in Figure 2, with tabled entries, the total number of responses for 10 subjects, and average percent correct identification for the 2 sessions. Figures 1 and 2 are arranged complementarily with water controls in the top rows followed by vanilla [V] identifications; and water controls in the bottom rows preceded by rose [R] identifications. The key identifications of components of mixtures are in the centers of the Figures 1 and 2. Graphed data presented in figures are mean (+standard error of the mean) percent identifications.
Experiment 1: adapting and test stimulus concentration
Identifications of 2 replicates of each test stimulus are tabled in Figure 1. When water (0) was the test stimulus, it was accurately identified following vanillin or PEA (rows 1–2, 35–36). The core of Figure 1 is identification frequencies for odors—vanilla (rows 3–12), vanilla and rose in the mixture (rows 13–24), and rose (rows 25–32). Numbers of correct identifications of odors are highlighted in gold.
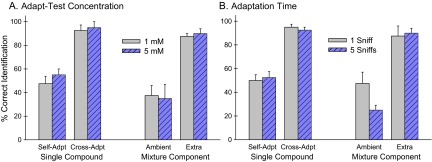
When adapt and test or mixture component concentrations were matched, single-compound and mixture component identifications were unaffected by stimulus intensity. This was not the case when the concentrations were unmatched. Cross-adapted and self-adapted single compounds were identified 16% less frequently after adapting with 5 than 1 mM stimuli or, looking at the same data points in the converse way, they were identified 16% more frequently when tested with 5 mM compared with 1 mM stimuli (F1,3 = 22.4, P = 0.02) (Supplementary Figure 1A). Also, selectively adapted ambient and new extra mixture components were identified more frequently at 5 than 1 mM (F1,3 = 14.5, P = 0.03). Extra components were identified 5% more frequently and ambient components identified 28% more frequently at the higher concentration in the unmatched concentration condition (Supplementary Figure 1B). And, as shown previously (Laing and Willcox 1983; Laing et al. 1984; Olsson 1994, 1998), on average, when concentrations of mixture components (sampled after water) were unmatched, 5 mM components (78 ± 9%) were better identified than 1 mM (52 ± 6%) components (F1,3 = 15.0, P = 0.03). Stimulus intensity significantly affected odor identification only on trials containing unmatched concentrations.
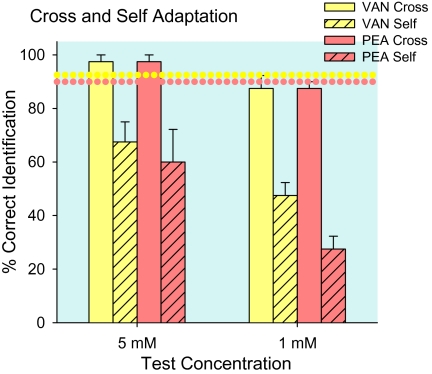
Figure 3 presents average single-compound odor identifications of 5 and 1 mM concentrations for concentration-matched and concentration-unmatched trials combined. Average water-adapted control identification of vanilla, 92 ± 2%, and rose, 90 ± 6% (dotted horizontal lines), were equally high; as was average cross-adapted identification, 93 ± 2%. Cross-adapted identification was higher than self-adapted (F1,3 = 125.8, P = 0.002); which was higher for vanilla, 58 ± 7%, than rose, 44 ± 10% (F1,3 = 13.4, P = 0.01). There were correspondingly more self-adapted, 42 ± 5 %, than cross-adapted, 6 ± 2%, misidentifications of the single compounds as water (F1,3 = 47.6, P = 0.006); and self-adapted PEA was misidentified as water (50 ± 9%) more often than was vanillin (35 ± 5%) (F1,3 = 10.8, P = 0.05). The equally high odor identification after cross-adaptation and water-adaptation are signs of the independence of the 2 odors. The association of self-adaptation with identifying the single compounds as water is a sign of odors fading after a few sniffs.
Figure 3.
Experiment 1—single component cross- and self-adapted stimuli. Regardless of concentration, characteristic odors of vanillin (VAN) and PEA were readily identifiable after water (dotted horizontal lines) or cross-adaptation (Cross); but, after self-adaptation (Self), odors of single compounds were less salient, the rose odor even less than the vanilla odor. Means (+standard error of the mean) are plotted.
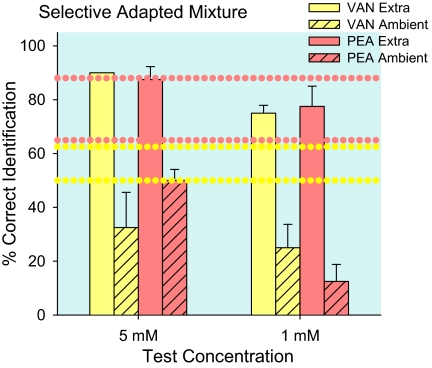
Figure 4 presents mixture component odor identifications of 5 and 1 mM concentrations for concentration-matched and concentration-unmatched trials combined. Mixture component controls are identifications of the mixed components sampled after water. The average control vanilla identification, 56 ± 5%, was lower than rose, 76 ± 6%, F1,3 = 32.0, P = 0.01. Identification of each compound in the mixture was better for high than low concentration: 62 ± 9 % for 5 mM and 50 ± 4% for 1 mM vanillin; 88 ± 5% for 5 mM and 65 ± 6% for 1 mM PEA (dotted horizontal lines). Following selective adaptation, identification of extra components, 82 ± 3%, was greater than ambient components, 30 ± 5%, F1,3 = 126.0, P = 0.002. Average identification of extra components was greater (F1,3 = 10.3, P = 0.05) and ambient components smaller (F1,3 = 11.2, P = 0.04) than the average 66 ± 4% control for the mixed components. Compared with identification of single compounds (Figure 3), ambient mixture component identification was even weaker than self-adapted, 50.6 ± 5% (F1,15 = 13.3, P = 0.002); and extra mixture component identification did not differ significantly from control single-compound identification after water, 91.2 ± 3%. Selective adaptation, by adjusting effective intensity, allows a characteristic odor suppressed in a mixture to emerge and further weakens other mixture components.
Figure 4.
Experiment 1—mixture component extra and ambient test stimuli. Within a binary mixture, the vanilla odor of vanillin (VAN) was less identifiable than the rose odor of PEA at high and at low concentrations (dotted horizontal lines, 2 for each compound). The 2 characteristic odors were more salient when they were extra components and less salient when they were ambient components, regardless of concentration. The dotted horizontal lines are separate controls for the 2 compounds at the 2 concentrations. Means (+standard error of the mean) are plotted.
Experiment 2: adapting stimulus time
Identifications of test stimuli at 1 and 5 mM after short and long adaptation times are tabled in Figure 2. Water (0) was accurately identified following itself, vanillin, PEA, or the mixture (rows 1–4, 29–32). The core of Figure 2 shows identification of vanilla (rows 5–12), vanilla or rose in the mixture (rows 13–20), and rose (rows 21–28) odors. Correct identifications of odor qualities are highlighted in gold. Adapting and test concentrations were matched at either 1 or 5 mM within a session.
Neither single-compound nor mixture component identification was influenced significantly by concentration, with overall identification 66.6 ± 4% at 1 mM and 67.2 ± 5% at 5 mM (Figure 5A). Adapting time had no significant overall effect on identification of single compounds or mixture components: identification after 1–2 natural sniffs was 68.4 ± 4% and after 5 timed sniffs was 65.3 ± 5%. The single significant effect was the 24% greater decrement in ambient component identification with more adapt-stimulus sniffing (P = 0.05); neither the corresponding extra mixture component identification nor self- or cross-adapted single-compound identification was affected (Figure 5B).
Figure 5.
Experiment 2—test stimulus identification, matched concentrations, and adapting times. (A) Concentration did not affect identification when concentrations of adapt–test stimulus pairs were matched. (B) Adapting time had little influence on identification, with one exception. Ambient mixture components were better identified after the shorter adaptation time. 1 sniff = 1–2 natural sniffs; 5 sniffs = 5 timed 2-s sniffs. Means (+standard error of the mean) are plotted.
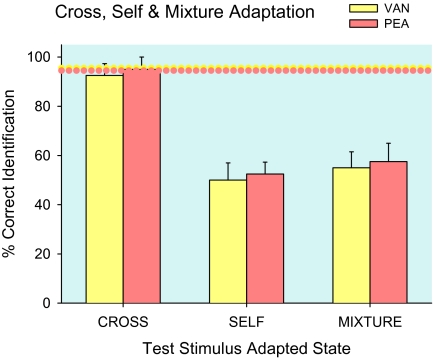
Figure 6 presents single-compound odor identifications combined across concentration and adapting time. Average water-adapted control identification was 95 ± 3% for vanilla and rose (dotted horizontal lines), values indistinguishable from cross-adapted identification of vanilla or rose, 94 ± 3 %. Average self-adapted identification of vanilla and rose, 51 ± 4%, and mixture-adapted identification of vanilla and rose, 56 ± 5%, were statistically equivalent (suggesting suppression in the adapting stimulus mixture did not significantly reduce self-adaptation). The strong effect of adapted state on identification, F3,9 = 45.5, P = 0.000009, distinguishes single and cross-adapted from self-adapted and mixture-adapted identifications, all P = 0.0002. There were correspondingly more self-adapted, 46 ± 5%, than cross-adapted, 6 ± 3%, misidentifications of single compounds as water F1,3 = 96.6, P = 0.002. As in Experiment 1, results are consistent with independent odors, and the fading of odors after a few sniffs, features of single compounds that allow testing of selective adaptation.
Figure 6.
Experiment 2—cross-adapted, self-adapted, and mixture-adapted single components. Characteristic odors of vanillin (VAN) and PEA were equally identifiable when preceded by water (dotted horizontal lines) or after cross-adaptation (CROSS). But, after self-adaptation (SELF) or mixture adaptation (MIXTURE) the rose odor and vanilla odors of single stimuli were less salient. Means (+standard error of the mean) are plotted.
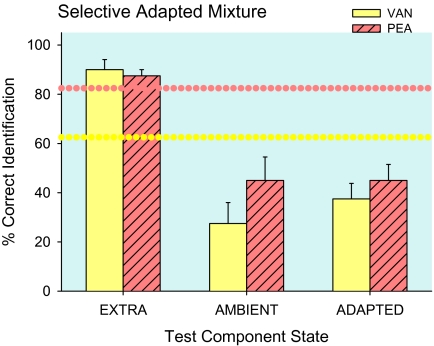
Figure 7 presents mixture component identifications (combined across concentration and adapting time), which differ with test component adaptation state, F3,9 = 29.3, P = 00001. Controls are identification of the mixed components after sampling water. As in Experiment 1, control vanilla identification, 62 ± 2%, was lower than rose, 82 ± 6% in the mixture (dotted horizontal lines), t3 = 4.9, P = 0.02. Following selective adaptation, the average extra component odor was identified, 89 ± 2%, more often (P = 0.0003), than the ambient, 36 ± 7%, and statistically equivalent mixture-adapted component odors, 41 ± 4%. Average control 72 ± 5% identification of mixture components was enhanced for extra components (P = 0.04), whereas ambient- and mixture-adapted components were diminished (both P = 0.001). Identification frequencies for extra and ambient mixture components were as distinct, F3,9 = 37.2, P = 0.00002, as water-adapted single and self-adapted single compounds (Figure 6). Extra odors and single-compound odors were equally well identified, and ambient odors were even less identifiable than odors of self-adapted single compounds, 51.2 ± 4 % (P = 0.05). The characteristic odor of one binary mixture component, effectively increased in intensity, emerges when the other mixture component is reduced in effective intensity by selective adaptation.
Figure 7.
Experiment 2—extra, ambient, and mixture-adapted mixture component test stimuli. Within a binary mixture, the vanilla odor of vanillin (VAN) was less identifiable than the rose odor of PEA (dotted horizontal lines). Characteristic odors of extra mixture components were as identifiable as unadapted single stimuli (cf. Figure 4). Characteristic odors of ambient mixture components were as identifiable as components presented after the mixture (ADAPTED) and less identifiable than self-adapted single components (cf. Figure 4). Means (+standard error of the mean) are plotted.
Discussion
The recognition of odor components in natural mixtures is limited to very few dominant components. In artificial mixtures with components of equal salience, only 2 or 3 components can be consistently identified when presented as mixtures, whereas in a mixture of 4 components, none are identified (Livermore and Laing 1996, 1998a, 1998b). We observed that mixture component identification is improved by prior adaptation to other components (Goyert et al. 2007). With our paradigm, characteristic odors of single extra components emerged from mixture suppression after 1 or 2 sniffs of an ambient mixture of the other components. At the same time, the ability to identify the ambient components decreased. The effects were observed whether the mixtures contained 2, 3, or 4 components and occurred in the course of a few seconds, implying there is adaptation on this short time scale. A key finding was that there was component recognition even when the extra component had chemical features in common with ambient components.
In the present study, we sought to determine the effects of varying component concentration and adapting time on component recognition with binary mixtures composed of PEA and vanillin. Using low (1 mM) and high (5 mM) concentrations of the 2 compounds as ambient and extra components, we found that adaptation and emergence occurred even when the salience of the 2 components was deliberately mismatched. Increasing the number of adapting sniffs did not affect identification of the extra component. These results show that the phenomenon of selective adaptation and extra component recognition is robust and is established rapidly in the course of natural sniffing.
Water controls, identifying water, and misidentifying single compounds as water
Within a complex balanced testing context that minimized subjects’ chances of guessing correctly, water stimuli served 3 important functions. Water was a test stimulus to evaluate guessing when preceded by water and assess recovery from adapting odors when preceded by single compounds and mixtures. Our subjects showed a remarkable median 95% accuracy in identifying water test stimuli whether sampled after water, single compound, or mixture suggesting correct guessing and carryover of adapting odors were not problems. Water also was an adapting stimulus control for single compounds and mixture components needed to assess effects of self-, cross-, and selective adaptation on identification rates. Our original selective adaptation study used 4 different compounds (Goyert et al. 2007), making it impossible to include these controls within a 1-h testing session. Water was also a trained response label available for subjects to use if a test stimulus smelled like water. The low median 5% identification of stimuli as water on cross-adaptation and mixture component identification trials contrasts with the high 40% water identifications on self-adapted and mixture-adapted trials. The stimuli did smell like water a high percentage of the time when self- or mixture-adapted.
Identifying stimuli differing in perceptual intensity and salience in mixtures
In our original study on the emergence of characteristic odors after selective adaptation (Goyert et al. 2007), we used 4 single compounds, each at a single concentration. To test the effect of varying concentration in the current study, the 4 stimuli used were high and low concentrations (differing by a factor of 5) of PEA and vanillin. Identification of mixture components or single compounds did not differ on trials when concentrations were matched, either at 1 or 5 mM. At either concentration, very similar results were obtained for self-adaptation and mixture component identification after water adaptation or component adaptation. Perceptions appeared concentration-invariant because neither stimulus had an intensity advantage. However, when 5 and 1 mM stimuli were presented serially or together within a mixture, an intensity advantage was created. In the self-adaptation trials, 5 mM adaptation reduced 1 mM identification much more than the reverse. Compared with 50% identification rates with matched concentrations (high or low), subjects identified 75% of 5 mM single compounds that were self-adapted by 1 mM but only 30% of 1 mM compounds self-adapted by 5 mM. In the mixture trials, the 5 mM components were better identified than the 1 mM in both water-adapted controls and component-adapted trials. Compared with ambient component identification rates of 35% for matched concentrations, subjects identified 20% of 1 mM ambient (adapted) mixture components but 45% of 5 mM ambient components. The intensity advantage has been reported previously for unmatched stimuli presented simultaneously in water-adapted mixtures (Laing and Willcox 1983; Laing et al. 1984; Olsson 1994, 1998). Nonetheless, it is also clear from our data that even with mismatched concentrations, component adaptation consistently leads to a decreased identification of the ambient component and increased identification of the extra component compared with water-adapted controls. Thus, like insect olfaction (Hildebrand 1995; Riffell et al. 2008), human olfaction and possibly taste (McBurney and Balaban 2009) may sample chemosensory environments every few seconds to dynamically track concentration change. The intensity changes will determine which few characteristic odors are detected on the same time scale.
The100% ceiling for identification clearly does not coincide with maximal perceptual intensity. Given the sensitivity to concentration change and the acute sensitivity of mixture suppression to component intensity (Laing and Willcox 1983; Laing et al. 1984; Olsson 1994, 1998), small differences in stimulus intensity would account for observed differences in identification of characteristic odors. Thus, rose odor may self-adapt more than vanilla odor on unmatched concentration trials because the 2 PEA concentrations differed more in odor intensity than the 2 vanillin concentrations; and rose may have been better identified than vanilla in PEA–vanillin mixtures because PEA concentrations both elicited higher intensities than corresponding vanillin concentrations. That is, small differences in intensities of the 2 compounds, not essential differences in the way 2 characteristic odors are dynamically coded, may account for observed differences in apparent salience of rose and vanilla odors.
Effective stimulus concentration and unequal mixture component intensities
Our studies rely on odor identification rather than scaled magnitude estimates of perceptual intensity; as a result, intensity factors generally refer to “effective” stimulus concentrations. After selectively adapting to the other component, identification of a binary mixture component increased to approach control identification of a single compound and, we reason, increased to the same effective concentration. Effective concentrations of a mixture component, self-adapted single-compound, and selectively adapted ambient mixture component were reduced compared with the control identification of a single compound. Effective stimulus concentrations may be estimated using these identification data and plots of probability of identification versus stimulus concentration, that is, psychometric functions. Change in concentration by a factor of 2 at the center of psychometric functions relating probability of detection to stimulus concentration produces a change in detection probability of about 30% of the entire dynamic range for esters (Cometto-Muñiz et al. 2008).
To simplify analysis, many studies on mixtures have used components at equal perceptual intensity, which would be equally effective in suppressing one another (Laing and Willcox 1983; Laing et al. 1984). Yet this condition rarely occurs in nature. If components were not intensity matched, the stronger would suppress the weaker component more than the weaker would suppress the stronger. A simple model stipulating equal average suppression for mixture components, matched and unmatched, predicts amounts of suppression for stronger and weaker stimuli. If intensity-matched unmixed components were to each be reduced by a factor of 2 in the mixture, the average intensity of each of the mixed components would be 50% of the intensity of the individual components. Whereas, if one component were 4 times stronger than is needed for a match to a weaker component; the stronger component would reduce the weaker to (1/2 × 1/4) or 1/8, when the weaker reduced the stronger by a factor of (1/4 × 1/2) or 1/8. The intensity of the stronger component would then be 88% and the weaker component 12% in the mixture, the average for the mixed components remaining 50% of the individual components. The numerical relationships of this simple model are readily testable using human subjects.
Adaptation time increased by extra sniffing
Identification of odors by humans is usually achieved during a single sniff. Subsequent natural sniffs are thought to be used to verify stimulus identity (Laing 1983, 1986). In rats, natural sniffing has been shown to “filter” inputs to the olfactory bulb (Verhagen et al. 2007; Wachowiak et al. 2009). Our subjects naturally sniffed to identify the test stimulus on all trials but adapting time was lengthened by having subjects increase natural sniffing from ∼5 s (Goyert et al. 2007) to ∼10 s (5, timed 2-s sniffs). The additional sniffing neither further decreased identification of self-adapted single compounds nor increased extra mixture component identification but reduced identification of the adapted ambient mixture components by an additional ∼20%. Rats quickly identify olfactory stimuli in natural settings; they use bouts of rapid 10-Hz sniffing to efficiently collect stimulus molecules (Wesson et al. 2009), but, when discriminating odors, slower “exploratory” sniffing is initiated within 150 ms. This early odor coding is associated with a phasic burst of OSN responding within a few olfactory bulb glomeruli (Wesson et al. 2008), a relationship that may be obscured by rapid sniffing (Carey et al. 2009). Thus, species-specific natural sniffing strategies may be critically involved in a dynamic olfactory coding.
Dynamic odor coding
Cooperative effects of mixture suppression and rapid adaptation associated with sniffing define dynamic olfactory coding. This inhibitory processing may occur across mammalian species in natural situations. The role of the segregation of hundreds of OR within independent restricted subsets of OSN became evident in engineered mice in which a single OR (M71) dominated all OSN. The mice could not discriminate the normal ligand of the dominant OR (acetophenone) from water (Fleischmann et al. 2008). This result, likely a sign of inhibition gone haywire, demonstrates the importance of organized inhibition among the independent inputs in odor coding.
Rapid adaptation in OSNs during transduction (Kelliher et al. 2003; Matthews and Reisert 2003; Song et al. 2008) is associated with sniffing strategies supporting odor recognition in rats (Wachowiak et al. 2009). In the olfactory bulb, inter-glomerular circuits, shown to operate in rabbits (Yokoi et al. 1995; Mori et al. 2006) and rodents (Schoppa and Urban 2003; Schoppa 2009; Soucy et al. 2009), would critically weaken less salient components in odor mixtures. Bulbar mutual inhibition may help explain a prevalence of general mixture suppression in mouse piriform cortex (Stettler and Axel 2009) as well as odor mixture suppression in human perception (Kurtz et al. 2009).
However, OR genes evolved rapidly, undoubtedly yielding tremendous species diversity in OR stimulus chemistry (Shi and Zhang 2009). About 1000 rodent OR chemoreceptors were discovered 20 years ago (Buck and Axel 1991), but relationships between stimulus chemistry and odor perceptions of nonhuman species’ remain mostly unknown (Youngentob et al. 2006). There may be hundreds of odors that rodents detect, each with rodent stimulus chemistry (Zhao et al. 1998; Lin et al. 2005, 2006). Yet researchers with newly available genetic techniques have had little recourse but to use “artificial chemicals” at “arbitrary” concentrations (Dulac 2006) to test similar, dissimilar, and limited odors judged from human perception (Malnic et al. 1999). Before discovery of the OR, olfactory coding was thought to involve patterns of activation of far fewer receptors; yet, similar “combinatorial” codes are embraced today for 1000 OR, each with its subset of labeled OSN; and searching continues for sites where several OSN may combine inputs in regions of the olfactory cortex (Zou and Buck 2006).
We developed a model for dynamic odor coding in humans based on realistic experimental studies of mixtures containing perceptually characterized independent, quickly self-adapting stimuli. Taking advantage of our own species’ well-appreciated odor perceptions (Moncrieff 1967; Wise et al. 2000; Bauer et al. 2001), we chose compounds that elicit clearly identifiable characteristic odors (Goyert et al. 2007). Human subjects perceived characteristic odors of single compounds when provided with a naturalistic experimental waxing and waning of stimuli (Goyert et al. 2007); in which multiple-component stimuli are effectively reduced in number by inhibitory physiological processing.
To summarize, our results show the following. 1) 1 and 5 mM vanillin and PEA elicited rapidly adapting independent odors that were not confused with each other and were mistaken for water when sufficiently reduced in effective concentration. 2) The 4 stimuli were nearly perfectly identified as single stimuli but PEA proved more effective than vanillin when tested in mixtures. 3) The 5-mM stimuli proved more effective only when in the context of 1-mM stimuli. 4) After adapting to one component, the new extra mixture component became more effective, whereas the adapted ambient component became less effective, more so when adapted for a longer time. We conclude that the olfactory system codes characteristic odors of critical compounds by adjusting the perceptual intensity of the many potential odor stimuli in dynamic natural settings.
Supplementary material
Supplementary material can be found at http//www.chemse.oxfordjournals.org/.
Funding
National Institutes of Health (5R01 DC004849 to M.E.F.).
Supplementary Material
Acknowledgments
The authors thank Janneane F. Gent for help with experimental design and approach to statistical analysis and Bradley K. Formaker for critically evaluating and editing previous versions of this manuscript.
References
- Axel R. Scents and sensibility: a molecular logic of olfactory perception (Nobel lecture) Angew Chem Int Ed. 2005;44:6111–6127. doi: 10.1002/anie.200501726. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. Taste mixtures: is mixture suppression related to compression? Physiol Behav. 1975;14:643–649. doi: 10.1016/0031-9384(75)90193-6. [DOI] [PubMed] [Google Scholar]
- Bauer K, Garbe D, Surburg H. Common fragrance and flavor materials: preparation, properties and uses. 4th ed. Weinheim (Germany): Wiley-VCH; 2001. [Google Scholar]
- Bell GA, Laing DG, Panhuber H. Odour mixture suppression: evidence for a peripheral mechanism in human and rat. Brain Res. 1987;426:8–18. doi: 10.1016/0006-8993(87)90419-7. [DOI] [PubMed] [Google Scholar]
- Berglund B, Berglund U, Lindvall T. Olfactory self- and cross-adaptation: effects of time of adaptation on perceived odor intensity. Sens Processes. 1978;2:191–197. [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Buck LB. Unraveling the sense of smell (Nobel lecture) Angew Chem Int Ed. 2005;44:6128–6140. doi: 10.1002/anie.200501120. [DOI] [PubMed] [Google Scholar]
- Carey RM, Verhagen JV, Wesson DW, Pírez N, Wachowiak M. Temporal structure of receptor neuron input to the olfactory bulb imaged in behaving rats. J Neurophysiol. 2009;101:1073–1088. doi: 10.1152/jn.90902.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS, Abraham MH, Gil-Lostes J. Concentration-detection functions for the odor of homologous n-acetate esters. Physiol Behav. 2008;95:658–667. doi: 10.1016/j.physbeh.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowart BJ. Relationships between taste and smell across the adult life span. Ann NY Acad Sci. 1989;561:39–55. doi: 10.1111/j.1749-6632.1989.tb20968.x. [DOI] [PubMed] [Google Scholar]
- Dalton P. Psychophysical and behavioral characteristics of olfactory adaptation. Chem Senses. 2000;25:487–492. doi: 10.1093/chemse/25.4.487. [DOI] [PubMed] [Google Scholar]
- Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav. 1978;20:175–185. doi: 10.1016/0031-9384(78)90070-7. [DOI] [PubMed] [Google Scholar]
- Dulac C. Sparse encoding of natural scents. Neuron. 2006;50:816–818. doi: 10.1016/j.neuron.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Fleischmann A, Shykind BM, Sosulski DL, Franks KM, Glinka ME, Mei DF, Sun Y, Kirkland J, Mendelsohn M, Albers MW, et al. Mice with a “monoclonal nose”: perturbations in an olfactory map impair odor discrimination. Neuron. 2008;60:1068–1081. doi: 10.1016/j.neuron.2008.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME. A perspective on chemosensory quality coding. In: Firestein S, Beauchamp GK, editors. The senses: a comprehensive reference, volume 4, olfaction and taste. San Diego (CA): Academic Press; 2008. pp. 339–344. [Google Scholar]
- Frank ME, Lundy RF, Jr, Contreras RJ. Cracking taste codes by tapping into sensory neuron impulse traffic. Prog Neurobiol. 2008;86:245–263. doi: 10.1016/j.pneurobio.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyert HF, Frank ME, Gent JF, Hettinger TP. Characteristic component odors emerge from mixtures after selective adaptation. Brain Res Bull. 2007;72:1–9. doi: 10.1016/j.brainresbull.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JG. Analysis of chemical signals by nervous systems. Proc Natl Acad Sci U S A. 1995;92:67–94. doi: 10.1073/pnas.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher KR, Ziesmann J, Munger SD, Reed RR, Zufall F. Importance of the CNGA4 channel gene for odor discrimination and adaptation in behaving mice. Proc Natl Acad Sci U S A. 2003;100:4299–4304. doi: 10.1073/pnas.0736071100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen S, Vento S, Malmberg H, Tuorila H. Correspondence between three olfactory tests and suprathreshold odor intensity ratings. Acta Otolaryngol. 2004;124:1072–1077. doi: 10.1080/00016480410015776. [DOI] [PubMed] [Google Scholar]
- Kurtz AJ, Lawless HT, Acree TE. Reference matching of dissimilar binary odor mixtures. Chem Percept. 2009;2:186–194. [Google Scholar]
- Laing DG. Natural sniffing gives optimum odour perception for humans. Perception. 1983;12:99–117. doi: 10.1068/p120099. [DOI] [PubMed] [Google Scholar]
- Laing DG. Identification of single dissimilar odors is achieved by humans with a single sniff. Physiol Behav. 1986;37:163–170. doi: 10.1016/0031-9384(86)90400-2. [DOI] [PubMed] [Google Scholar]
- Laing DG, Eddy A, Best DJ. Perceptual characteristics of binary, trinary, and quaternary odor mixtures consisting of unpleasant constituents. Physiol Behav. 1994;56:81–93. doi: 10.1016/0031-9384(94)90264-x. [DOI] [PubMed] [Google Scholar]
- Laing DG, Panhuber H, Willcox ME, Pittman EA. Quality and intensity of binary odor mixtures. Physiol Behav. 1984;33:309–319. doi: 10.1016/0031-9384(84)90118-5. [DOI] [PubMed] [Google Scholar]
- Laing DG, Willcox ME. Perception of components in binary odour mixtures. Chem Senses. 1983;7:249–264. [Google Scholar]
- Lin DY, Shea SD, Katz LC. Representation of natural stimuli in the rodent main olfactory bulb. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Lin DY, Zhang S-Z, Block E, Katz LC. Encoding social signals in the mouse main olfactory bulb. Nature. 2005;434:470–477. doi: 10.1038/nature03414. [DOI] [PubMed] [Google Scholar]
- Livermore A, Laing DG. Influence of training and experience on the perception of multicomponent odor mixtures. J Exp Psychol Hum Percept Perform. 1996;22:267–277. doi: 10.1037//0096-1523.22.2.267. [DOI] [PubMed] [Google Scholar]
- Livermore A, Laing DG. The influence of chemical complexity on the perception of multicomponent odor mixtures. Percept Psychophys. 1998a;60:650–661. doi: 10.3758/bf03206052. [DOI] [PubMed] [Google Scholar]
- Livermore A, Laing DG. The influence of odor type on the discrimination and identification of odorants in multicomponent odor mixtures. Physiol Behav. 1998b;65:311–320. doi: 10.1016/s0031-9384(98)00168-1. [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Matthews HR, Reisert J. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr Opin Neurobiol. 2003;13:469–475. doi: 10.1016/s0959-4388(03)00097-7. [DOI] [PubMed] [Google Scholar]
- McBurney DH, Balaban CD. A heuristic model of sensory adaptation. Atten Percept Psychophys. 2009;71:1941–1961. doi: 10.3758/APP.71.8.1941. [DOI] [PubMed] [Google Scholar]
- Moncrieff RW. Olfactory adaptation and odour likeness. J Physiol. 1956;133:301–316. doi: 10.1113/jphysiol.1956.sp005587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncrieff RW. The chemical senses. 3rd ed. London: Leonard Hill; 1967. [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Olsson MJ. An interaction model for odor quality and intensity. Percept Psychophys. 1994;55:363–372. doi: 10.3758/bf03205294. [DOI] [PubMed] [Google Scholar]
- Olsson MJ. An integrated model of intensity and quality of odor mixtures. Ann NY Acad Sci. 1998;855:837–840. doi: 10.1111/j.1749-6632.1998.tb10672.x. [DOI] [PubMed] [Google Scholar]
- Philpott CM, Gaskin JA, McClelland L, Goodenough PC, Clark A, Robinson AM, Murty GE. The Leicester semi-automated olfactory threshold test—a psychophysical olfactory test for the 21st century. Rhinology. 2009;47:248–253. doi: 10.4193/Rhin08.232. [DOI] [PubMed] [Google Scholar]
- Riffell JA, Abrell L, Hildebrand JG. Physical processes and real-time chemical measurement of the insect olfactory environment. J Chem Ecol. 2008;34:837–853. doi: 10.1007/s10886-008-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic I. Processing of odorous signals in humans. Brain Res Bull. 2001;54:307–312. doi: 10.1016/s0361-9230(00)00439-1. [DOI] [PubMed] [Google Scholar]
- Schoppa NE. Making scents out of how olfactory neurons are ordered in space. Nat Neurosci. 2009;12:103–104. doi: 10.1038/nn0209-103. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 2003;26:501–506. doi: 10.1016/S0166-2236(03)00228-5. [DOI] [PubMed] [Google Scholar]
- Shi P, Zhang J. Extraordinary diversity of chemosensory receptor gene repertoires among vertebrates. Results Probl Cell Differ. 2009;47:1–23. doi: 10.1007/400_2008_4. [DOI] [PubMed] [Google Scholar]
- Song Y, Cygnar KD, Sagdullaev B, Valley M, Hirsh S, Stephan A, Reisert J, Zhao H. Olfactory CNG channel desensitization by Ca2+/CaM via the B1b subunit affects response termination but not sensitivity to recurring stimulation. Neuron. 2008;58:374–386. doi: 10.1016/j.neuron.2008.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci. 2007;10:631–639. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Wachowiak M, Wesson DW, Pírez N, Verhagen JV, Carey RM. Low level mechanisms for processing odor information in the behaving animal. Ann NY Acad Sci. 2009;1170:286–292. doi: 10.1111/j.1749-6632.2009.04015.x. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Carey RM, Verhagen JV, Wachowiak M. Rapid encoding and perception of novel odors in the rat. PloS Biol. 2008;6:e82. doi: 10.1371/journal.pbio.0060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Verhagen JV, Wachowiak M. Why sniff fast? The relationship between sniff frequency, odor discrimination, and receptor neuron activation in the rat. J Neurophysiol. 2009;101:1089–1102. doi: 10.1152/jn.90981.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Olsson MJ, Cain WS. Quantification of odor quality. Chem Senses. 2000;25:429–443. doi: 10.1093/chemse/25.4.429. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Johnson BA, Leon M, Sheehe PR, Kent PF. Predicting odorant quality perceptions from multidimensional scaling of olfactory bulb glomerular activity patterns. Behav Neurosci. 2006;120:1337–1345. doi: 10.1037/0735-7044.120.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ivic L, Otaki JM, Hashimoto M, Mikoshiba K, Firestein S. Functional expression of a mammalian odorant receptor. Science. 1998;279:237–242. doi: 10.1126/science.279.5348.237. [DOI] [PubMed] [Google Scholar]
- Zou Z, Buck LB. Combinatorial effects of odorant mixes in olfactory cortex. Science. 2006;311:1477–1481. doi: 10.1126/science.1124755. [DOI] [PubMed] [Google Scholar]
- Zufall F, Leinders-Zufall T. The cellular and molecular basis of odor adaptation. Chem Senses. 2000;25:473–481. doi: 10.1093/chemse/25.4.473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.