Abstract
Expression of herpes simplex virus genes at the initiation of replication involves two steps that take place at ND10 nuclear bodies. These are suppression of cellular repressors that attempt to silence viral DNA and remodeling of the viral chromatin to make it accessible for transcription. In earlier studies we reported on the mechanism by which viral proteins ICP0 and US3 protein kinase modify and disrupt the HDAC1/CoREST/REST/LSD1 repressor complex. The remodeling step requires in addition acetylation of histones bound to DNA. In an attempt to identify the enzyme, we took note of the observation that ICP0 physically and functionally interacts with Bmal1, a partner of the CLOCK histone acetyl transferase, and key members of the bHLH–PAS family of transcriptional factors. The Bmal11 and CLOCK heterodimer is best known as a regulator of the circadian oscillation in the mammalian CLOCK system. In this article we report the following: (i) in infected cells both Bmal1 and CLOCK localize at ND10 bodies; (ii) wild-type virus stabilizes the CLOCK protein; (iii) overexpression of CLOCK partially compensates for the absence of ICP0 and enables higher yields in cells infected with a ΔICP0 mutant and this activity is not expressed by CLOCK mutants lacking histone acetyl transferase activity; and (iv) depletion of CLOCK in cells infected with wild-type virus results in significant decrease in the expression of all viral proteins tested. We conclude that ICP0 interacts with Bmal1 and by extension with CLOCK histone acetyl transferase to remodel viral chromatin.
Keywords: Bmal1, chromatin, ICP0
On release from capsids at the nuclear pore, herpes simplex virus 1 (HSV1) DNA aggregates in the nucleus with histones, repressors, and complexes of proteins known as ND10 bodies. The objective of the bound histones and repressor complexes is to silence the viral DNA. Current models postulate that activation of the viral transcriptional program involves two steps. In the first, VP16, a viral tegument protein released into the cells during viral entry, interacts with the cellular proteins HCF1, Oct1, demethylases, and their partners to activate the α genes (1–3). The second step of the activation process involves the α protein ICP0. At low multiplicities of infection in the absence of ICP0, transcription of downstream β and γ genes does not ensue (4). ICP0, a multifunction protein performs two key functions to enable activation of transcription of downstream genes. Thus ICP0 acts as an ubiquitin ligase that interacts with the UbcH5a-conjugating enzyme to degrade PML and SP100, key components of the ND10 bodies (5–7). ICP0 in addition, binds to corepressor of resilencing transcriptional factor, REST (CoREST) and dislodges histone deacetylase (HDAC) 1 or 2 from the repressor complex whose key constituents are HDAC1 and -2, CoREST, REST, and the lysine-dependent demethylase 1 (LSD1) (8, 9). The dislocation of the repressor complex ensues. Ultimately, at least a fraction of the components of the complex is translocated to the cytoplasm (8, 9). The suppression of silencing predicts that in addition to the disruption of the repressor complex the virus must bring a histone acetyl transferase (HAT) to bear on the histones bound to the viral DNA to enable effective transcription. In this report we show that the CLOCK HAT plays an essential role in this process.
The circumstances that led us to consider CLOCK HAT were as follows. In collaborative studies with Y. Kawaguchi, this laboratory reported that ICP0 interacts with BMAL1 in the yeast two-hybrid system and also in vitro (10). In pulldown assays the interaction was mapped to residues 20–241 of ICP0 (10). The data also showed that ICP0 enhances the BMAL1-dependent transcription of p21 in vitro (10). The interest in BMAL1 stems from its association with the CLOCK HAT known to acetylate histone H3 (11). Both proteins are members of the bHLH–PAS family of transcriptional factors (12–14). Although Bmal1 plays a role in adaptation to environmental changes by partnering with other members of the family, it is best known for its interactions with the CLOCK HAT to regulate circadian oscillations of the mammalian CLOCK system (11, 15, 16). Dual modification of BMAL1 by SUMO and ubiquitin localizes the protein exclusively to ND10 bodies and simultaneously promotes its transactivation and ubiquitin-dependent degradation (17). During the transcriptional activation stage BMAL1 promotes E-box–dependent CLOCK genes transcription and nuclear translocation of CLOCK that is coupled with rapid proteolysis of both proteins (15). A recent report suggested also that BMAL1 mediates the nuclear translocation of CLOCK in a circadian manner and that this BMAL1-dependent translocation provides a regulatory mechanism by generating periodic availability of the heterodimeric BMAL1–CLOCK transactivation complex (16). CLOCK activity is also regulated by posttranslational modifications (16, 18). In addition, it has been demonstrated that the absolute molar concentration of BMAL1 is much lower than that of CLOCK (18). The observation that BMAL1 interacts with ICP0 in physical and functional screens raised the question whether the real target of HSV is the recruitment and utilization of CLOCK HAT.
We report that HSV-1 stabilizes and blocks degradation of CLOCK, that overexpression of wild-type CLOCK but not of a mutated CLOCK lacking HAT activity partially complements ΔICP0 mutants and finally, that in cells depleted of CLOCK by siRNA, the accumulation of viral proteins is severely reduced.
Results
Bmal1 and CLOCK Localize at ND10 Bodies.
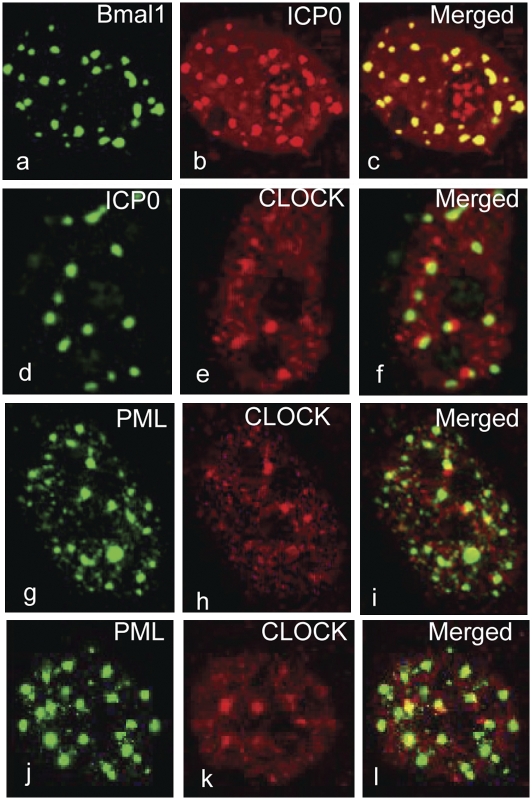
In this series of experiments we sought to determine whether Bmal1 and CLOCK are recruited to ND10 bodies identified by the presence of PML and in infected cells by the presence of both PML and ICP0. Bmal1 was barely visible in untreated uninfected cells. To determine the localization of Bmal1, HEp-2 cells were transfected with a plasmid encoding an HA-tagged Bmal1 gene and superinfected with wild-type virus. To stabilize Bmal1, MG132 was added to the cultures 2 h after infection. The design of the experiment took advantage of the observation that in cells transfected with DNA and then infected, the disappearance of ICP0 from the nucleus was delayed in proportion to the amount of transfected DNA (19). Hence in these experiments the cells were fixed 6 h after infection and scanned by confocal microscopy. The results in Fig. 1 A–C show that Bmal1 colocalizes with ICP0. To determine the localization of CLOCK in infected cells, HEp-2 cells were transfected first with irrelevant DNA and then infected with wild-type virus for 6 h prior to fixation. The results in Fig. 1 D–F show that in contrast to Bmal1, CLOCK surrounds or abuts the structures containing ICP0.
Fig. 1.
In infected cells CLOCK and Bmal1 localize at ND10 bodies. (A–C) HEp-2 cells were exposed to 10 pfu of HSV-1(F) per cell 24 h after transfection of a plasmid encoding HA–Bmal1. The cells were fixed 6 h after infection and reacted with the mouse monoclonal antibody to HA and the rabbit polyclonal antibody to ICP0. (D–F) HEp-2 cells were transfected with the empty vector pcDNA3.1Zeo(+) for 24 h prior to infection with the HSV-1(F) (10 pfu/cell). The cells were fixed at 6 h after infection and the cells reacted with the rabbit polyclonal antibody against CLOCK and the mouse monoclonal antibody against ICP0. (G–I) HEp-2 cells were infected with the wild-type virus (10 pfu/cell). The cells were fixed at 4 h after infection and reacted with the rabbit polyclonal antibody against CLOCK and the mouse monoclonal antibody against PML. (J–L) Mock-infected HEp-2 cells fixed and reacted with antibody as above. All of the images were captured with the same settings of a Zeiss confocal microscope.
To verify that the structures abutting CLOCK protein are ND10 bodies, HEp-2 cells mock infected or infected with the wild-type virus were fixed at 4 h after infection and reacted with antibodies against CLOCK and PML. The results (Fig. 1 G–I) show that CLOCK surrounds or abuts structures containing PML. Similar images were observed in mock-infected cells that reacted with antibodies to PML and CLOCK (Fig. 1 J–L).
We conclude that the stabilized form of Bmal1 colocalizes with ICP0 at the ND10 structures whereas CLOCK abuts or partially overlaps ND10 structures containing ICP0 and PML. The localization of CLOCK at ND10 structures takes place in both infected and uninfected cells.
HSV-1(F) Modifies the Endogenous CLOCK.
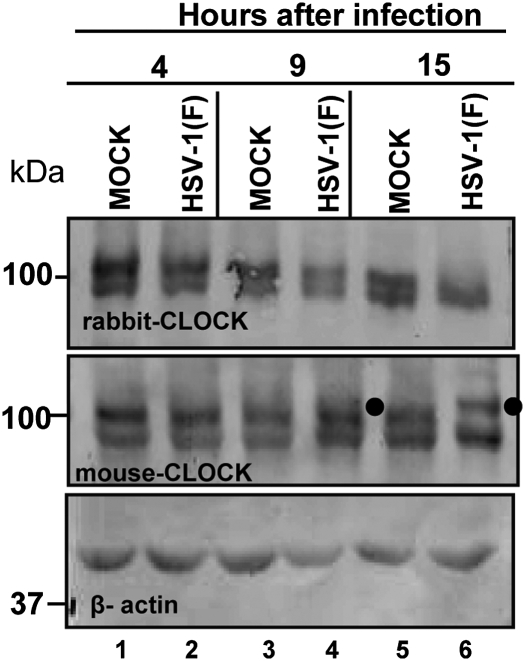
The objective of this experiment was to determine whether CLOCK is stable or posttranslationally modified during infection with wild-type virus. HEp-2 cells were mock infected or exposed to 10 pfu of wild-type virus per cell. The cells were harvested at 4, 9, or 15 h after infection and equal amounts of total lysates were solubilized, electrophoretically separated in a denaturing gel, and probed with a rabbit polyclonal antibody that was raised against the CLOCK residues 571–846 or a mouse monoclonal antibody that was raised against the amino acids 497–597 of the protein. The results shown in Fig. 2 may be summarized as follows: In samples collected at 4 or 9 h after infection or mock infection each of the two antibodies reacted with two protein bands (lanes 1–4). At later times (15 h) the polyclonal antibody to residues 571–846 reacted with the fast migrating band only. In contrast, the monoclonal antibody reacted both with the fast migrating lock band and also with a slower migrating band identified by the filled circle (lanes 4 and 6). The slow migrating band was barely visible in the immunoblot of the sample collected at 9 h after infection (lane 4).
Fig. 2.
Clock is modified in HSV-1(F)–infected cells. HEp-2 cells were mock infected or exposed to 10 pfu of HSV-1(F) per cell. The cells were harvested and lysed at 4, 9, or 15 h after infection. The electrophoretically separated denatured proteins were transferred to nitrocellulose sheets and immunoblotted either with the rabbit CLOCK antibody (Upper) or with the mouse CLOCK antibody (Lower). β-Actin served as a loading control. The filled circles point to a modified form of CLOCK.
We conclude that CLOCK is posttranslationally modified at 9 h or later. One hypothesis that could explain the difference obtained with the two antibodies is that the posttranslational modifications in residues 571–846 precluded the interaction of the polyclonal rabbit antibody but did not affect the interaction of the mouse anti-CLOCK antibody with the modified CLOCK.
HSV-1(F) Stabilizes CLOCK Independently and Additively upon Bmal1 Overexpression and/or Proteasome Inhibition.
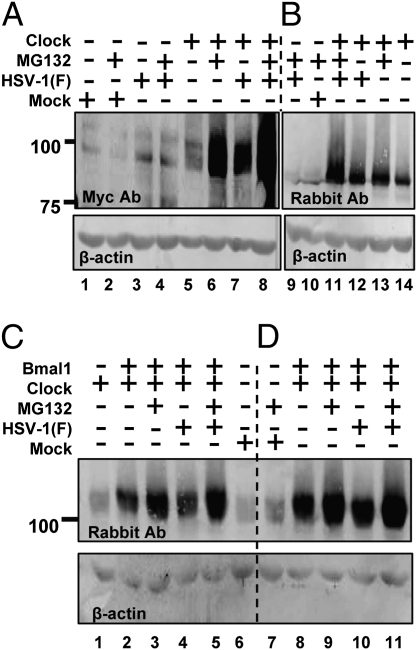
In these series of experiments, we sought to determine whether viral gene products mediate alterations in the stability of CLOCK. In these experiments, Vero cells were transfected with a plasmid-encoding myc-tagged CLOCK. After 24 h the cells were exposed to 10 pfu of HSV-1(F) per cell. Replicate cultures were exposed to MG132 (5 μM) at 2 h after infection. The cells were harvested at 9 h after infection and equal amounts of total cell lysates were electrophoretically separated on a denaturing gel and probed with antibodies to myc or to CLOCK. The results shown in Fig. 3 A and B may be summarized as follows:
(i) Significantly more CLOCK protein was detected in transfected cells treated with MG132 than in transfected untreated cells (A, compare lane 6 with lane 5 or B, compare lane 14 to lane 13).
(ii) Wild-type virus dramatically increased the stability of ectopically expressed CLOCK in transfected cells. The amount of CLOCK detected in transfected/infected cells attained levels comparable to those detected in cells uninfected/treated with MG132 (A, compare lane 7 with lanes 6 and 5 or B, compare lanes 12, 13, and 14).
(iii) Treatment of the infected cells with the MG132 inhibitor during the course of infection had an additive effect in the stability of CLOCK (A, compare lane 8 with lane 7 and 6 or B, lane 11 with 12 or 13).
Fig. 3.
HSV-1(F) stabilizes the exogenous CLOCK. (A and B) Vero cells were exposed to 10 pfu of HSV-1(F) per cell 24 h after transfection of a plasmid encoding myc–CLOCK. MG132 (5 μM) was added to the cultures 2 h after infection. The cells were harvested at 9 h after infection, and equal amounts of cellular proteins were analyzed for the abundance of the CLOCK either with the myc antibody (A) or with the CLOCK antibody (B). (C and D) Vero cells transfected either with the expressing myc–CLOCK only or cotransfected with plasmids encoding myc–CLOCK and HA–Bmal1 were infected with the HSV-1(F) virus and/or treated with MG132 as above. The cells were harvested at 4 or 9 h after infection. The electrophoretically separated proteins were immunoblotted with a CLOCK antibody. In all panels β-actin served as a loading control.
We conclude that HSV-1(F) stabilized the ectopically expressed CLOCK and the effect was enhanced in the presence of MG132.
In the next series of experiments we examined the effect of HSV-1(F) on the stability of CLOCK in the presence of Bmal1. For this purpose Vero cells were cotransfected with the myc–CLOCK- and the HA–Bmal1-expressing plasmids for 24 h prior to exposure to the wild-type virus. MG132 was added to the cultures 2 h after infection. The cells were harvested either 4 h (Fig. 3C) or 9 h (Fig. 3D) after exposure to the virus and equal amounts of lysates were subjected to electrophoresis on denaturing gel and probed for the abundance of the CLOCK protein. The results shown in Fig. 3 C and D may be summarized as follows:
(i) Bmal1 stabilized the ectopically expressed CLOCK (Fig. 3C, compare lanes 2 and 8 with lane 1).
(ii) Addition of MG132 inhibitor to the medium of cells cotransfected with Bmal1, and CLOCK had an additive effect in the stability of CLOCK (Fig. 3 C and D, compare lanes 3 and 2 with lanes 9 and 8).
(iii) Infection of cotransfected cells has only a small additive effect on the stability of CLOCK (Fig. 3 C and D, compare lanes 4 and 2 with lanes 10 and 8).
(iv) The highest levels of CLOCK were observed in transfected/infected cells treated with MG132 (Fig. 3 C and D, compare lanes 5 and 3 with lanes 11 and 9).
We conclude that CLOCK is stabilized by HSV-1 independently of Bmal1 or MG132.
Bmal1 Is Less Stable than CLOCK in HSV-1(F)–Infected Cells.
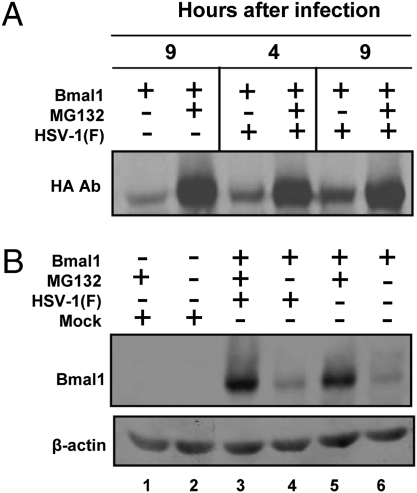
In this series of experiments, Vero cells were infected with HSV-1(F) 24 h after transfection with a plasmid-encoding HA-tagged Bmal1. MG132 was added to the culture 2 h after infection. The cells were harvested either 4 h or 9 h after infection, solubilized, subjected to electrophoresis in denaturing gels, and probed with either HA (Fig. 4A) or anti-Bmal1 (Fig. 4B) antibody. The results shown in Fig. 4 may be summarized as follows:
(i) The exogenous Bmal1 is unstable as evidenced by the observation that significantly more Bmal1 protein accumulated in cells treated with MG132 (Fig. 4A, compare lanes 1 and 2 and Fig. 4B, compare lanes 5 and 6).
(ii) The stabilization of Bmal1 during infection with HSV-1(F) may be evaluated by the comparison of lanes 1 and 5 in Fig. 4A and lanes 4 and 6 in Fig. 4B. It is, at best, much less dramatic than the stabilization of CLOCK described above.
Fig. 4.
The stability of ectopically expressed Bmal1 is not significantly affected by superinfection with wild-type virus. Vero cells transfected with the plasmid encoding HA–Bmal1 were infected with HSV-1(F) and/or treated with MG132, as above. The cells were harvested 4 h or 9 h after infection. The immunoblots were reacted with the HA (A) or with the Bmal1 antibodies (B).
Wild-Type CLOCK but Not a Mutant Impaired for the HAT Activity Partially Complemented the ΔICP0 Mutant Virus.
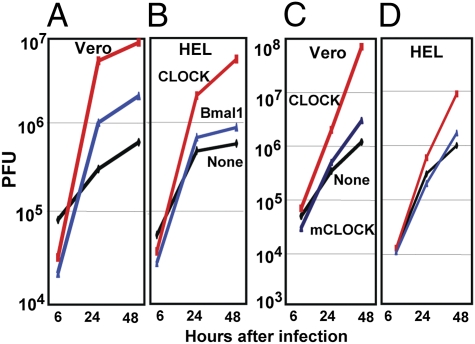
The fundamental hypothesis of these studies is that CLOCK is recruited to the ND10 bodies to enable the expression of viral genes. To test this hypothesis two series of experiments were done. In the first, we constructed three ΔICP0 mutant viruses carrying Bmal1 (R6705), wild-type CLOCK (R6703), or a CLOCK carrying substitutions G673A, S674A, and V676A (R6704), which have been reported to inactivate the HAT activity of CLOCK (11). Vero or HEL cells were infected with these viruses at a multiplicity of 0.1 pfu/cell. The results shown in Fig. 5 were as follows. (i) The mutant carrying wild-type CLOCK yielded more than 10-fold higher titers in Vero cells (Fig. 5A) and ≈10-fold higher in HEL cells (Fig. 4B) than the ΔICP0 mutant virus. The recombinant virus encoding Bmal1 was significantly less effective in rescuing the ΔICP0 mutant than the virus encoding the CLOCK gene. (ii) The yields of recombinant virus encoding the mutated CLOCK gene were not significantly different from that of ΔICP0 parent virus in either Vero (Fig. 5C) or HEL cells (Fig. 5D). We conclude that CLOCK partially rescues the ΔICP0 mutant and that the partial complementation required the HAT activity of the protein.
Fig. 5.
Insertion of the CLOCK gene but not of mutant CLOCK gene enhances the replication of ΔICP0 mutant virus. Vero (A and C) or HEL (B and D) cells were exposed to 0.1 pfu per cell to ΔICP0 virus (None, A–D), the R6703 ΔICP0 virus carrying the CLOCK gene (CLOCK, all panels), the R6704 ΔICP0 virus carrying the mutated CLOCK (mCLOCK, C and D) or the R6705 ΔICP0 virus carrying the Bmal1 gene (Bmal1, A and B). The viruses harvested at 6, 24, or 48 h after infection were titered on U2OS cells.
Knockdown of CLOCK Reduces Viral Protein Accumulation.
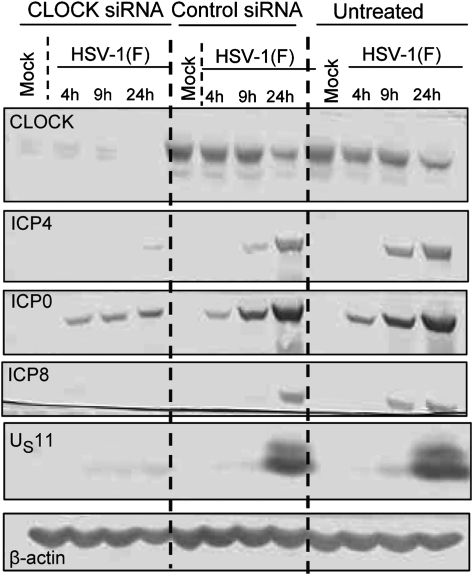
In this series of experiments we tested the effect of silencing of CLOCK on viral genes expression. Thus, HEp-2 cells were transfected with CLOCK or control siRNA as described in Materials and Methods. Approximately 80 h after transfection the cells were exposed to 10 pfu of HSV-1(F) virus per cell. The results in Fig. 6 show that silencing of CLOCK significantly affected the accumulation of ICP4, ICP8, and US11 proteins and reduced the accumulation of ICP0 at late times after infection. The effect of control siRNA was minimal: it affected the accumulation of ICP4 at 9 h but not at 24 h after infection.
Fig. 6.
Depletion of CLOCK attenuates viral genes expression. HEp-2 cells were either mock transfected, transfected with the CLOCK siRNA, or the control siRNA for 80 h before infection with the HSV-1(F) (10 pfu/cell). The cells were harvested at 4 h, 9 h, or 24 h after infection. Lysates containing 60 μg of total proteins were separated on denaturing polyacrylamide gels, electrically transferred to nitrocellulose sheets, and immunoblotted for Clock, ICP4, ICP0, ICP8, US11, and β-actin, as described in Materials and Methods.
We conclude that depletion of CLOCK severely impairs the expression of viral genes.
Discussion
Central to the model of activation of HSV-1 genes expression is that the host cell attempts to silence viral DNA by converging to its surface histones, repressors, and ND10 bodies. HSV remodels the ensuing viral heterochromatin in at least two stages. As cited in the introduction, cellular and viral proteins repress the silencing machinery at the promoters of α genes and enable their transcription (1, 2). In the second stage, ICP0, a multifunctional α protein, acts directly or recruits cellular factors to render the genome accessible to transcriptional factors. The available data suggest that ICP0 binds to CoREST and dislodges HDAC1 or -2 from the CoREST/REST/LSD1 complex (8, 9). The histones and CoREST are independently phosphorylated by US3 and both US3 and UL13 viral kinases, respectively (20). The histones bound to the DNA are demethylated (1), acetylated, and render the DNA available for transcription. A central question posed in the studies that led to this report is the identity of the HAT enzyme involved in rendering the viral chromatin accessible to transcription. In this article, we report that the CLOCK HAT plays an essential role in the expression of HSV genes. In brief, we showed that (i) CLOCK turns over rapidly in a proteasome-dependent fashion but is stabilized in HSV-1 infected cells; (ii) CLOCK localizes in structures that abut ND10 bodies. The localization of CLOCK vis-a-vis its partner, Bmal1, is similar to that of CoREST and ICP0, respectively. Whereas Bmal1 and ICP0 colocalize with the PML marker of ND10 bodies, CLOCK and CoREST abut the ND10 bodies presumably at the sites of accumulation of DNA introduced by infection or transfection; and (iii) ΔICP0 mutants carrying a wild-type CLOCK gene replicate to higher yields than the parent mutant or a ΔICP0 mutant carrying a CLOCK devoid of HAT activity. Lastly, depletion of CLOCK resulted in grossly diminished accumulations of most viral proteins tested to date. The sum total of the data presented here makes a strong case for the involvement of CLOCK HAT in the remodeling of viral chromatin.
We initiated these studies on the basis of the observation that ICP0 interacts with Bmal1 (10) but the studies reported here focused on CLOCK, a partner of Bmal1. We did not, however, specifically focus on the chain linking ICP0 with CLOCK HAT. Both Bmal1 and CLOCK accumulate at ND10 bodies in cells transfected with irrelevant DNA in the absence of infection. Because ND10 bodies form at the sites of accumulation of transfected DNA, the data suggest that both proteins are also recruited to those sites and that the recruitment is not virus specific. In light of this observation, one effect of virus infections appears to be on the stability of CLOCK and Bmal1. The results and their implications are as follows:
(i) CLOCK appears to be completely shielded from proteolytic degradation during virus infection. In contrast, Bmal1 is not similarly shielded. If Bmal1 and CLOCK were to function in both infected and uninfected cells as heterodimers, it could be predicted that both proteins would be equally stabilized in the course of infection with wild-type virus. It is conceivable that the active enzyme is not bound to Bmal1 and turns over very rapidly. If this were the case, the stabilization of CLOCK would require an interaction with a viral gene product in infected cells.
(ii) CLOCK and Bmal1 are normally associated as heterodimers. The Bmal1/CLOCK heterodimer would be expected to colocalize in nuclei of infected cells. Whereas Bmal1 localizes with PML in ND10 structure, CLOCK is found in structures that abut, but only occasionally totally overlap with the ND10 structures.
(iii) Unlike overexpression of CLOCK, overexpression of Bmal1 does not significantly increase the yields of ΔICP0 mutant virus.
(iv) It is difficult to reconcile the E-box promoters on whose histones CLOCK normally acetylates with the wild range of promoters in the HSV genome without invoking partners that expand the range of promoters that would be activated by CLOCK.
These observations suggest that in infected cells CLOCK is sequestered, stabilized, and possibly redirected to acetylate histones on novel promoters. This model is not unprecedented but rather, it reflects a basic strategy evolved by HSV. Thus, HSV has evolved a phosphatase accessory factor, ICP34.5, whose major function is to bind and redirect cellular protein phosphatase 1α to dephosphorylate the α subunit of the translation initiation factor eIF-2 (21, 22). It remains to be seen whether another viral protein stabilizes and redirects CLOCK to acetylate histones on a wide range of viral promoters. If our hypothesis is tenable, the role of ICP0 may be to facilitate the activation of CLOCK by binding to Bmal1.
It is of interest to note that in the initial stages of infection of cells depleted of CLOCK, the levels of ICP0 are similar to those of untreated cells or cells exposed to control siRNA. At that time, the accumulation of all other proteins is retarded. Only at later time points is the accumulation of ICP0 reduced. This observation is consistent with the hypothesis that activation of CLOCK follows initial synthesis of ICP0. Conversely, depletion of CLOCK could have a global effect on the transcription of all genes including those of the α genes.
Materials and Methods
Cells and Viruses.
Elsewhere were reported the sources and propagation of HEp-2, Vero, HEL, and U2OS cells (23) the isolation of HSV-1(F), a limited passage isolate that is the HSV-1 prototype strain used in this laboratory (24), and the derivation and properties of a BAC encoding the ΔICP0 DNA (25). All of the titrations of the ΔICP0 or the ΔICP0 derived viruses were done in U2OS.
Plasmid Construction and Site-Directed Mutagenesis.
The full-length CLOCK was PCR amplified from the KIAA0334 clone, digested with EcoRV, and inserted into the EcoRV site of pCDNA3.1(+)ZeoMyc to generate pRB6721. The PmeI fragment from pRB6721 containing the myc-tagged CLOCK was inserted into the SpeI–XhoI sites of pRB5162 (26) to generate pRB6722. The SphI fragment of myc-tagged CLOCK under the control of the Egr-1 promoter followed by the bidirectional UL21/UL22 poly(A) from pRB6722 was inserted into the unique BamHI site between UL3 and UL4 of pRB6717 (23) to generate pRB6723. The CLOCK codon substitutions G673A, S674A, and V676A were done with the aid of the site-directed mutagenesis kit (Stratagene) according to the supplier's instructions, using the pRB6721 as template and the primers forward 5′ TGGTGATTTCTCAGCCTGCAGCCGCAGCCATGG CCCAGATTCCAT CTAGTATGCCACAA 3′ and reverse 5′ TTGTGGCATACTAGATGGA ATCTGGGCCATGG CTGCGGCTGCAGGCTGAGAAATCACCA 3′ to generate pRB6724. The PmeI fragment from pRB6724 containing the myc-tagged mutated CLOCK was inserted into the SpeI–XhoI sites of pRB5162 to generate pRB6725. The SphI fragment of the myc-tagged mutated CLOCK under the Egr-1 promoter followed by the bidirectional UL21/UL22 poly(A) from pRB6725 was inserted into the BamHI site between UL3 and UL4 of pRB6717 to generate pRB6726.
The HA-tagged Bmal1 in the pcDNA3 vector was kindly provided by Kimitoshi Kohno (University of Occupational and Environmental Health, Kitakyushu, Japan) (27). The NruI–XhoI fragment of HA–Bmal1 from pcDNA3 was inserted into the SpeI–XhoI sites of pRB5162 to generate pRB6727. The PCR-amplified fragment of Bmal1 under CMV promoter followed by the bidirectional UL21/UL22 poly(A) from pRB6727 was digested with XhoI and inserted into the BamHI site of pRB6717 (23) to generate pRB6728.
Construction of Recombinant Viruses.
The procedures were described elsewhere (23). Briefly, Escherichia coli RR1 strain harboring ΔICP0 BAC was electroporated with pRB6723, or pRB6726, or pRB6728 and incubated at 43 °C on LB plates containing 25 μg/mL of zeosin (Zeo) and 20 μg/mL of chloramphenicol. The colonies were diluted and plated on LB plates containing chloramphenicol (20 μg/mL) and 5% sucrose. Colonies grown on sucrose plates were screened by colony hybridization. Plasmids isolated from positive colonies were transfected into U2OS cells. The incorporation of WT CLOCK, mutated CLOCK, or Bmal1 at the desired location of the recombinant viruses R6703, R6704, and R6705, respectively, was verified by PCR.
Transfection and Infections.
Cells grown in four-well slides (Erie Scientific) were transfected when 60–70% confluent with 300 ng per well of DNA in mixtures of 1 μL of Lipofectamine and 1.5 μL of Plus reagents, as specified by the supplier (Invitrogen). Cells grown in six-well plates were transfected with 1 μg total DNA in mixtures of 6 μL of Plus reagents and 4 μL of Lipofectamine per well. At 3 h after transfection, the medium was removed, and the cells were rinsed extensively with DMEM supplemented with 10% FBS and further incubated for 24 h. The cells were exposed to 10 pfu of virus per cell in medium 199V, consisting of mixture 199 (Sigma) supplemented with 1% calf serum 24 h after transfection. MG132 was added at a final concentration 5 μM 2 h after infection.
Immunoblot Analysis.
The procedures were described elsewhere (28). Briefly, the cells were harvested at the indicated times after infection, rinsed with PBS, solubilized in triple detergent buffer [50 mM Tris-HCl (pH 8), 150 mM NaCl, 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 100 μg·mL−1 of phenylmethylsulfonyl fluoride) supplemented with phosphatase inhibitors (10 mM NaF, 10 mM β-glycerophosphate, 0.1 mM sodium vanadate) and protease inhibitor mixture (Sigma) as specified by the manufacturer, and briefly sonicated. The proteins concentration in total cell lysates was determined with the aid of Bio-Rad protein assay (Bio-Rad Laboratories). Approximately 60 μg of proteins per sample were subjected to further analysis.
Proteins were electrophoretically separated in denaturing polyacrylamide gels, electrically transferred to nitrocellulose sheets, blocked with PBS supplemented with 0.02% (vol/vol) Tween 20 (PBST) and 5% nonfat milk, and reacted overnight at 4 °C with the appropriate primary antibodies diluted in PBST 1% nonfat milk. The rabbit polyclonal antibodies to Clock (Santa Cruz), Bmal1 (10), and the mouse monoclonal antibodies to Clock (Abnova), HA-probe and c-myc (Santa Cruz) were used at 1:500 dilution. The mouse monoclonal antibody to β-actin (Sigma) was used in a 1:1,000 dilution. The ICP4, ICP0, ICP8, and US11 mouse monoclonal antibodies (Goodwin Institute for Cancer Research, Plantation, FL) were used in a 1:1,000 dilution. After several rinses with PBST 1% nonfat milk, the membranes were reacted with the appropriate secondary antibody conjugated either to alkaline phosphatase or to horseradish peroxidase. Finally, protein bands were visualized with 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium (Denville Scientific) or with ECL Western blotting detection reagents (Amersham Biosciences) according to manufacturer's instruction.
Immunofluorescence Analysis.
The procedures were described elsewhere (28). Briefly, the cells were fixed in 4% paraformaldehyde, at times indicated in Results, permeabilized, blocked with PBS–TBH solution consisting of 0.1% Triton X-100 in PBS, 10% horse serum, and 1% BSA, and reacted with primary antibodies diluted in PBS–TBH. The ICP0 mouse monoclonal antibody (Goodwin Institute for Cancer Research) was used at a 1:500 dilution. The ICP0 exon 2 rabbit polyclonal antibody was used in a dilution 1:2,000 (26). The CLOCK rabbit polyclonal antibody, the HA probe and PML mouse monoclonal antibodies (Santa Cruz Biotechnology) were used in a dilution 1:500. The four-well cultures were then rinsed several times with PBS–TBH and reacted with Alexa-Fluor-594–conjugated goat anti-rabbit or Alexa-Fluor-488–conjugated goat anti-mouse, diluted 1:1,000 in PBS–TBH. After several rinses, first with PBS–TBH and then with PBS, the samples were mounted and examined with a Zeiss confocal microscope equipped with software provided by Zeiss.
Depletion of CLOCK by siRNA.
The CLOCK small interfering RNA (siRNA), 5′-UAAAGUCUGUUGUUGUAUCAUGUGC-3′ (sense) and 5′-GCACAUGAUACAACAA CAGACUUUA-3′ (antisense) were obtained from Invitrogen. The negative control was also purchased from Invitrogen. siRNA transfections were performed according to manufacturer's instruction with minor modifications. Briefly, 10 μL of Lipofectamine 2000 was diluted in 250 μL Opti-MEM I medium and incubated for 5 min at room temperature. Next, 150 pmol of either CLOCK siRNA or control siRNA diluted in Opti-MEM I were added gently to the Lipofectamine mixture and incubated for 20 min at room temperature. Oligomer–Lipofectamine complexes were added to cells seeded on six-well plates at a confluency of 40–50%. Approximately 80 h after the transfection, the cells were infected with wild-type virus (10 pfu/cell), harvested at the indicated time points, and equal amounts of total proteins were analyzed for the abundance of the viral proteins and the efficiency of CLOCK depletion.
Acknowledgments
We thank Dr. Kimitoshi Kohno (University of Occupational and Environmental Health, Kitakyushu, Japan) for kindly providing the pcDNA 3 HA-BMAL1 encoding plasmid. These studies were aided by National Cancer Institute Grant R37 CA78766.
Footnotes
The authors declare no conflict of interest.
References
- 1.Liang Y, Vogel JL, Narayanan A, Peng H, Kristie TM. Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency. Nat Med. 2009;15:1312–1317. doi: 10.1038/nm.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kristie TM, Liang Y, Vogel JL. Control of alpha-herpesvirus IE gene expression by HCF-1 coupled chromatin modification activities. Biochim Biophys Acta. 2010;1799:257–265. doi: 10.1016/j.bbagrm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKnight JL, Kristie TM, Roizman B. Binding of the virion protein mediating alpha gene induction in herpes simplex virus 1-infected cells to its cis site requires cellular proteins. Proc Natl Acad Sci USA. 1987;84:7061–7065. doi: 10.1073/pnas.84.20.7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao F, Schaffer PA. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol. 1995;69:6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boutell C, Sadis S, Everett RD. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J Virol. 2002;76:841–850. doi: 10.1128/JVI.76.2.841-850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu H, Roizman B. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc Natl Acad Sci USA. 2003;100:8963–8968. doi: 10.1073/pnas.1533420100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagglund R, Van Sant C, Lopez P, Roizman B. Herpes simplex virus 1-infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin-conjugating enzymes. Proc Natl Acad Sci USA. 2002;99:631–636. doi: 10.1073/pnas.022531599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu H, Liang Y, Mandel G, Roizman B. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc Natl Acad Sci USA. 2005;102:7571–7576. doi: 10.1073/pnas.0502658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu H, Roizman B. Engagement of the lysine-specific demethylase/HDAC1/CoREST/REST complex by herpes simplex virus 1. J Virol. 2009;83:4376–4385. doi: 10.1128/JVI.02515-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaguchi Y, et al. Herpes simplex virus 1 alpha regulatory protein ICP0 functionally interacts with cellular transcription factor BMAL1. Proc Natl Acad Sci USA. 2001;98:1877–1882. doi: 10.1073/pnas.041592598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Gekakis N, et al. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- 13.Hogenesch JB, Gu YZ, Jain S, Bradfield CA. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc Natl Acad Sci USA. 1998;95:5474–5479. doi: 10.1073/pnas.95.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King DP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon I, et al. BMAL1 shuttling controls transactivation and degradation of the CLOCK/BMAL1 heterodimer. Mol Cell Biol. 2006;26:7318–7330. doi: 10.1128/MCB.00337-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondratov RV, et al. BMAL1-dependent circadian oscillation of nuclear CLOCK: Posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev. 2003;17:1921–1932. doi: 10.1101/gad.1099503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J, et al. Dual modification of BMAL1 by SUMO2/3 and ubiquitin promotes circadian activation of the CLOCK/BMAL1 complex. Mol Cell Biol. 2008;28:6056–6065. doi: 10.1128/MCB.00583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 19.Kalamvoki M, Roizman B. Nuclear retention of ICP0 in cells exposed to HDAC inhibitor or transfected with DNA before infection with herpes simplex virus 1. Proc Natl Acad Sci USA. 2008;105:20488–20493. doi: 10.1073/pnas.0810879105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon AP, Gu H, Roizman B. ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc Natl Acad Sci USA. 2006;103:9993–9998. doi: 10.1073/pnas.0604142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci USA. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He B, Gross M, Roizman B. The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J Biol Chem. 1998;273:20737–20743. doi: 10.1074/jbc.273.33.20737. [DOI] [PubMed] [Google Scholar]
- 23.Kalamvoki M, Roizman B. ICP0 enables and monitors the function of D cyclins in herpes simplex virus 1 infected cells. Proc Natl Acad Sci USA. 2009;106:14576–14580. doi: 10.1073/pnas.0906905106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 25.Gu H, Roizman B. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc Natl Acad Sci USA. 2007;104:17134–17139. doi: 10.1073/pnas.0707266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyamoto N, et al. Tip60 is regulated by circadian transcription factor clock and is involved in cisplatin resistance. J Biol Chem. 2008;283:18218–18226. doi: 10.1074/jbc.M802332200. [DOI] [PubMed] [Google Scholar]
- 28.Kalamvoki M, Roizman B. The interwoven roles of cyclin D3 and CDK4 recruited by ICP0 and ICP4 in the expression of herpes simplex virus genes. J Virol. 2010;84:9709–9717. doi: 10.1128/JVI.01050-10. [DOI] [PMC free article] [PubMed] [Google Scholar]