Abstract
Peripheral taste receptor cells depend on distinct calcium signals to generate appropriate cellular responses that relay taste information to the central nervous system. Some taste cells have conventional chemical synapses and rely on calcium influx through voltage-gated calcium channels. Other taste cells lack these synapses and depend on calcium release from stores to formulate an output signal through a hemichannel. Despite the importance of calcium signaling in taste cells, little is known about how these signals are regulated. This review summarizes recent studies that have identified 2 calcium clearance mechanisms expressed in taste cells, including mitochondrial calcium uptake and sodium/calcium exchangers (NCXs). These studies identified a unique constitutive calcium influx that contributes to maintaining appropriate calcium homeostasis in taste cells and the role of the mitochondria and exchangers in this process. The additional role of NCXs in the regulation of evoked calcium responses is also discussed. Clearly, calcium signaling is a dynamic process in taste cells and appears to be more complex than has previously been appreciated.
Keywords: calcium clearance mechanisms, calcium signaling, mitochondria, sodium/calcium exchangers, taste
Introduction
When chemical stimuli are detected by peripheral taste cells, one of several different calcium signaling mechanisms may be activated, depending on the nature of the stimulus. Some taste stimuli have simple chemical structures and interact directly with ionotropic receptors located on the apical ends of the cell's microvilli. When ionotropic receptors are activated, they open and cause a cell depolarization. This cell depolarization generates an action potential, which causes voltage-gated calcium channels (VGCCs) to open, and a calcium influx that is necessary for vesicular neurotransmitter release (Yang, Crowley et al. 2000; Medler et al. 2003; Purves 2007). Other taste stimuli, including most bitter, sweet, and umami tastants are more chemically complex and activate G protein–coupled receptors (GPCRs) to cause a cellular response (Adler et al. 2000; Chandrashekar et al. 2000; Chaudhari et al. 2000; Matsunami et al. 2000; Max et al. 2001; Nelson et al. 2001, 2002). These taste-specific GPCRs initiate a signaling cascade that causes calcium release from internal calcium stores to activate a transient receptor potential cation channel (TRPM5) on the plasma membrane. TRPM5 opens and causes cell depolarization via an influx of monovalent cations, mostly sodium (Hofmann et al. 2003; Liu and Liman 2003; Prawitt et al. 2003; Kaske et al. 2007; Liman 2007). Taste cells that express this signaling pathway lack conventional synapses and rely on the opening of a hemichannel to release adenosine triphosphate (ATP) as a neurotransmitter (Finger et al. 2005; Huang et al. 2007; Romanov et al. 2007, 2008; Dando and Roper 2009).
Regardless of the signaling mechanism used, an increase in intracellular calcium is required for a normal output signal to be generated in taste cells. All cells tightly regulate cytosolic calcium levels to prevent nonspecific responses and taste cells are no exception. Although changes in cytosolic calcium are critical for peripheral taste transduction across most, if not all species, very little is currently known about the role of calcium regulation in this system. We have begun identifying the calcium clearance mechanisms (CCMs) expressed in taste cells and their role in calcium signaling. This review will be focused on our mouse studies, but CCMs are expected to have important functions in the peripheral taste signaling of all species.
Calcium responses in taste cells
In most cells, including excitable and nonexcitable cell types, calcium is a critical second messenger for many processes. Calcium signals are temporally and spatially complex; they can be transient or oscillatory and global or localized. As calcium enters the cytoplasm, there is a brief calcium increase at the entry site before it diffuses into the cell. Calcium can remain localized and activate effectors or can summate to yield global signals (Bootman et al. 2002). The complexity of calcium signaling depends on both the mechanisms used to increase calcium as well as multiple clearance mechanisms that function to return calcium to prestimulation levels.
Although increases in intracellular calcium are required for normal signaling to occur in taste receptor cells, few studies have focused on understanding any of the potential complexities within these signals. Several years ago, my laboratory set out to identify any differences in the calcium signals that depend on calcium release from internal stores compared with the calcium signals that depend on calcium influx. In other cell types, including neurons, these types of calcium signals are different from each other; however, this question had not previously been addressed in taste cells. For these studies, we measured global cytosolic calcium changes in acutely isolated individual taste receptor cells using the calcium sensitive dye, Fura 2-AM. We used 50 mM KCl to cause cell depolarization, which opens VGCCs and allows for calcium influx into the cells expressing these channels. To activate calcium release from internal stores, we used bitter compounds at concentrations we had previously determined would maximally activate calcium release. Therefore, any differences in the calcium signals were due to real differences in the calcium responses and not due to a partial stimulus response (Hacker et al. 2008).
Bitter, sweet, and umami taste stimuli activate membrane bound apically located GPCRs. When ligand binds, the taste GPCRs trigger the G protein βγ subunits to activate a phospholipase C (PLCβ) signaling pathway that produces inositol trisphosphate (IP3) and diacylglycerol (Huang et al. 1999; Ming et al. 1999; Yan et al. 2001). The IP3 binds to an IP3 receptor on the endoplasmic reticulum (ER) and causes calcium release from internal stores (Akabas et al. 1988; Rossler et al. 1998; Clapp et al. 2001, 2004; Miyoshi et al. 2001). Elevated intracellular calcium activates TRPM5 which opens and causes cell depolarization (Hofmann et al. 2003; Liu and Liman 2003; Prawitt et al. 2003; Zhang et al. 2007). Subsequent to this cell depolarization, a hemichannel opens and ATP is released which acts as a neurotransmitter (Finger et al. 2005; Huang et al. 2007; Romanov et al. 2007, 2008; Dando and Roper 2009). Disruption of this signaling pathway impairs the ability to detect multiple taste stimuli (Zhang et al. 2003; Damak et al. 2006).
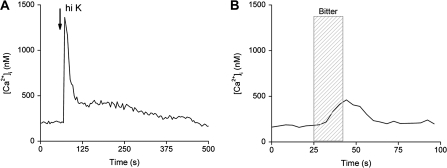
Analyses of these 2 different types of calcium signals revealed significant differences between them (Figure 1). On average, the bitter-induced calcium release from internal stores generates a peak calcium value around 200 nM, whereas the influx through VGCCs produces an average peak value of 3 μM calcium. In addition, the peak rise times for the calcium influx signals are significantly faster (average time = 6.2 s) than the peak rise times for the calcium signals due to calcium release from stores (average time = 42.6 s, P < 0.001) (Hacker et al. 2008). These calcium signals are clearly different from each other and appear to correspond with their specialized physiological roles: The calcium influx signal needs to be large to trigger vesicular release of neurotransmitter, whereas the calcium release from stores that activates the opening of TRPM5 is a smaller and slower signal.
Figure 1.
Examples of evoked calcium responses to either 50 mM KCl or 10 mM denatonium benzoate in isolated taste receptor cells. (A) A 10-s application of 50 mM KCl (arrow) caused a large increase over baseline values in taste cells that express VGCCs. After an initial rapid clearance of calcium, a plateau phase was present that eventually returned to baseline. (B) A taste receptor cell responded to a 20-s application of 10 mM denatonium benzoate (hatched column) with a small slow calcium increase.
Calcium clearance mechanisms
Although the source of the calcium signal is essential to forming an appropriate cellular response, another key element that contributes to generating a normal calcium signal is the mechanism(s) used by the cell to reduce elevated calcium. Without these CCMs, cells would be unable to return to baseline calcium levels and would not be responsive to further stimulation. In addition, prolonged calcium elevations generate nonspecific or inappropriate cellular responses to the initial stimulus (Blaustein 1988; Berridge and Bootman 1996; Berridge et al. 1998; Bootman et al. 2002; Augustine et al. 2003). Therefore, the functions of CCMs are crucial to the formation of the correct output signals in cells.
There are 5 known mechanisms that contribute to regulating calcium loads either by reducing cytosolic calcium or by temporarily buffering calcium elevations that are later removed. Two CCMs located on the plasma membrane are the sodium/calcium exchangers (NCXs) and the plasma membrane calcium ATPases (PMCAs), which extrude calcium out of the cell. PMCAs use ATP hydrolysis to pump calcium against its concentration gradient and out of the cell, whereas NCXs remove cytosolic calcium by exchanging 1 calcium ion for 3 external sodium ions. Exchangers take advantage of the strong inward electrochemical gradient for sodium to move a calcium ion against its concentration gradient and out of the cell. These actions result in a net positive charge across the membrane as the exchangers function. The excess sodium is later pumped out of the cell by the sodium/potassium ATPase (Blaustein 1988; Berridge et al. 1998; Blaustein et al. 2002; Bootman et al. 2002; Kim et al. 2005).
A third CCM is comprised of calcium ATPases that are associated with internal calcium stores and sequester cytosolic calcium into these stores. These ATPases reduce elevated cytosolic calcium but also function to maintain appropriate calcium levels inside the stores. The ER is probably the best-characterized internal calcium store and uses the sarco-ER calcium ATPase (SERCA) to take up cytosolic calcium. The ER is not the only calcium store, and in many cells, the nuclear envelope, synaptic vesicles, and mitochondria can also function in this capacity. Interactions between these different calcium stores can also impact calcium signaling in the cell (Verkhratsky and Petersen 1998; Parys et al. 2000).
Calcium-binding proteins are cytosolic proteins that bind free calcium ions in the cytosol to help control the magnitude of the calcium signal. There are approximately 240 calcium-binding proteins, some of which are pure calcium buffers, whereas others, such as calmodulin, function as calcium sensors and have additional signaling functions. Both types of calcium-binding proteins reversibly sequester calcium when cytosolic calcium levels are high (Rogers et al. 1990; Burgoyne and Weiss 2001; Haeseleer et al. 2002; Burgoyne 2007; Braunewell and Klein-Szanto 2009). In addition to calcium-binding proteins, mitochondria also act as CCMs and buffer cytosolic calcium. During large calcium loads, mitochondria take up calcium through a calcium selective uniporter. There is a large electrochemical gradient for calcium in the mitochondria due to the negative membrane potential and low calcium concentrations within the mitochondrial matrix. As excess calcium is removed from the cytosol, the calcium-binding proteins and mitochondria release the bound calcium, which is then extruded from the cell. These buffering mechanisms play key roles in calcium signaling because they can prevent calcium-dependent inactivation of calcium sensitive processes but can also prolong a calcium signal by slowly releasing the bound calcium back into the cytosol (Blaustein 1988; Budd and Nicholls 1996, 1998; Kits et al. 1997; Verkhratsky and Petersen 1998; Friel 2000; Nicholls and Budd 2000).
Increasingly, studies are finding that calcium-dependent signaling is affected by interactions between the ER and the mitochondria. These interactions impact the ER calcium stores, the intensity of the calcium influx through store-operated channel (SOC) channels, and even affect the calcium signal directly. In many systems, interactions between the ER and the mitochondria influence the activation of IP3-dependent calcium release (Hajnoczky et al. 1999; Gilabert et al. 2001; Montero, Alonso, Albillos, Cuchillo-Ibanez, et al. 2001; Montero, Alonso, Albillos, Garcia-Sancho, and Alvarez 2001) and can also modulate the store-operated response that is activated when calcium stores are depleted (Gilabert et al. 2001; Parekh 2003; Jackson and Thayer 2006). In some cells, mitochondria are vital to maintaining ER calcium stores, independent of the store-operated response (Landolfi et al. 1998; Pacher et al. 2000; Arnaudeau et al. 2001; Malli et al. 2005). Thus, the complexity of the calcium response depends on both the mitochondria and ER, and the responses vary depending on how these organelles interact with each other (Verkhratsky and Petersen 1998; Hajnoczky et al. 1999; Gilabert et al. 2001; Montero, Alonso, Albillos, Cuchillo-Ibanez, et al. 2001; Montero, Alonso, Albillos, Garcia-Sancho, and Alvarez 2001).
Basal calcium levels in taste cells
Our studies were performed on acutely isolated taste receptor cells from mice. In these preparations, the tongue epithelium was enzymatically disassociated from the underlying muscle and individual taste receptor cells were plated onto coverslips and kept under constant perfusion of Tyrode's solution. This experimental design allows us to measure individual cellular responses, but the cells are no longer in their native configuration, which may potentially affect their responses. For that reason, taste cells with a resting baseline calcium level greater than 200 nM were not used in these studies to ensure that we were not including damaged cells in our analyses (Hacker and Medler 2008; Laskowski and Medler 2009; Szebenyi et al. 2010). This value was based on the fact that most neurons have a resting calcium value around 100 nM and taste cells from other studies have reported similar values (Ogura et al. 1997; Baryshnikov et al. 2003).
In their native configuration, taste receptor cells are housed together in taste buds and extend microvilli into the oral cavity to detect chemicals from food. Although most of the cell is housed in a normal extracellular ionic environment, the microvilli are exposed to variable conditions. When an organism is not eating, the microvilli are bathed in saliva and oral fluids that have an overall low ionic content (Malamud 1993; Mandel 1993; Schramm et al. 1993), but during consumption, the oral environment changes and the microvilli are subjected to this variable milieu. In our experimental setup, the entire cell is kept in extracellular solution and the microvilli are no longer in a low ionic environment.
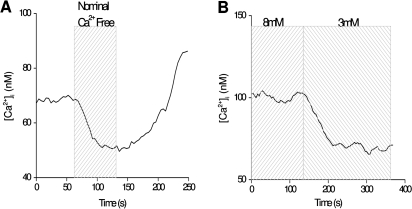
Despite being in a nonnative environment, isolated taste receptor cells maintain a resting calcium level at approximately 100 nM which is comparable to most neurons (Purves 2007). However, when external calcium is removed, baseline calcium levels in taste cells drop, even when the cell has not otherwise been stimulated (Figure 2A). Similar findings have been seen in other studies of taste cells (Ogura et al. 2002), but this is not a general characteristic of other cell types (White and Reynolds 1995; Lawrie et al. 1996; Mironov et al. 2005), which suggests that taste cells may have unique mechanisms to regulate cytosolic calcium. When the external calcium concentration is increased, baseline calcium levels also increase (Hacker and Medler 2008) with corresponding decreases if external calcium is decreased (Figure 2B).
Figure 2.
External calcium concentrations affect internal calcium levels in taste receptor cells. (A) Removing external calcium caused a decrease in cytosolic calcium levels that recovered when external calcium was replaced. Nominal calcium-free external solution consisted of Tyrode's solution with no calcium or calcium chelators added. (B) Changing external calcium concentrations from 8 to 3 mM decreased basal calcium levels, even in the absence of cell stimulation.
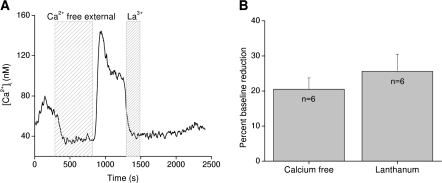
As cytosolic calcium levels return to equilibrium after the removal of external calcium, there is often a transient spike in cytosolic calcium as external calcium is replaced (Figure 3A). These data suggest a dynamic relationship between internal and external calcium that likely depends on a channel or channels at the plasma membrane. SOC blockers do not affect these calcium changes, but when the trivalent ion lanthanum chloride is applied, basal calcium levels decrease to levels comparable with removing external calcium (Figure 3A,B) (Hacker and Medler 2008). Lanthanum is a nonselective cation and TRP channel blocker (Zhu et al. 1996; Halaszovich et al. 2000; Jung et al. 2003), so these data suggest that the channel or channels responsible for the constitutive calcium influx may belong to the TRP family of channels.
Figure 3.
Cytosolic calcium is significantly influenced by constitutively active channels that are blocked by lanthanum. (A) Removing external calcium caused a transient decrease in cytosolic calcium levels that “rebounded” when external calcium was replaced. Addition of lanthanum chloride (La3+) to the external solution caused a comparable reduction in basal calcium levels. (B) The percent reduction in baseline calcium levels in calcium-free external solution or 100 μM LaCl3 in normal external were not significantly different. Reprinted from Hacker and Medler (2008) J Neurophys with permission from The Am Physiol Soc.
There appears to be an unusually dynamic relationship between the external and internal calcium in taste cells. It is important for cells to keep cytosolic calcium levels low to prevent nonspecific activation of calcium-dependent processes, and it is quite unusual that taste cells actively increase as well as decrease cytosolic calcium to maintain homeostasis. We began investigating this relationship and found that multiple CCMs contribute to the regulation of basal calcium in taste cells.
Mitochondrial regulation of basal calcium levels
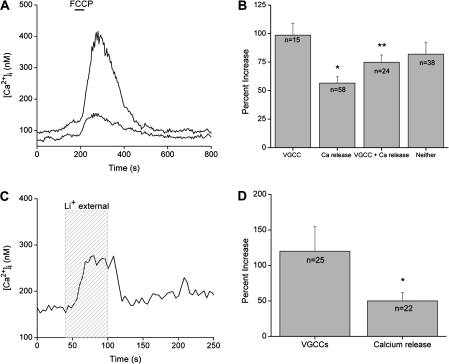
Mitochondria are well known calcium “sinks” that temporarily sequester calcium when a cell undergoes a large calcium load (Budd and Nicholls 1996, 1998; Nicholls and Budd 2000). In most cells, cell stimulation is required to generate a sufficient calcium load to activate mitochondrial calcium uptake, but in unstimulated taste cells, simply inhibiting this mitochondrial function causes an increase in cytosolic calcium. This indicates that the mitochondria are functioning to buffer cytosolic calcium even when the taste cell is not stimulated. Mitochondrial calcium uptake can be inhibited by the protonophore p-trifluoromethoxy-phenylhydrazone (FCCP) that dispels the large negative membrane potential across the inner mitochondrial membrane and greatly reduces the driving force for calcium to enter the mitochondria through the calcium uniporter (Ricken et al. 1998; Boitier et al. 1999). In other cell types, applying FCCP to unstimulated cells has no effect on baseline calcium (Budd and Nicholls 1996, 1998; Nicholls and Budd 2000). However, when taste cells are exposed in FCCP, there are variable calcium responses, as seen in Figure 4A, with peak calcium increases ranging from 32% to 826% over baseline calcium levels (average = 215% increase over baseline). As long as the mitochondria are disabled, cytosolic calcium is elevated, but it returns to baseline values when mitochondrial function is restored.
Figure 4.
CCMs routinely regulate cytosolic calcium levels in taste cells to maintain calcium homeostasis. (A) Application of the protonophore, FCCP (black bar), reversibly eliminated the mitochondria's ability to buffer calcium. FCCP caused increases in cytosolic calcium levels in 94% of previously unstimulated taste cells (n = 536). The amplitude of the response varied among different cells as shown here. Two taste cells simultaneously stimulated by FCCP had very different amplitudes in their calcium responses. (B) Amplitudes of the FCCP-induced calcium responses varied significantly across different taste cell populations. Taste cells that release calcium from internal stores in response to bitter stimuli but lack VGCCs had significantly smaller FCCP-dependent calcium responses compared with taste cells with VGCCs (*P < 0.001) or taste cells that were not responsive to taste stimuli and did not express VGCCs (labeled “neither,” *P = 0.02). Taste cells that responded to bitter stimuli (via calcium release from internal stores) and express VGCCs had significantly smaller FCCP-induced calcium elevations compared with taste cells that only have VGCCs (**P < 0.05). No other significant differences in the FCCP-dependent calcium responses were found. Reprinted from Hacker and Medler (2008) J Neurophys with permission from The Am Physiol Soc. (C) Replacing external sodium with lithium (light gray column, 60 s) caused a reversible increase in cytosolic calcium levels in 92% of taste cells tested (n = 146). (D) Taste cells that release calcium from internal stores in response to bitter taste stimuli but lack VGCCs had significantly smaller lithium-dependent calcium elevations compared with taste cells with VGCCs (*P < 0.05). Adapted from Laskowski and Medler (2009).
During these studies, we had to consider that one of the primary functions of mitochondria is to produce ATP and support the metabolic needs of the taste cell. In addition, both PMCAs and SERCA pumps require ATP to function, so it is possible that the mitochondria were not directly buffering cytosolic calcium but were indirectly inhibiting the calcium ATPases through ATP depletion. However, multiple control experiments demonstrated that within the time frame of the experiments, ATP depletion does not significantly contribute to these calcium elevations and that mitochondrial calcium uptake was the only process responsible for the measured effect (Hacker and Medler 2008). Based on these data, we concluded that mitochondria actively regulate cytosolic calcium in taste cells, even in unstimulated cells.
In an effort to identify the source of variability in the amplitudes of the calcium elevations due to FCCP, we evaluated the effect of different exposure times and concentrations of FCCP on the taste cells. There were no significant differences in the amplitude of the responses that correlated with either time or concentration. There were also no differences in the amplitude of the FCCP-induced calcium elevation that correlated with papillae type. However, there is a significant correlation between the signaling mechanisms used by the taste cells and the amplitude of the FCCP-induced calcium response. Taste cells with stimulus-induced calcium release from internal stores have significantly smaller changes in cytosolic calcium levels when the mitochondria are inhibited compared with taste cells that express VGCCs (Figure 4B; *P < 0.001). Taste cells that did not respond to either stimulus have comparable FCCP-dependent calcium responses with the taste cells that express VGCCs. In taste cells that respond to both cell depolarization and taste stimuli, the FCCP-dependent calcium responses are not significantly different from the taste cells that depend on calcium release from stores but are significantly smaller than the FCCP-dependent responses in taste cells that only express VGCCs (Figure 4B; **P < 0.05) (Hacker and Medler 2008). These data suggest a relationship between the amplitude of the FCCP-evoked calcium responses and the signaling mechanisms used by the taste cells.
It is not clear what is driving this relationship between mitochondrial calcium clearance and the signaling mechanisms that the taste cells use to respond to stimuli. It is possible that numbers of mitochondria vary across cell type due to the different metabolic needs of the cells or due to the different calcium-buffering requirements needed to appropriately control the evoked calcium signals. It is also possible that these different responses are due to distinct mitochondrial localizations within the cells. Mitochondria may cluster near the plasma membrane in taste cells with VGCCs but are preferentially localized close to the ER in taste cells that release calcium from stores in response to taste stimuli. Clustering near the ER would result in fewer mitochondria contributing to the regulation of any calcium influx signals, including a constitutive calcium influx. It is equally possible that there are different channels contributing to the constitutive calcium influx in the different cell types, and it is the magnitude of the calcium influx that is changing rather than any difference in the mitochondrial calcium buffering.
One of the most intriguing potential differences across cell types that may be significantly influencing the role of mitochondrial calcium buffering in taste cells is the presence of atypical mitochondria in some taste cells. These atypical mitochondria were anatomically identified in mouse taste cells over 20 years ago but have not been well characterized. Atypical mitochondria are much larger than normal mitochondria, are near sites of nerve contact, and often associate with subsurface cisternae (Royer and Kinnamon 1988). Subsurface cisternae have been reported to be predominantly found in type II taste cells (Clapp et al. 2004), which respond to bitter, sweet, or umami taste stimuli via calcium release from internal stores (Zhang et al. 2003). Taken together, these studies suggest a potential connection between a preponderance of atypical mitochondria in taste cells that use calcium release from stores and the role of mitochondrial calcium buffering in these taste cells. Currently, more studies are needed to characterize the role of these atypical mitochondria. It will be interesting to see if they are contributing to the differences in the calcium response amplitudes that occur when mitochondria are inhibited.
It was quite unexpected to discover that the mitochondria contribute to the regulation of basal calcium levels in the absence of cell stimulation. Mitochondria are known to modulate calcium signals in many cell types, including both excitable and nonexcitable cells. However, the mitochondrial calcium uptake mechanism, the calcium uniporter, has a relatively low affinity for calcium with a Kd between 10 and 20 μM, and mitochondria only begin accumulating calcium when the extramitochondrial calcium concentration is greater than 400 nM (Gunter and Pfeiffer 1990; Duszynski et al. 2006; Contreras et al. 2010). Because baseline calcium levels in taste cells are maintained close to 100 nM, it is not expected that mitochondria would take up calcium under these conditions. In many cell types, mitochondria do not actively take up calcium unless the cell undergoes a large stimulus-induced calcium load (Budd and Nicholls 1996, 1998; Nicholls and Budd 2000; Nicholls 2005).
In general, the localized calcium concentrations close to the initiation site of calcium signals can be many times higher than the bulk cytosolic calcium concentration (Rizzuto et al. 1993, 1999). Although it is not possible to accurately measure these calcium microdomains, it is thought that calcium levels within these microdomains could get high enough to activate calcium uptake by mitochondria that are located near the calcium signal site. Our data suggest that taste cells likely have localized microdomains of high calcium that are sufficient to activate the mitochondrial uniporter.
NCXs contribute to the regulation of basal calcium levels
Because mitochondria function as temporary calcium sinks and cannot take up calcium indefinitely, there has to be at least one calcium extrusion mechanism to remove excess calcium from the cytosol and prevent nonspecific calcium activation of cellular processes. We hypothesized that NCXs may be important in the regulation of basal calcium levels in taste cells. NCXs are localized on the plasma membrane in cells and have a low calcium affinity but high capacity for calcium removal. When cells have large calcium loads, PMCA activity becomes saturated and cells then rely on NCXs to remove the majority of the excess calcium from the cytosol (Monteith and Roufogalis 1995; Berridge and Bootman 1996; Sedova and Blatter 1999).
Even though lithium can substitute for sodium through many sodium channels, it is a good blocker of NCX activity because it cannot substitute for sodium in these exchangers (Blaustein and Santiago 1977). In unsimulated taste cells, inhibiting NCX activity by substituting lithium for external sodium increases cytosolic calcium. This calcium elevation is maintained as long as external sodium is absent (Figure 4C) and can range from 4% up to 400% increases in baseline calcium levels. When the exchangers are inhibited, the calcium elevation usually plateaus at a new concentration level where it is maintained until the exchangers are again functional. These elevations are due to the inhibition of the exchanger's role in removing cytosolic calcium and do not depend on time of inhibition or the ion used to substitute for sodium in the extracellular solution (Laskowski and Medler 2009).
Similar to the effects of inhibiting mitochondrial calcium uptake, there is variability in the magnitude of the calcium elevation when NCXs are inhibited that correlates with the calcium signaling mechanisms used by the taste cells. In taste cells that rely on calcium release from internal stores, the amplitude of the calcium elevation is significantly smaller than the increase in cytosolic calcium when the exchangers are inhibited in taste cells expressing VGCCs (Figure 4D) (Laskowski and Medler 2009). These data parallel our findings from inhibiting mitochondrial calcium buffering and strongly suggests that different taste cell populations have significant differences in the way they regulate cytosolic calcium. The reason for this variability is unclear but since exchangers are restricted to the plasma membrane, their cellular localization is less likely to be driving these differences. However, there may be a correlation between the channel(s) responsible for the calcium influx and the calcium signaling mechanism used by the taste cell. Taste cells express multiple exchanger isoforms (Laskowski and Medler 2009) and there may be some specificity in exchanger isoform expression that is driving the differences we have measured. Further studies are necessary to address these questions.
The mitochondria and exchangers synergistically regulate basal calcium levels
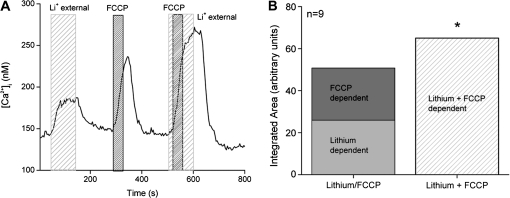
Because there were similar correlations in the response amplitudes when either the mitochondria or the exchangers were inhibited, we postulated that these 2 calcium regulatory mechanisms may work synergistically to regulate cytosolic calcium. To address this question, we analyzed the effect on cytosolic calcium when each CCM was individually inhibited and when they were simultaneously inhibited. When both CCMs are inhibited, the overall calcium response significantly increases compared with the magnitude of the calcium responses when the CCMs were individually inhibited (Figure 5A) (Laskowski and Medler 2009). There are 2 possible explanations for these findings. The first possibility is that the functions of these 2 CCMs are completely independent from the other, including the calcium loads they are regulating. If this is the case, then when both CCMs are inhibited simultaneously, the subsequent calcium response should equal the sum of these 2 independent events. The second possibility is that these 2 CCMs work together to regulate cytosolic calcium and if one mechanism is inhibited, the other CCM can at least partially compensate for the additional calcium load. If both mechanisms are inhibited, the overall calcium response will increase even more because neither mechanism is available to compensate for the other. Our analysis revealed that when both CCMs are inhibited, the calcium load is significantly greater than the sum of the individual calcium loads from inhibiting either NCX or mitochondrial calcium uptake (Figure 5B). Therefore, the mitochondria and NCXs appear to work jointly to keep cytosolic calcium levels low.
Figure 5.
Exchangers and mitochondrial calcium transport work together to regulate cytosolic calcium levels in taste cells. (A) Replacement of external sodium with lithium (gray column) to inhibit NCX activity caused an elevation in cytosolic calcium. Applying FCCP (1 μM, dark gray column) to inhibit mitochondrial calcium uptake also elevated cytosolic calcium. Adding FCCP when NCXs were inhibited caused a much larger increase in cytosolic calcium compared with FCCP or lithium alone. (B) The integrated area under the FCCP + lithium curve was significantly larger than the combination of the integrated areas for the calcium elevations that occurred when FCCP and lithium were applied individually (*P < 0.05). Adding the area of the FCCP-dependent calcium response to the integrated area of the calcium response when NCXs were inhibited was only 78% of the integrated area of the calcium response that was generated when FCCP was applied while NCXs were inhibited. Adapted from Laskowski and Medler (2009).
Channels involved in the constitutive calcium influx
Our initial data indicated that a TRP channel or channels may be involved in the constitutive calcium influx found in taste cells (Figure 3). Three TRP channels that have been identified in taste cells: the TRPM5 channel, a taste variant of TRPV1, and a heteromer of polycystic kidney disease PKD1L3/PKD2L1 (Liu and Liman 2003; Prawitt et al. 2003; Lyall et al. 2004; Lyall, Heck, Vinnikova, et al. 2005; Ishimaru et al. 2006; LopezJimenez et al. 2006; Kaske et al. 2007; Kataoka et al. 2008). The TRPM5 channel is monovalent selective (Hofmann et al. 2003; Ullrich et al. 2005) and could not contribute to the constitutive calcium influx. The PKD heteromer is only expressed in a subset of taste cells (Ishimaru et al. 2006; LopezJimenez et al. 2006; Kataoka et al. 2008; Ishii et al. 2009) but the constitutive calcium influx is observed in most taste cells, so the PKDs are unlikely to be important in regulating cytosolic calcium. Currently, the role of TRPV1 in taste transduction is not well defined. Initial studies proposed a role for this channel in salt taste (Lyall et al. 2004; Lyall, Heck, Phan, et al. 2005; Lyall, Heck, Vinnikova, et al. 2005), but behavioral studies concluded that TRPV1 was not necessary for salt detection (Ruiz et al. 2006; Treesukosol et al. 2007). Therefore, we hypothesized that TRPV1 may contribute to the constitutive calcium influx in mouse taste cells.
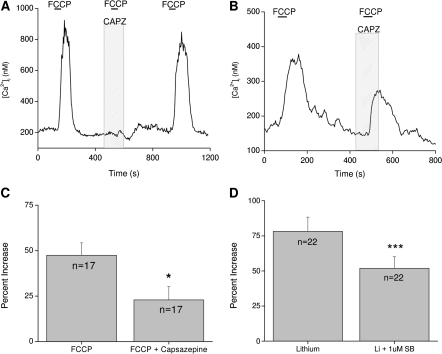
We initially used a TRPV1 antagonist capsazepine (10 μM) to determine if TRPV1 contributes to the FCCP-induced calcium elevations. In some taste cells, capsazepine completely abolishes the FCCP-dependent response (Figure 6A) while significantly reducing calcium elevations in other cells (Figure 6B,C) (Hacker and Medler 2008). These data suggest that TRPV1 is contributing to this constitutive calcium influx and for some taste cells is the only channel involved in this process. In other taste cells, multiple channels contribute to this calcium influx. Our findings were confirmed using another TRPV1 antagonist SB-366791 (Hacker and Medler 2008), and similar results were obtained when NCXs were inhibited in the presence of TRPV1 antagonists (Figure 6D) (Laskowski and Medler 2009). Taken together, these studies demonstrate that TRPV1 or a taste variant of TRPV1 is one of the channels contributing to the constitutive calcium influx across the plasma membrane in some taste cells. Additional channels appear to contribute to this constitutive calcium influx but they have not yet been identified. Future studies using the TRPV1 knockout mouse would provide valuable insights into cellular mechanisms controlling this constitutive calcium influx. Because it appears that other unidentified channels also contribute to this constitutive calcium influx, a great deal of study is needed to identify all the channels involved, ascertain their relative participation in the constitutive calcium influx and then relate how these findings affect calcium homeostasis in taste cells.
Figure 6.
TRPV1 contributes to the constitutive calcium influx that is regulated by CCMs in taste cells. (A) Application of the TRPV1 antagonist capsazepine (CAPZ, 10 μM) abolished the FCCP-dependent cytosolic calcium increases in some taste cells. (B) In other taste cells, capsazepine inhibited, but did not abolish, the rise in intracellular calcium when mitochondria were disabled with FCCP. (C) Capsazepine significantly inhibited the peak of the FCCP-induced calcium response in taste cells (*P < 0.05). Reprinted from Hacker and Medler (2008) J Neurophys with permission from The Am Physiol Soc. (D) Application of 1 μM SB-366791, another TRPV1 antagonist, significantly reduced the amplitude of the calcium response due to inhibition of NCX activity (***P < 0.0001). Adapted from Laskowski and Medler (2009).
Evoked calcium responses in taste cells
Although the CCMs in taste cells play a critical role in the regulation of basal calcium in the absence of cell stimulation, there is still a need for calcium clearance when cells are stimulated and have an acute rise in intracellular calcium. Our initial studies focused on the role of NCXs in terminating 2 different types of calcium responses: signals that depend on calcium influx through VGCCs and signals that depend on calcium release from internal stores.
When taste cells are depolarized and calcium enters the cell through VGCCs, there is an initial fast signal that reaches the peak value very quickly and then enters a plateau phase that slowly returns to prestimulus levels (Figure 1A). The time to return to baseline calcium can be quite prolonged and is likely due to the slow release of cytosolic calcium by the mitochondria and calcium-buffering proteins (White and Reynolds 1995, 1997; Medler and Gleason 2002). We performed experiments to determine if NCXs contribute to the regulation of this calcium influx signal in taste cells. When NCXs are inhibited by removing external sodium, the initial peak amplitude of the response does not change but the duration of the initial response significantly increases (Szebenyi et al. 2010). These data indicate that NCXs have a critical role in limiting the length of time that the cell is exposed to very high calcium levels due to open VGCCs but appear to be less important in regulating the initial amplitude of the peak calcium response.
Another possible explanation is that the peak amplitudes are not changing when NCXs are inhibited because most of the calcium influx through the VGCCs is coming through the L-type calcium channels that are calcium sensitive and are inactivated by elevated cytosolic calcium (Hille 2001). The additional calcium load due to NCX inhibition may be sufficient to at least partially inactivate these L-type calcium channels and thus reduce the overall peak calcium response that can occur during cell depolarization. The functional distribution of VGCCs in taste cells has not been well characterized, but studies have established that multiple VGCCs are expressed in taste cells, including L-type calcium channels (Behe et al. 1990; DeFazio et al. 2006; Roberts et al. 2009). It is clear that L-type calcium channels are not the only VGCCs in taste cells (Behe et al. 1990; DeFazio et al. 2006; Roberts et al. 2009), and although there was not a lot of variation in the measurements taken in our study (n = 35 cells) (Szebenyi et al. 2010), it would be useful to confirm these findings in taste cells with identified VGCCs. Data from the Roberts et al. (2009) study indicate that there is some segregation in the expression of VGCC isoforms within taste cells, so these studies should be possible. If L-type calcium channel inactivation was significantly affecting the response peak amplitude, the NCXs may actually have an important role in controlling the initial response magnitude that we were not able to measure in our earlier study.
Because NCXs routinely contribute to the regulation of the constitutive calcium influx, this generates an additional calcium load on the taste cells when NCXs are inhibited that has to be considered in the analysis. To evaluate the role of NCXs in the termination of the calcium influx signal, we integrated the area under the response curve to get a proportional measure of the amount of calcium that enters during cell stimulation. Once we accounted for the calcium load due to inhibiting NCXs alone, we compared the calcium loads due to opening VGCCs when NCXs are functional with the calcium load that occurs when VGCCs are open and NCXs are inhibited. Adding these individual calcium loads generates an overall calcium load that is significantly less than the load that occurs when both events happen at the same time. This suggests that the NCXs significantly regulate the calcium influx signal through VGCCs because when NCXs are nonfunctional, the calcium load becomes significantly bigger even after we account for the constitutive calcium influx (Szebenyi et al. 2010).
Similar experiments were performed to determine if NCXs significantly regulate the calcium signals depending on calcium release from internal stores. Taste cells were stimulated with denatonium to activate the PLCβ signaling pathway and cause calcium release from internal stores in bitter-sensitive taste cells (Figure 1B). Adding the integrated calcium loads for the denatonium-evoked calcium elevations and the calcium loads that develop when the exchangers are inhibited resulted in an overall calcium load that was approximately equal to the calcium load on the taste cell when both events occurred simultaneously. These data suggest that the NCXs do not significantly contribute to the termination of these calcium release signals (Szebenyi et al. 2010).
Because many bitter-responsive taste cells are type II taste cells and many taste cells with VGCCs are type III cells (Yang, Crowley, et al. 2000; Yang, Tabata, et al. 2000; Clapp et al. 2001, 2004, 2006; Miyoshi et al. 2001; Yee et al. 2001; DeFazio et al. 2006), we postulated that taste cell type was responsible for the different roles of NCXs in terminating these diverse calcium signals. We tested this hypothesis on a recently identified taste cell population that is bitter sensitive but also expresses VGCCs. These dual-responsive taste cells use both calcium release from stores and calcium influx through VGCCs (Hacker et al. 2008), so if cell type is controlling NCX activity, it should be similar regardless of the calcium signal in this taste cell population. In these taste cells, NCXs significantly regulate the calcium influx signals but do not affect the calcium signals that depend on release from internal stores (Szebenyi et al. 2010). Therefore, it does not appear that taste cell type influences the different roles for NCXs.
Because NCXs have a relatively low affinity for calcium (Matsuda et al. 1997), another potential reason for the differences is that the calcium load due to calcium release from stores is not large enough to activate the NCXs while the calcium influx signal is sufficient. It is also possible that the initiation site of the calcium signal is driving NCX activity because calcium influx occurs at the plasma membrane where NCXs are located, whereas calcium release occurs at the internal stores. Subsequent experiments determined that signal location rather than signal-induced calcium load underlies the selectivity of the NCX response. Weak depolarizations that generate much smaller calcium loads on the cell are still affected by NCX activity while comparable calcium loads due to calcium release from stores are not (Szebenyi et al. 2010). It is likely that either mitochondrial calcium uptake, SERCA pump activity on the ER, or a combination of both of these mechanisms are more important in the regulation of calcium loads due to the release from stores. Future studies will be focused on addressing this hypothesis.
Conclusions/future directions
To my knowledge, these are the only published studies addressing the complexity of calcium signaling in the peripheral taste receptor cells and specifically the role of CCMs in these processes. These studies have shown that calcium regulation is important in the formation of appropriate calcium responses and that evoked calcium responses can significantly change if CCMs are not functional. To date, mitochondrial calcium buffering and NCX activity have been identified but it is expected that other CCMs are also important in taste cells. PMCAs are expressed in taste cells (Medler 2005), but their role in regulating calcium in these cells has not been identified. PMCAs are important in regulating calcium in olfactory neurons (Weeraratne et al. 2006; Kleene 2009; Saidu et al. 2009) and they likely also have important roles in taste cells. It would be interesting to determine if PMCAs are similar to NCXs in taste cells and have the same selectivity in the evoked calcium signals they regulate. Calcium-binding proteins have also been identified in taste cells (Ichikawa et al. 1992; Johnson et al. 1992; Yamagishi et al. 1993; Barlow et al. 1996; Miyawaki et al. 1996, 1998; Ichikawa and Helke 1997; Yamamoto et al. 2000; Diaz-Regueira et al. 2005; Northcutt 2005; Germana et al. 2007; Ohkubo et al. 2007; Barreiro-Iglesias et al. 2008), their expression varies by species, and no functional studies have addressed their role in taste cells.
One of the most unexpected findings from these studies is the identification of the constitutive calcium influx in taste cells, even in the absence of cell stimulation. This constitutive calcium influx adds a complicating factor to studies focused on understanding calcium signaling in these cells. Because this calcium influx that must be regulated to maintain appropriate basal calcium levels, the CCMs have to be continuously activated. The dynamics of these processes are not known, but because there is also a need to regulate evoked calcium signals, we can assume that the CCMs are not maximally activated by the need to regulate this calcium influx. It is possible, but not currently known, that there is selectivity between isoform expression and function, i.e., certain exchangers regulate the calcium influx, while others are responsible for removing excess calcium due to stimulus signaling. It is equally possible that the CCMs are partially activated and are fully activated by a stimulus-induced calcium load. Many questions remain regarding this constitutive calcium influx and its influence on taste cell signaling. We postulate that this influx may have evolved to compensate for the variable external environment that taste cell microvilli are kept. It would be interesting to determine if similar characteristics are present in other species, especially marine organisms that are subject to such different external environments compared with mice.
Funding
The work discussed in this review was supported by a grant from the National Institutes of Health (DC006358) to KM.
Acknowledgments
Thanks to Dr S. Medler and M.R. Rebello for their helpful comments.
References
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988;242:1047–1050. doi: 10.1126/science.3194756. [DOI] [PubMed] [Google Scholar]
- Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca(2+) to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Barlow LA, Chien CB, Northcutt RG. Embryonic taste buds develop in the absence of innervation. Development. 1996;122:1103–1111. doi: 10.1242/dev.122.4.1103. [DOI] [PubMed] [Google Scholar]
- Barreiro-Iglesias A, Villar-Cervino V, Villar-Cheda B, Anadon R, Rodicio MC. Neurochemical characterization of sea lamprey taste buds and afferent gustatory fibers: presence of serotonin, calretinin, and CGRP immunoreactivity in taste bud bi-ciliated cells of the earliest vertebrates. J Comp Neurol. 2008;511:438–453. doi: 10.1002/cne.21844. [DOI] [PubMed] [Google Scholar]
- Baryshnikov SG, Rogachevskaja OA, Kolesnikov SS. Calcium signaling mediated by P2Y receptors in mouse taste cells. J Neurophysiol. 2003;90:3283–3294. doi: 10.1152/jn.00312.2003. [DOI] [PubMed] [Google Scholar]
- Behe P, DeSimone JA, Avenet P, Lindemann B. Membrane currents in taste cells of the rat fungiform papilla. Evidence for two types of Ca currents and inhibition of K currents by saccharin. J Gen Physiol. 1990;96:1061–1084. doi: 10.1085/jgp.96.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M, Bootman M. Calcium signaling. London: Chapman and Hall; 1996. [Google Scholar]
- Berridge MJ, Bootman MD, Lipp P. Calcium—a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- Blaustein MP. Calcium transport and buffering in neurons. Trends Neurosci. 1988;11:438–443. doi: 10.1016/0166-2236(88)90195-6. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Juhaszova M, Golovina VA, Church PJ, Stanley EF. Na/Ca exchanger and PMCA localization in neurons and astrocytes: functional implications. Ann N Y Acad Sci. 2002;976:356–366. doi: 10.1111/j.1749-6632.2002.tb04762.x. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Santiago EM. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977;20:79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ, Roderick HL. Calcium signalling: more messengers, more channels, more complexity. Curr Biol. 2002;12:R563–R565. doi: 10.1016/s0960-9822(02)01055-2. [DOI] [PubMed] [Google Scholar]
- Braunewell KH, Klein-Szanto AJ. Visinin-like proteins (VSNLs): interaction partners and emerging functions in signal transduction of a subfamily of neuronal Ca2+ -sensor proteins. Cell Tissue Res. 2009;335:301–316. doi: 10.1007/s00441-008-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996;66:403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. Mitochondria in the life and death of neurons. Essays Biochem. 1998;33:43–52. doi: 10.1042/bse0330043. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 2007;8:182–193. doi: 10.1038/nrn2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 2000;3:113–119. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta. 2010;1797(6–7):607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr, Glendinning JI, Ninomiya Y, et al. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–5906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Regueira SM, Lamas I, Anadon R. Calretinin immunoreactivity in taste buds and afferent fibers of the grey mullet Chelon labrosus. Brain Res. 2005;1031:297–301. doi: 10.1016/j.brainres.2004.10.039. [DOI] [PubMed] [Google Scholar]
- Duszynski J, Koziel R, Brutkowski W, Szczepanowska J, Zablocki K. The regulatory role of mitochondria in capacitative calcium entry. Biochim Biophys Acta. 2006;1757:380–387. doi: 10.1016/j.bbabio.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- Friel DD. Mitochondria as regulators of stimulus-evoked calcium signals in neurons. Cell Calcium. 2000;28:307–316. doi: 10.1054/ceca.2000.0172. [DOI] [PubMed] [Google Scholar]
- Germana A, Paruta S, Germana GP, Ochoa-Erena FJ, Montalbano G, Cobo J, Vega JA. Differential distribution of S100 protein and calretinin in mechanosensory and chemosensory cells of adult zebrafish (Danio rerio) Brain Res. 2007;1162:48–55. doi: 10.1016/j.brainres.2007.05.070. [DOI] [PubMed] [Google Scholar]
- Gilabert JA, Bakowski D, Parekh AB. Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx. EMBO J. 2001;20:2672–2679. doi: 10.1093/emboj/20.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol. 2008;99:1503–1514. doi: 10.1152/jn.00892.2007. [DOI] [PubMed] [Google Scholar]
- Hacker K, Medler KF. Mitochondrial calcium buffering contributes to the maintenance of basal calcium levels in mouse taste cells. J Neurophysiol. 2008;100:2177–2191. doi: 10.1152/jn.90534.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Sokal I, Filipek S, Palczewski K. Calcium-binding proteins: intracellular sensors from the calmodulin superfamily. Biochem Biophys Res Commun. 2002;290:615–623. doi: 10.1006/bbrc.2001.6228. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- Halaszovich CR, Zitt C, Jungling E, Luckhoff A. Inhibition of TRP3 channels by lanthanides. Block from the cytosolic side of the plasma membrane. J Biol Chem. 2000;275:37423–37428. doi: 10.1074/jbc.M007010200. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion channels of excitable membranes. Sunderland (MA): Sinauer Associates, Inc; 2001. [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, Rosenzweig S, Spielman AI, Max M, Margolskee RF. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H, Helke CJ. Coexistence of calcium-binding proteins in vagal and glossopharyngeal sensory neurons of the rat. Brain Res. 1997;768:349–353. doi: 10.1016/s0006-8993(97)00822-6. [DOI] [PubMed] [Google Scholar]
- Ichikawa H, Jacobowitz DM, Sugimoto T. Calretinin-immunoreactivity in the oro-facial and pharyngeal regions of the rat. Neurosci Lett. 1992;146:155–158. doi: 10.1016/0304-3940(92)90066-g. [DOI] [PubMed] [Google Scholar]
- Ishii S, Misaka T, Kishi M, Kaga T, Ishimaru Y, Abe K. Acetic acid activates PKD1L3-PKD2L1 channel—a candidate sour taste receptor. Biochem Biophys Res Commun. 2009;385:346–350. doi: 10.1016/j.bbrc.2009.05.069. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M, Zhuang H, Tominaga M, Matsunami H. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JG, Thayer SA. Mitochondrial modulation of Ca2+ -induced Ca2+ -release in rat sensory neurons. J Neurophysiol. 2006;96:1093–1104. doi: 10.1152/jn.00283.2006. [DOI] [PubMed] [Google Scholar]
- Johnson EW, Eller PM, Jafek BW, Norman AW. Calbindin-like immunoreactivity in two peripheral chemosensory tissues of the rat: taste buds and the vomeronasal organ. Brain Res. 1992;572:319–324. doi: 10.1016/0006-8993(92)90493-s. [DOI] [PubMed] [Google Scholar]
- Jung S, Muhle A, Schaefer M, Strotmann R, Schultz G, Plant TD. Lanthanides potentiate TRPC5 currents by an action at extracellular sites close to the pore mouth. J Biol Chem. 2003;278:3562–3571. doi: 10.1074/jbc.M211484200. [DOI] [PubMed] [Google Scholar]
- Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci. 2007;8:49. doi: 10.1186/1471-2202-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S, Yang R, Ishimaru Y, Matsunami H, Sevigny J, Kinnamon JC, Finger TE. The candidate sour taste receptor, PKD2L1, is expressed by type III taste cells in the mouse. Chem Senses. 2008;33:243–254. doi: 10.1093/chemse/bjm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Korogod N, Schneggenburger R, Ho WK, Lee SH. Interplay between Na+/Ca2+ exchangers and mitochondria in Ca2+ clearance at the calyx of Held. J Neurosci. 2005;25:6057–6065. doi: 10.1523/JNEUROSCI.0454-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kits KS, Dreijer AM, Lodder JC, Borgdorff A, Wadman WJ. High intracellular calcium levels during and after electrical discharges in molluscan peptidergic neurons. Neuroscience. 1997;79:275–284. [PubMed] [Google Scholar]
- Kleene SJ. Limits of calcium clearance by plasma membrane calcium ATPase in olfactory cilia. PLoS One. 2009;4:e5266. doi: 10.1371/journal.pone.0005266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolfi B, Curci S, Debellis L, Pozzan T, Hofer AM. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured In situ in intact cells. J Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski AI, Medler KF. Sodium/calcium exchangers contribute to the regulation of cytosolic calcium levels in mouse taste cells. J Physiol. 2009;587(Pt 16):4077–4089. doi: 10.1113/jphysiol.2009.173567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie AM, Rizzuto R, Pozzan T, Simpson AWM. A role for calcium influx in the regulation of mitochondrial calcium in endothelial cells. J Biol Chem. 1996;271:10753–10759. doi: 10.1074/jbc.271.18.10753. [DOI] [PubMed] [Google Scholar]
- Liman ER. TRPM5 and taste transduction. Handb Exp Pharmacol. 2007;179:287–298. doi: 10.1007/978-3-540-34891-7_17. [DOI] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LopezJimenez ND, Cavenagh MM, Sainz E, Cruz-Ithier MA, Battey JF, Sullivan SL. Two members of the TRPP family of ion channels, Pkd1l3 and Pkd2l1, are co-expressed in a subset of taste receptor cells. J Neurochem. 2006;98:68–77. doi: 10.1111/j.1471-4159.2006.03842.x. [DOI] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Phan TH, Mummalaneni S, Malik SA, Vinnikova AK, Desimone JA. Ethanol modulates the VR-1 variant amiloride-insensitive salt taste receptor. II. Effect on chorda tympani salt responses. J Gen Physiol. 2005;125:587–600. doi: 10.1085/jgp.200509264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Alam RI, Russell OF, Malik SA, Bigbee JW, DeSimone JA. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 2004;558:147–159. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall V, Heck GL, Vinnikova AK, Ghosh S, Phan TH, Desimone JA. A novel vanilloid receptor-1 (VR-1) variant mammalian salt taste receptor. Chem Senses. 2005 doi: 10.1093/chemse/bjh104. (30 Suppl)1: i42–i43. [DOI] [PubMed] [Google Scholar]
- Malamud D. Guidelines for saliva nomenclature and collection. Ann N Y Acad Sci. 1993;694:xi–xii. [Google Scholar]
- Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J Biol Chem. 2005;280:12114–12122. doi: 10.1074/jbc.M409353200. [DOI] [PubMed] [Google Scholar]
- Mandel ID. Salivary diagnosis: promises, promises. Ann N Y Acad Sci. 1993;694:1–10. doi: 10.1111/j.1749-6632.1993.tb18336.x. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Takuma K, Baba A. Na(+)-Ca2+ exchanger: physiology and pharmacology. Jpn J Pharmacol. 1997;74:1–20. doi: 10.1254/jjp.74.1. [DOI] [PubMed] [Google Scholar]
- Matsunami H, Montmayeur JP, Buck LB. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- Medler K, Gleason EL. Mitochondrial Ca(2+) buffering regulates synaptic transmission between retinal amacrine cells. J Neurophysiol. 2002;87:1426–1439. doi: 10.1152/jn.00627.2001. [DOI] [PubMed] [Google Scholar]
- Medler KF. Regulating calcium in taste cells: expression of calcium ATPases. Sarasota (FL): Achems XXVII; 2005. pp. #240. [Google Scholar]
- Medler KF, Margolskee RF, Kinnamon SC. Electrophysiological characterization of voltage-gated currents in defined taste cell types of mice. J Neurosci. 2003;23:2608–2617. doi: 10.1523/JNEUROSCI.23-07-02608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming D, Ninomiya Y, Margolskee RF. Blocking taste receptor activation of gustducin inhibits gustatory responses to bitter compounds. Proc Natl Acad Sci U S A. 1999;96:9903–9908. doi: 10.1073/pnas.96.17.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL, Ivannikov MV, Johansson M. [Ca2+]i signaling between mitochondria and endoplasmic reticulum in neurons is regulated by microtubules. From mitochondrial permeability transition pore to Ca2+-induced Ca2+ release. J Biol Chem. 2005;280:715–721. doi: 10.1074/jbc.M409819200. [DOI] [PubMed] [Google Scholar]
- Miyawaki Y, Morisaki I, Tabata MJ, Kurisu K, Wakisaka S. Calbindin D28k-like immunoreactivity in the gustatory epithelium in the rat. Neurosci Lett. 1996;214:29–32. doi: 10.1016/0304-3940(96)12871-8. [DOI] [PubMed] [Google Scholar]
- Miyawaki Y, Morisaki I, Tabata MJ, Maeda T, Kurisu K, Wakisaka S. Calbindin D28k-like immunoreactivity in the developing and regenerating circumvallate papilla of the rat. Cell Tissue Res. 1998;291:81–90. doi: 10.1007/s004410050981. [DOI] [PubMed] [Google Scholar]
- Miyoshi MA, Abe K, Emori Y. IP(3) receptor type 3 and PLCbeta2 are co-expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses. 2001;26:259–265. doi: 10.1093/chemse/26.3.259. [DOI] [PubMed] [Google Scholar]
- Monteith GR, Roufogalis BD. The plasma membrane calcium pump—a physiological perspective on its regulation. Cell Calcium. 1995;18:459–470. doi: 10.1016/0143-4160(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Montero M, Alonso MT, Albillos A, Cuchillo-Ibanez I, Olivares R, AGG, Garcia-Sancho J, Alvarez J. Control of secretion by mitochondria depends on the size of the local [Ca2+] after chromaffin cell stimulation. Eur J Neurosci. 2001;13:2247–2254. doi: 10.1046/j.0953-816x.2001.01602.x. [DOI] [PubMed] [Google Scholar]
- Montero M, Alonso MT, Albillos A, Garcia-Sancho J, Alvarez J. Mitochondrial Ca(2+)-induced Ca(2+) release mediated by the Ca(2+) uniporter. Mol Biol Cell. 2001;12:63–71. doi: 10.1091/mbc.12.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondria and calcium signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80:315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Taste bud development in the channel catfish. J Comp Neurol. 2005;482:1–16. doi: 10.1002/cne.20425. [DOI] [PubMed] [Google Scholar]
- Ogura T, Mackay-Sim A, Kinnamon SC. Bitter taste transduction of denatonium in the mudpuppy Necturus maculosus. J Neurosci. 1997;17:3580–3587. doi: 10.1523/JNEUROSCI.17-10-03580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Margolskee RF, Kinnamon SC. Taste receptor cell responses to the bitter stimulus denatonium involve Ca2+ influx via store-operated channels. J Neurophysiol. 2002;87:3152–3155. doi: 10.1152/jn.2002.87.6.3152. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y, Yokosuka H, Kumakura M, Yoshie S. Existence of subtypes of gustducin-immunoreactive cells in the vallate taste bud of guinea pigs. Arch Histol Cytol. 2007;70:291–296. doi: 10.1679/aohc.70.291. [DOI] [PubMed] [Google Scholar]
- Pacher P, Csordas P, Schneider T, Hajnoczky G. Quantification of calcium signal transmission from sarco-endoplasmic reticulum to the mitochondria. J Physiol. 2000;529(Pt 3):553–564. doi: 10.1111/j.1469-7793.2000.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh AB. Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane. J Physiol. 2003;547:333–348. doi: 10.1113/jphysiol.2002.034140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys JB, Vanlingen S, Raeymaekers L, De Smedt H, Missiaen L. Subcellular fractionation and intracellular calcium stores. In: Putney J, editor. Calcium signaling. Boca Raton (FL): CRC Press; 2000. pp. 71–110. [Google Scholar]
- Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, Penner R. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci U S A. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Neuroscience. Sunderland (MA): Sinauer Associates, Inc; 2007. [Google Scholar]
- Ricken S, Leipziger J, Greger R, Nitschke R. Simultaneous measurements of cytosolic and mitochondrial Ca2+ transients in HT29 cells. J Biol Chem. 1998;273:34961–34969. doi: 10.1074/jbc.273.52.34961. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Brini M, Chiesa A, Filippin L, Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–199. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- Roberts CD, Dvoryanchikov G, Roper SD, Chaudhari N. Interaction between the second messengers cAMP and Ca2+ in mouse presynaptic taste cells. J Physiol. 2009;587:1657–1668. doi: 10.1113/jphysiol.2009.170555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Khan M, Ellis J. Calretinin and other CaBPs in the nervous system. Adv Exp Med Biol. 1990;269:195–203. doi: 10.1007/978-1-4684-5754-4_32. [DOI] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossler P, Kroner C, Freitag J, Noe J, Breer H. Identification of a phospholipase C beta subtype in rat taste cells. Eur J Cell Biol. 1998;77:253–261. doi: 10.1016/s0171-9335(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Royer SM, Kinnamon JC. Ultrastructure of mouse foliate taste buds: synaptic and nonsynaptic interactions between taste cells and nerve fibers. J Comp Neurol. 1988;270:11–24. doi: 10.1002/cne.902700103. 58–59. [DOI] [PubMed] [Google Scholar]
- Ruiz C, Gutknecht S, Delay E, Kinnamon S. Detection of NaCl and KCl in TRPV1 knockout mice. Chem Senses. 2006;31:813–820. doi: 10.1093/chemse/bjl024. [DOI] [PubMed] [Google Scholar]
- Saidu SP, Weeraratne SD, Valentine M, Delay R, Van Houten JL. Role of plasma membrane calcium ATPases in calcium clearance from olfactory sensory neurons. Chem Senses. 2009;34:349–358. doi: 10.1093/chemse/bjp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm W, Smith RH, Craig PA. Methods of simplified saliva collection for the measurement of drugs of abuse, therapeutic drugs, and other molecules. Ann N Y Acad Sci. 1993;694:311–313. doi: 10.1111/j.1749-6632.1993.tb18374.x. [DOI] [PubMed] [Google Scholar]
- Sedova M, Blatter LA. Dynamic regulation of [Ca2+]i by plasma membrane Ca(2+)-ATPase and Na+/Ca2+ exchange during capacitative Ca2+ entry in bovine vascular endothelial cells. Cell Calcium. 1999;25:333–343. doi: 10.1054/ceca.1999.0036. [DOI] [PubMed] [Google Scholar]
- Szebenyi SA, Laskowski AI, Medler KF. Sodium/calcium exchangers selectively regulate calcium signaling in mouse taste receptor cells. J Neurophysiol. 2010;104(1):529–538. doi: 10.1152/jn.00118.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treesukosol Y, Lyall V, Heck GL, Desimone JA, Spector AC. A psychophysical and electrophysiological analysis of salt taste in Trpv1 null mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1799–R1809. doi: 10.1152/ajpregu.00587.2006. [DOI] [PubMed] [Google Scholar]
- Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, Nilius B. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium. 2005;37:267–278. doi: 10.1016/j.ceca.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Verkhratsky AJ, Petersen OH. Neuronal calcium stores. Cell Calcium. 1998;24:333–343. doi: 10.1016/s0143-4160(98)90057-4. [DOI] [PubMed] [Google Scholar]
- Weeraratne SD, Valentine M, Cusick M, Delay R, Van Houten JL. Plasma membrane calcium pumps in mouse olfactory sensory neurons. Chem Senses. 2006;31:725–730. doi: 10.1093/chemse/bjl014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Reynolds IJ. Mitochondria and Na+/Ca2+ exchange buffer glutamate-induced calcium loads in cultured cortical neurons. J Neurosci. 1995;15:1318–1328. doi: 10.1523/JNEUROSCI.15-02-01318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Reynolds IJ. Mitochondria accumulate Ca2+ following intense glutamate stimulation of cultured rat forebrain neurones. J Physiol. 1997;498(Pt 1):31–47. doi: 10.1113/jphysiol.1997.sp021839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi M, Ishizuka Y, Fujiwara M, Nakamura H, Igarashi S, Nakano Y, Kuwano R. Distribution of calcium binding proteins in sensory organs of the ear, nose and throat. Acta Otolaryngol Suppl. 1993;506:85–89. doi: 10.3109/00016489309130248. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Atoji Y, Suzuki Y. Calbindin D28k-immunoreactive afferent nerve endings in the laryngeal mucosa. Anat Rec. 2000;259:237–247. doi: 10.1002/1097-0185(20000701)259:3<237::AID-AR20>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Yan W, Sunavala G, Rosenzweig S, Dasso M, Brand JG, Spielman AI. Bitter taste transduced by PLC-beta(2)-dependent rise in IP(3) and alpha-gustducin-dependent fall in cyclic nucleotides. Am J Physiol Cell Physiol. 2001;280:C742–C751. doi: 10.1152/ajpcell.2001.280.4.C742. [DOI] [PubMed] [Google Scholar]
- Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. J Comp Neurol. 2000;424:205–215. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Yang R, Tabata S, Crowley HH, Margolskee RF, Kinnamon JC. Ultrastructural localization of gustducin immunoreactivity in microvilli of type II taste cells in the rat. J Comp Neurol. 2000;425:139–151. doi: 10.1002/1096-9861(20000911)425:1<139::aid-cne12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. J Comp Neurol. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhao Z, Margolskee R, Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27:5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jiang M, Peyton M, Boulay G, Hurst R, Stefani E, Birnbaumer L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]