Abstract
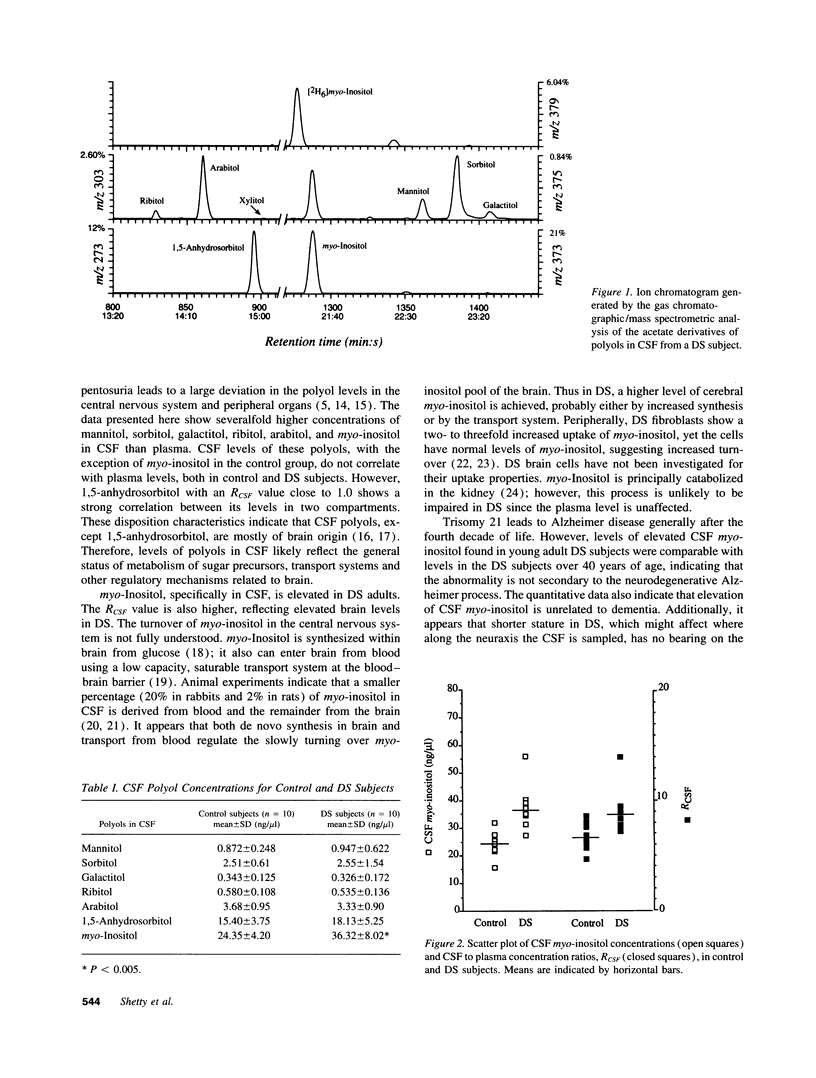
Polyols are reduction products of aldoses and ketoses; their concentrations in tissues can reflect carbohydrate metabolism. Several polyol species were quantitated in cerebrospinal fluid (CSF) and plasma from 10 Down Syndrome (trisomy 21) subjects between the ages of 22 and 63 years (3 of whom were demented) and from 10 healthy age-matched controls, using a gas chromatographic/mass spectrometric technique. The mean CSF concentration and the mean CSF/plasma concentration ratio of myo-inositol were significantly elevated in Down syndrome compared with controls, but were not correlated with the presence of dementia in the Down subjects. Plasma myo-inositol was not significantly altered in these subjects. No significant difference between Down syndrome and controls was found for CSF concentrations of mannitol, sorbitol, galactitol, ribitol, arabitol, or 1,5-anhydrosorbitol, but plasma mannitol, ribitol and arabitol were elevated in Down syndrome. The present observation provides new impetus for studying synthesis and transport of myo-inositol as well as phosphatidylinositol cycle in trisomy 21 disorder.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkai A. I. Myo-inositol turnover in the intact rat brain: increased production after d-amphetamine. J Neurochem. 1981 Apr;36(4):1485–1491. doi: 10.1111/j.1471-4159.1981.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, DeJesus P. V., Jr, Winegrad A. I. Raised plasma-myoinositol levels in uraemia and experimental neuropathy. Lancet. 1973 May 26;1(7813):1137–1141. doi: 10.1016/s0140-6736(73)91143-4. [DOI] [PubMed] [Google Scholar]
- Duara R., Margolin R. A., Robertson-Tchabo E. A., London E. D., Schwartz M., Renfrew J. W., Koziarz B. J., Sundaram M., Grady C., Moore A. M. Cerebral glucose utilization, as measured with positron emission tomography in 21 resting healthy men between the ages of 21 and 83 years. Brain. 1983 Sep;106(Pt 3):761–775. doi: 10.1093/brain/106.3.761. [DOI] [PubMed] [Google Scholar]
- Egan T. J., Wells W. W. Alternate metabolic pathway in galactosemia. Observations in patient with galactosemia. Am J Dis Child. 1966 Apr;111(4):400–405. doi: 10.1001/archpedi.1966.02090070098013. [DOI] [PubMed] [Google Scholar]
- Fruen B. R., Lester B. R. Down's syndrome fibroblasts exhibit enhanced inositol uptake. Biochem J. 1990 Aug 15;270(1):119–123. doi: 10.1042/bj2700119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruen B. R., Lester B. R. Inositol and inositol 1,4,5-trisphosphate content of Down syndrome fibroblasts exhibiting enhanced inositol uptake. FEBS Lett. 1991 Dec 16;295(1-3):43–47. doi: 10.1016/0014-5793(91)81380-q. [DOI] [PubMed] [Google Scholar]
- Ghalayini A., Eichberg J. Purification of phosphatidylinositol synthetase from rat brain by CDP-diacylglycerol affinity chromatography and properties of the purified enzyme. J Neurochem. 1985 Jan;44(1):175–182. doi: 10.1111/j.1471-4159.1985.tb07128.x. [DOI] [PubMed] [Google Scholar]
- HAUSER G., FINELLI V. N. THE BIOSYNTHESIS OF FREE AND PHOSPHATIDE MYO-INOSITOL FROM GLUCOSE BY MAMMALIAN TISSUE SLICES. J Biol Chem. 1963 Oct;238:3224–3228. [PubMed] [Google Scholar]
- Haxby J. V. Neuropsychological evaluation of adults with Down's syndrome: patterns of selective impairment in non-demented old adults. J Ment Defic Res. 1989 Jun;33(Pt 3):193–210. doi: 10.1111/j.1365-2788.1989.tb01467.x. [DOI] [PubMed] [Google Scholar]
- Holub B. J. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- Hsia D. Y., Smith G. F., Dowben R. M., Justice P. Down's syndrome. A critical review of the biochemical and immunological data. Am J Dis Child. 1971 Feb;121(2):153–161. [PubMed] [Google Scholar]
- Kinoshita J. H. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974 Oct;13(10):713–724. [PubMed] [Google Scholar]
- Liveson J. A., Gardner J., Bornstein M. B. Tissue culture studies of possible uremic neurotoxins: myoinositol. Kidney Int. 1977 Aug;12(2):131–136. doi: 10.1038/ki.1977.90. [DOI] [PubMed] [Google Scholar]
- Nahorski S. R., Ragan C. I., Challiss R. A. Lithium and the phosphoinositide cycle: an example of uncompetitive inhibition and its pharmacological consequences. Trends Pharmacol Sci. 1991 Aug;12(8):297–303. doi: 10.1016/0165-6147(91)90581-c. [DOI] [PubMed] [Google Scholar]
- Schapiro M. B., Atack J. R., Hanin I., May C., Haxby J. V., Rapoport S. I. Lumbar cerebrospinal fluid choline in healthy aging and in Down's syndrome. Arch Neurol. 1990 Sep;47(9):977–980. doi: 10.1001/archneur.1990.00530090047012. [DOI] [PubMed] [Google Scholar]
- Schapiro M. B., Grady C. L., Kumar A., Herscovitch P., Haxby J. V., Moore A. M., White B., Friedland R. P., Rapoport S. I. Regional cerebral glucose metabolism is normal in young adults with Down syndrome. J Cereb Blood Flow Metab. 1990 Mar;10(2):199–206. doi: 10.1038/jcbfm.1990.35. [DOI] [PubMed] [Google Scholar]
- Schapiro M. B., Haxby J. V., Grady C. L., Duara R., Schlageter N. L., White B., Moore A., Sundaram M., Larson S. M., Rapoport S. I. Decline in cerebral glucose utilisation and cognitive function with aging in Down's syndrome. J Neurol Neurosurg Psychiatry. 1987 Jun;50(6):766–774. doi: 10.1136/jnnp.50.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro M. B., Haxby J. V., Grady C. L. Nature of mental retardation and dementia in Down syndrome: study with PET, CT, and neuropsychology. Neurobiol Aging. 1992 Nov-Dec;13(6):723–734. doi: 10.1016/0197-4580(92)90096-g. [DOI] [PubMed] [Google Scholar]
- Servo C., Bergström L., Fogelholm R. Cerebrospinal fluid sorbitol and myoinositol in diabetic polyneuropathy. Acta Med Scand. 1977;202(4):301–304. doi: 10.1111/j.0954-6820.1977.tb16831.x. [DOI] [PubMed] [Google Scholar]
- Spector R., Lorenzo A. V. Myo-inositol transport in the central nervous system. Am J Physiol. 1975 May;228(5):1510–1518. doi: 10.1152/ajplegacy.1975.228.5.1510. [DOI] [PubMed] [Google Scholar]
- Spector R. Myo-inositol transport through the blood-brain barrier. Neurochem Res. 1988 Aug;13(8):785–787. doi: 10.1007/BF00971603. [DOI] [PubMed] [Google Scholar]
- TOUSTER O., SHAW D. R. Biochemistry of the acyclic polyols. Physiol Rev. 1962 Apr;42:181–225. doi: 10.1152/physrev.1962.42.2.181. [DOI] [PubMed] [Google Scholar]
- Tippett P., Kaplan J. C. Report of the Committee on the Genetic Constitution of Chromosomes 20, 21, and 22. Cytogenet Cell Genet. 1985;40(1-4):268–295. doi: 10.1159/000132177. [DOI] [PubMed] [Google Scholar]
- Varma S. D., Chakrapani B., Reddy V. N. Intraocular transport of myoinositol. II. Accumulation in the rabbit lens in vitro. Invest Ophthalmol. 1970 Oct;9(10):794–800. [PubMed] [Google Scholar]
- Yagi K., Kotaki A. The effect of massive doses of myo-inositol on hepatic phospholipid metabolism. Ann N Y Acad Sci. 1969 Oct 17;165(2):710–725. [PubMed] [Google Scholar]



