Abstract
Fasting promotes hepatic gluconeogenesis to maintain glucose homeostasis. The cAMP-response element binding protein (CREB)-regulated transcriptional coactivator 2 (CRTC2) is responsible for transcriptional activation of gluconeogenic genes and is critical for conveying the opposing hormonal signals of glucagon and insulin in the liver. Here, we show that suppressor of MEK null 1 (SMEK1) and SMEK2 [protein phosphatase 4 (PP4) regulatory subunits 3a and 3b, respectively] are directly involved in the regulation of hepatic glucose metabolism in mice. Expression of hepatic SMEK1/2 is up-regulated during fasting or in mouse models of insulin-resistant conditions in a Peroxisome Proliferator-Activated Receptor-gamma Coactivator 1α (PGC-1α)-dependent manner. Overexpression of SMEK promotes elevations in plasma glucose with increased hepatic gluconeogenic gene expression, whereas depletion of the SMEK proteins reduces hyperglycemia and enhances CRTC2 phosphorylation; the effect is blunted by S171A CRTC2, which is refractory to salt-inducible kinase (SIK)-dependent inhibition. Taken together, we would propose that mammalian SMEK/PP4C proteins are involved in the regulation of hepatic glucose metabolism through dephosphorylation of CRTC2.
Keywords: liver, glucose, insulin resistance
Under fasting conditions, hepatic glucose production is enhanced to maintain glucose homeostasis. Transcriptional activation of hepatic gluconeogenic genes is partially responsible for the phenomenon, and the cAMP/cAMP-response element binding protein (CREB)-dependent signaling cascade is critical for the process (1–4). Recently, the role of a previously undescribed CREB-regulated transcriptional coactivator 2 (CRTC2, also known as TORC2) and its involvement in hepatic gluconeogenesis was described (5–8). Briefly, fasting hormone glucagon and cAMP-mediated dephosphorylation of CRTC2 lead to its nuclear localization and subsequent activation of gluconeogenic gene expression. AMP-activated protein kinases (AMPKs) and the related kinases, salt-inducible kinase 1 (SIK1) and SIK2, are involved in the phosphorylation of CRTC2 at serine 171, causing its cytoplasmic retention through 14-3-3 binding. Insulin and Akt are responsible for the inactivation of this process by activating SIK2 under feeding conditions. Activation of SIK2 reduces the level of active CRTC2, thus turning off the transcription of gluconeogenic genes and reducing glucose production from the liver (9). Such regulation is perturbed in the case of peripheral insulin resistance, and it was postulated that the accumulation of CRTC2 in the nucleus results in increased glucose production from the liver in diabetic animal models.
Factors involved in the regulation of longevity and stress resistance in lower eukaryotes are often found to function in caloric restriction and energy metabolism in mammals. Previously, Caenorhabditis elegans SMK-1, a homolog of a Dictyostelium suppressor of MEK null (SMEK), was shown to be responsible for stress resistance phenotypes and for lifespan extension observed with reduced insulin/insulin-like growth factor (IGF)-1 receptor signaling and with diet restriction (10, 11). Later, it was shown that Saccharomyces cerevisiae Psy2, an ortholog of SMK-1, is a unique regulatory subunit for phosphatase family 4 (PP4), and is involved in the cisplatin-based anticancer therapeutical resistance in this organism (12). In mammalians, two PP4R3 subunits were identified, termed PP4R3a (SMEK1) and PP4R3b (SMEK2). The resulting active PP4 complex includes protein phosphatase 4 catalytic subunit (PP4C), PP4R3, and PP4R2, and belongs to the type 2A family of phosphatases (13).
In this study, we show that mammalian orthologs for C. elegans SMK-1, SMEK1 and SMEK2, function as PP4R3s for a PP4 complex that enhances hepatic glucose production. We observed increased expression of both PP4R3 isoforms under fasting conditions or in mouse models of insulin resistance. In vitro phosphatase assay revealed that SMEK/PP4C is able to dephosphorylate phosphoserine 171 directly in CRTC2. Overexpression of SMEK promotes elevations in plasma glucose with increased hepatic gluconeogenic gene expression, whereas knockdown of hepatic SMEK proteins improves hyperglycemia by reducing hepatic glucose production via promotion of CRTC2 phosphorylation. These data support the proposal that SMEK/PP4C regulates hepatic glucose production by controlling CRTC2-dependent transcriptional events.
Results
SMEK Expression Is Induced on Fasting or by Insulin Resistance in the Liver.
In C. elegans, SMK-1 was shown to be required for stress responses and lifespan extension because of reduced insulin/IGF signaling (11). The convergence of downstream target genes by SMK-1 and DAF-16 suggests a possible genetic interaction between two factors in nematodes (14, 15). Mammalian forkhead transcription factors such as Forkhead box O (FOXO) 1a are known to regulate hepatic energy metabolism, including gluconeogenesis (16), prompting us to investigate whether SMEK1 and SMEK2 are involved in the regulation of glucose metabolism in the liver. Interestingly, hepatic expression levels of SMEK1 and SMEK2 are elevated during fasting conditions (Fig. 1A and Fig. S1A Upper) or in mouse models of diet-induced or genetic insulin resistance (Fig. 1B and Fig. S1A Lower and B) in a manner similar to that of gluconeogenic genes. Glucagon-mediated induction of SMEK1 and SMEK2 mRNA levels is reduced by cotreatment with insulin in hepatocytes (Fig. S1C), a trend that is similar to the expression pattern of Peroxisome Proliferator-Activated Receptor-gamma Coactivator 1α (PGC-1α), a major regulator for hepatic transcription in response to fasting or insulin resistance (2, 17–21). This led us to test whether SMEK family members are transcriptionally regulated by PGC-1α. Indeed, both SMEK1 and SMEK2 mRNA and protein levels are reduced in livers of PGC-1α knockdown mice compared with control groups (Fig. S1 D and E). Conversely, overexpression of PGC-1α enhances expression of SMEK1 and SMEK2 (Figs. S1F and S2A). Promoter analysis revealed a potential hepatocyte nuclear factor 4α (HNF4α)-binding site, which would allow transcriptional regulation of both SMEK genes by PGC-1α, and this notion was confirmed by ChIP studies that showed binding of HNF4α and PGC-1α and demonstrated that SMEK2 promoter reporter activity is stimulated by HNF4α and PGC-1α (Fig. S2B). These data suggest that expression of both SMEK1 and SMEK2 is regulated by nutrient status in a PGC-1α–dependent manner.
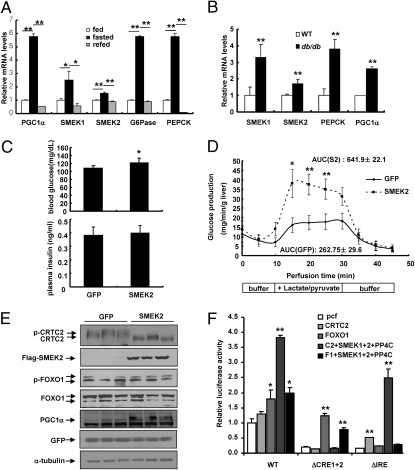
Fig. 1.
SMEK enhances hepatic gluconeogenesis in vivo. (A) Q-PCR analysis showing SMEK1, SMEK2, PEPCK, G6Pase, and PGC-1α mRNA levels in ad libitum-fed (fed), 24-h fasted (fasted), or 24-h fasted and 5-h refed (refed) mice (*P < 0.05 and **P < 0.01, t test; n = 8). (B) Q-PCR analysis showing SMEK1, SMEK2, PEPCK, and PGC-1α mRNA in WT and db/db mice under ad libitum conditions (**P < 0.01, t test; n = 5). (C) Effects of Ad-SMEK2 or Ad-GFP on 4-h fasting glucose levels (Upper) or insulin levels (Lower) in WT mice (*P < 0.05, t test; n = 4). (D) Effects of Ad-SMEK2 or Ad-GFP on in situ hepatic glucose production in WT mice following 24 h of fasting (**P < 0.01 and *P < 0.05, t test; n = 10). The area under the curve (AUC) during the perfusion period for each condition is indicated. (E) Western blot analysis showing effects of Ad-SMEK2 on CRTC2, FOXO1a, or PGC-1α. (F) Transfection analysis was performed to determine the effects of SMEK/PP4C expression on CRTC2- or FOXO1a-dependent activation of G6Pase promoter activity in HepG2 cells (**P < 0.01 and *P < 0.05, t test; n = 3). pcf, pcDNA-flag empty vector. Data in A, B, and F represent the mean ± SD, and data in C and D represent the mean ± SEM.
SMEK Enhances Hepatic Gluconeogenesis in Vivo.
To assess the functional role of SMEK1/2 in hepatic glucose metabolism, we generated adenoviruses for expression of SMEK1 and SMEK2 and used them to infect hepatocytes. SMEK1/2 overexpression enhances mRNA levels for phosphoenol pyruvate carboxykinase (PEPCK) and glucose-6-phosphatase catalytic subunit (G6Pase) (Fig. S2C). Furthermore, SMEK2 overexpression results in increased glucose production from hepatocytes, suggesting that increased expression of SMEK in hepatocytes might promote hepatic gluconeogenesis (Fig. S2D). To test the effects of hepatic SMEK expression on glucose homeostasis in vivo, we injected adenovirus for SMEK2 into the tail veins of WT mice. Surprisingly, 2-fold overexpression of SMEK2 promotes higher glucose levels in mice, with elevated expression of gluconeogenic genes (Fig. 1C and Fig. S3 A and B). Similar results were also obtained with SMEK1 adenovirus infection, suggesting that SMEK1 and SMEK2 might be functionally redundant for regulating gluconeogenic gene expression. In addition, a pyruvate challenge test revealed that Ad-SMEK2 mice exhibited higher blood glucose levels than control mice by means of i.p. injection of pyruvate (Fig. S3C). Interestingly, coinfection of SMEK2 with PP4R2 did not significantly affect the glucose excursion curve in this test compared with SMEK2 infection alone. The effect of SMEK2 on hepatic glucose production was further assessed by an in situ glucose production assay, and we confirmed that the rate of hepatic glucose production in response to gluconeogenic precursors was significantly higher for Ad-SMEK2 mice compared with the control mice (Fig. 1D). These data suggest that SMEK could control gluconeogenic potential to promote glucose output in the liver.
We then tested whether elevated gluconeogenic gene expression induced by SMEK overexpression is attributable to the modification of transcriptional machinery. We thus investigated whether regulatory phosphorylation of CRTC2 and FOXO1a, two major transcriptional circuits for regulation of hepatic gluconeogenesis, is affected by SMEK expression in mouse liver. Neither FOXO1a protein levels nor its phosphorylation status is changed by SMEK overexpression (Fig. 1E). In contrast, CRTC2 phosphorylation is greatly reduced by hepatic SMEK expression, as evidenced by increased formation of dephosphorylated CRTC2 in livers of Ad-SMEK mice compared with that of control mice. In line with this result, SMEK expression enhances CRTC2-dependent activation of G6Pase (−231/+57) luciferase or cAMP-response element (CRE) luciferase reporter gene activity (Fig. 1F and Fig. S3D). This effect was completely blunted with G6Pase (−231/+57) luciferase lacking CRE (ΔCRE1+2) (22). Conversely, SMEK expression does not promote FOXO1a transcriptional potential of FOXO1a, and a FOXO1a binding site mutation [Δinsulin-response element (IRE)] (38) did not affect CRTC2-dependent induction of G6Pase promoter activity by SMEK/PP4C (Fig. 1F).
SMEKs Are Crucial Components of PP4 Complex.
Recently, two previously undescribed PP4 regulatory subunit families, PP4R3a and PP4R3b, involved in the formation of active PP4 complexes with PP4C, a catalytic subunit, and another regulatory subunit, PP4R2, have been reported (24, 25). Because SMEK1 and SMEK2 correspond to PP4R3a and PP4R3b, respectively, we wanted to confirm whether SMEK could associate with PP4C in the mammalian liver. Indeed, both SMEK1 and SMEK2 interact with PP4C and PP4R2 (but not with PP2B) by coimmunoprecipitation analysis after transient transfection in 293T cells (Fig. S3 E and F). Furthermore, endogenous SMEK1 and SMEK2 interact with PP4C and PP4R2 by coimmunoprecipitation analysis in hepatocytes (Fig. 2A) and in mouse liver (Fig. 2B), showing that SMEK1/2 and PP4C potentially form a complex. PP4 has been linked to the various cellular signaling cascades both in the nucleus and in the cytoplasmic compartments (25–29). To gain insight into the potential role of SMEK/PP4C in the regulation of CRTC2, we chose to dissect the subcellular localization of this complex in the liver. Unlike the case in HeLa cells or human osteosarcoma U2OS cells (28, 30), SMEK2 was found predominantly in cytoplasmic fractions in both rodent hepatocytes and mouse liver (Fig. 2C). SMEK1, PP4R2, and PP4C were distributed equally in nuclear and cytoplasmic fractions, suggesting that the PP4 complex could be active both in the nucleus and in the cytoplasm in the liver.
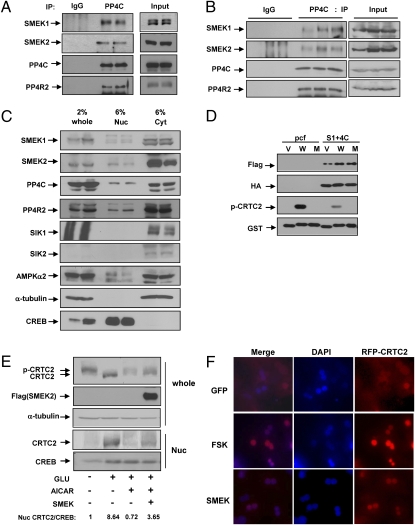
Fig. 2.
SMEKs are crucial components of the PP4 complex. (A) Interaction of SMEK and PP4C or PP4R2 in rat primary hepatocytes. Coimmunoprecipitation assay was performed with anti-PP4C antibody or IgG control and blotted with antibodies against SMEK1, SMEK2, PP4C, and PP4R2. IP, immunoprecipitation. (B) Coimmunoprecipitation assay was performed with anti-PP4C antibody as in Fig. 2A to confirm the in vivo interaction with SMEK1 in db/db mouse liver. (C) Western blot analysis using whole-cell lysates (whole), nuclear fractions (Nuc), and cytoplasmic fractions (Cyt) showing subcellular localization of SMEK, PP4C, and PP4R2 in livers from normal lean mice. α-Tubulin and heat shock protein 90 (HSP90) was used for a cytoplasmic marker, whereas CREB was used for a nuclear marker. Several serine/threonine kinases known to regulate CRTC2 phosphorylation (AMPKα1, AMPKα2, SIK1, and SIK2) were also shown. (D) Effects of SMEK/PP4C on CRTC2 phosphorylation in an in vitro phosphatase assay. GST and GST-CRCT2 161–181 (WT and S171A) fusion proteins were isolated from bacteria and phosphorylated in vitro with AMPK. V, W, and M represent GST vector only, GST-WT CRTC2 161–181 amino acids, and GST-S171A CRTC2 161–181 amino acids, respectively. pcf, pcDNA-flag empty vector. (E) Effects of SMEK on CRTC2 translocation in primary hepatocytes. Effects of glucagon (GLU, 50 nM for 15 min) or aminoimidazole carboxamide ribonucleotide (AICAR) (500 μM for 30 min) on the nuclear localization of CRTC2 was shown. Ratio of nuclear CRTC2/CREB is indicated below. CREB is used as a marker for nuclear localization. (F) Effects of SMEK on CRTC2 translocation in primary hepatocytes. Ad-red fluorescent protein (RFP)-CRTC2 was coinfected with either Ad-GFP or Ad-SMEK2 in mouse primary hepatocytes. Effect of forskolin (FSK, 10 μM for 15 min) on nuclear localization of CRTC2 was shown as a control. DAPI staining was performed to indicate the location of nuclei.
Next, we addressed whether the increased CRTC2 dephosphorylation promoted by SMEK expression is attributable to the phosphatase activity of the PP4 complex. Indeed, the immunoisolated SMEK/PP4C complex is able to dephosphorylate WT CRTC2 161–181 phosphorylated at S171 in an in vitro phosphatase assay, corroborating the notion that SMEK/PP4C could directly regulate the CRTC2 phosphorylation status (Fig. 2D). Furthermore, SMEK overexpression in hepatocytes enhances nuclear localization of CRTC2 as confirmed by Western blot analysis (Fig. 2E) or microscopic visualization of cells expressing red fluorescent protein (RFP)-CRTC2 (Fig. 2F); this leads to increased occupancy of CRTC2 over CRE on the PEPCK and G6Pase promoters (Fig. S4A). These data suggest that SMEK could promote hepatic glucose production by its involvement in the formation of PP4, which regulates dephosphorylation of CRTC2 in the cytoplasm.
SMEK Knockdown Reduces Blood Glucose Levels and Increases Hepatic CRTC2 Phosphorylation.
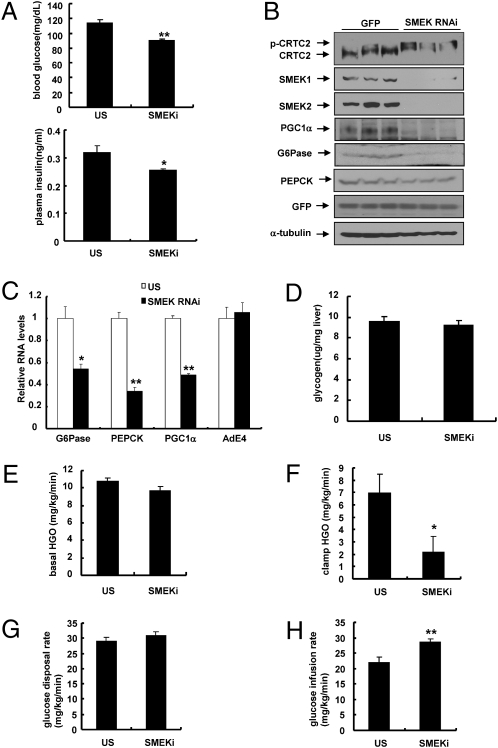
To test directly whether SMEK1/2 regulates hepatic glucose metabolism via CRTC2 phosphorylation, we prepared shRNA adenoviruses for SMEK1 and SMEK2 and tested them in hepatocytes. As expected, we observed increased cytoplasmic localization and reduced occupancy of CRTC2 over gluconeogenic promoters on SMEK knockdown (Fig. S4 B and C). Combined knockdown of SMEK1 and SMEK2 in mouse liver significantly reduces blood glucose levels in WT mice in a 4-h fasted condition (Fig. 3A Upper) or a 16-h fasted condition (Fig. S4D Upper). Four-hour fasting plasma insulin levels are also lower in SMEK-knockdown mice compared with controls (Fig. 3A Lower), although no significant change was observed under a 16-h fasting condition (Fig. S4D Lower). Because SMEKs were found to be components of a PP4 complex that is able to dephosphorylate CRTC2, we expected to observe an increased level of phosphorylated CRTC2 (p-CRTC2) in livers of SMEK-knockdown mice. Indeed, the p-CRTC2 form is highly enriched on SMEK knockdown (Fig. 3B). Furthermore, hepatic mRNA and protein levels for gluconeogenic enzymes and regulators are significantly reduced in SMEK-knockdown mice (Fig. 3 B and C). Conversely, no significant changes are observed in hepatic glycogen levels on SMEK knockdown (Fig. 3D). To assess the effect of SMEK knockdown on glucose homeostasis in vivo directly, we performed euglycemic-hyperinsulinemic clamp studies. Knockdown of SMEK was shown to reduce endogenous hepatic glucose output under basal conditions (Fig. 3E). Furthermore, during the clamp period, endogenous hepatic glucose output was significantly reduced on SMEK knockdown without changes in overall glucose disposal rates (Fig. 3 F and G). The increased glucose infusion rate with SMEK-knockdown mice could reflect the corresponding changes in hepatic glucose production during the clamp period (Fig. 3H and Fig. S4 E and F). Collectively, these data suggest that changes in blood glucose levels on SMEK knockdown are largely attributable to the changes in CRTC2-mediated hepatic glucose production.
Fig. 3.
SMEK knockdown reduces blood glucose levels and increases CRTC2 phosphorylation. (A) Effects of Ad-SMEK RNAi or control [Ad-unspecific (US)] RNAi on 4-h fasting glucose levels (Upper) and 4-h fasting insulin levels (Lower) in WT mice (**P < 0.01 and *P < 0.05, t test; n = 10). (B) Immunoblot analysis showing effects of Ad-SMEK RNAi on CRTC2 phosphorylation status or PGC-1α levels in WT mice. (C) Effects of Ad-SMEK RNAi or Ad-US RNAi on gluconeogenic gene expression in WT mice (**P < 0.01 and *P < 0.05, t test; n = 10). (D) Effects of Ad-SMEK RNAi or Ad-US RNAi on liver glycogen contents in WT mice (n = 10). Six-week-old mice were fed a high-fat diet for 6 wk, and hyperinsulinemic-euglycemic clamp studies were performed (**P < 0.01 and *P < 0.05, t test; n = 13–14). Basal hepatic glucose output (basal HGO) (E), clamp hepatic glucose output (clamp HGO) (F), glucose disposal (G), and infusion rates (H) were determined. Data in A and D–H represent the mean ± SEM, and data in C represent the mean ± SD.
SMEK Regulates CRTC2-Dependent Hepatic Gluconeogenesis in Vivo.
Increased activation of CRTC2-dependent transcription has been linked to the hyperglycemic phenotype in rodent models of type 2 diabetes (9). Enhanced expression of SMEK proteins is observed in db/db mice or WT mice feed a high-fat diet, which led us to test whether knockdown of SMEK also normalizes elevated blood glucose levels in these settings. Indeed, depletion of hepatic SMEK1/2 reduces hyperglycemia, with a slight decline in plasma insulin levels in db/db mice (Fig. 4A and Fig. S5A) or in mice fed a high-fat diet (Fig. S5B). Amelioration of hyperglycemia might be largely attributable to changes in hepatic gluconeogenesis, because SMEK knockdown greatly improves pyruvate intolerance as well (Fig. 4B). Indeed, SMEK knockdown induces CRTC2 phosphorylation in the livers of db/db mice (Fig. 4C). No changes are observed in serine phosphorylation of AKT or GSK3β on SMEK knockdown, excluding potential nonspecific effects of loss of SMEKs and the PP4 complex on other phosphoserine/threonine-containing substrates (Fig. S5C). No significant change compared with controls was found in insulin tolerance tests on SMEK-knockdown WT or db/db mice (Fig. S5D). In addition, liver kinase B1 (LKB1), phosphorylated LKB1, AMPK, phosphorylated AMPK, and SIK levels were not altered by SMEK knockdown, excluding potential indirect modification of kinase activity in the regulation of CRTC2 phosphorylation (Fig. S6A). These data support the notion that the reduced glycemia observed with SMEK deficiency is mainly attributable to the inactivation of CRTC2 via reduced PP4 phosphatase activity but not attributable to improved hepatic insulin signaling or activation of serine/threonine kinase pathways.
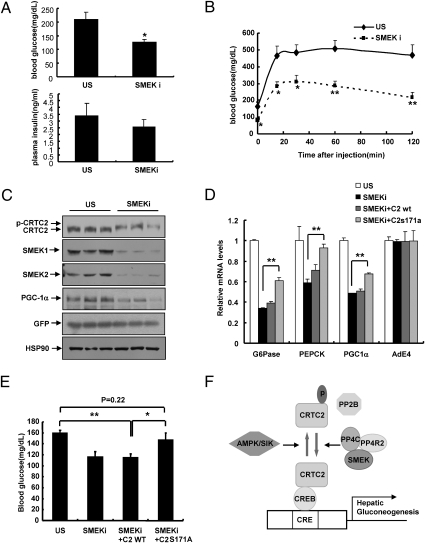
Fig. 4.
SMEK regulates CRTC2-dependent hepatic glucose metabolism in vivo. (A) Effects of Ad-SMEK RNAi or Ad-US RNAi on 4-h fasting glucose levels (Upper) or insulin levels (Lower) in db/db mice (*P < 0.05, t test; n = 5). (B) Pyruvate challenge analysis showing effects of SMEK knockdown on hepatic gluconeogenesis in db/db mice (**P < 0.01 and *P < 0.05, t test; n = 7). (C) Western blot analysis showing effects of Ad-SMEK RNAi or Ad-US on CRTC2 phosphorylation status, hepatic SMEK1, SMEK2, and PGC-1α expression in db/db mice. HSP90, heat shock protein 90. (D) Effects of Ad-WT CRTC2 or Ad-S171A CRTC2 on 4-h fasting gluconeogenic gene expression in db/db mice infected with Ad-SMEK RNAi (**P < 0.01, t test; n = 5). (E) Effects of Ad-WT CRTC2 or Ad-S171A CRTC2 on 4-h fasting glucose levels in db/db mice infected with Ad-SMEK RNAi (**P < 0.01 and *P < 0.05, t test; n = 5). (F) Proposed model for the regulation of CRTC2 phosphorylation and hepatic glucose production by interplay of serine/threonine kinases and phosphatases. Data in A, B, and E represent the mean ± SEM, and data in D represent the mean ± SD.
As in the case of WT mice, SMEK deficiency greatly reduces gluconeogenic gene expression and CRE activity in livers of db/db mice or mice fed a high-fat diet (Fig. S6 B–D), demonstrating that SMEKs are essential in the cAMP-mediated transcriptional regulation of hepatic gluconeogenesis in vivo. Even though knockdown of PP4C, per se, was sufficient to block accumulation of non-p-CRTC2 and CRE-reporter activity in primary hepatocytes (Fig. S7A) or to reduce blood glucose levels in db/db mice (Fig. S7B), knockdown of PP4C in combination with SMEK knockdown in db/db mice does not further affect blood glucose levels or hepatic gluconeogenic gene expression (Fig. S7 C–E). These data further corroborate the idea that SMEKs and PP4C act in the same complex. Finally, to confirm whether SMEK knockdown affects hepatic gluconeogenesis via CRTC2 modification, we coinjected adenoviruses for either WT or S171A mutant CRTC2 together with SMEK RNAi adenoviruses. Transduction of mice with S171A mutant but not with WT CRTC2 significantly negates the effect of SMEK knockdown on blood glucose levels as well as on hepatic gluconeogenic gene expression (Fig. 4 D and E and Fig. S7F), showing that SMEK proteins indeed regulate hepatic gluconeogenesis via modulating the phosphorylation status of CRTC2 at the serine 171 residue.
Discussion
In C. elegans, SMK-1, a SMEK1/2 ortholog, was shown to be required for stress responses and for lifespan extension attributable to reduced insulin/IGF signaling (11). In the present study, SMEK proteins are shown to function as regulatory components of a PP4 complex that controls hepatic glucose production by regulating CRTC2 phosphorylation levels (a proposed model is presented in Fig. 4F).
Prior work has shown that the PP4 complex consists of the PP4C catalytic subunit and various combinations of regulatory subunits, including PP4R1, PP4R2, or PP4R3 (SMEKs), and is involved in recovery from the DNA damage checkpoint (24, 28, 29). Surprisingly, in mouse liver, we found that both endogenous SMEK1/2 and PP4C could colocalize in the cytoplasm (Fig. 2C), where the dephosphorylation of the 14-3-3/pS171 CRTC2 complex should occur to release CRTC2 to enter the nucleus. Given the cytosolic localization, SMEK/PP4C may have specific physiological roles in the liver by regulating cytosolic targets, including CRTC2.
In summary, our study demonstrated a critical role of SMEK/PP4C in the regulation of hepatic gluconeogenesis as a bona fide hepatic phosphatase for CRTC2. Interestingly, we observed increased expression of SMEK genes on fasting and during diet-induced or genetically derived insulin resistance (Fig. 1 A and B and Fig. S1 A and B). The transcriptional regulation of these genes is shown to be regulated by PGC-1α. It is noteworthy that increased expression of PGC-1α itself is a key for the pathogenesis of hepatic insulin resistance in rodents (16, 17, 20). Thus, it is plausible that increased SMEK expression increases efficiency of PP4 complex-mediated dephosphorylation of CRTC2 and, in combination with reduced SIK2 activity, promotes hyperglycemia in that setting. In support of this notion, we observed decreased CRTC2 phosphorylation on SMEK overexpression in WT mice (Fig. 2E). Further studies are needed to determine the relative contribution of these proteins in the regulation of glucose metabolism and CRTC2 activity in type 2 diabetes.
Materials and Methods
Culture of Primary Hepatocytes.
Rodent primary hepatocytes were prepared from either Sprague–Dawley rats or C57BL/6 mice as described (31). After 16 h of adenoviral infection, cells were maintained in medium 199 (MediaTech) for an additional 24–48 h. Cells were exposed to 10 μM forskolin or 100 nM glucagon for 10 min (for Western blot analysis), 2 h [for quantitative PCR (Q-PCR) analysis], or 4 h (for reporter assays).
Adenoviruses.
Ad-GFP, Ad-US control RNAi, Ad WT CRTC2, Ad S171A CRTC2, Ad-CRE-luc (luciferase), and Ad-rous sarcoma virus (RSV)-β-gal (galactosidase) adenoviruses were described previously (6, 9, 32). Adenoviruses for SMEK1, SMEK2, SMEK1 RNAi, SMEK2 RNAi, and PP4C RNAi were generated as described (20).
Animal Experiments.
Male, 7-wk-old C57BL/6 mice or db/db diabetic mice were purchased from Charles River Laboratories. Recombinant adenovirus (0.5 × 109 plaque-forming unit/mice) was delivered by tail vein injection to mice. Fasting blood glucose levels were measured from animals that were fasted for 16 or 4 h with free access to water. Plasma insulin levels were measured using insulin assay ELISA kits (SHIBAYAGI). For the glucose tolerance test or pyruvate challenge, mice were injected i.p. with glucose or pyruvate (2 g/kg of body weight for WT lean mice and 1 g/kg of body weight for db/db mice) after 16 h of fasting on 4–6 d after injection of adenovirus as described (33). All procedures were performed under guidelines that were approved by the Sungkyunkwan University School of Medicine Institutional Animal Care And Use Committee.
Western Blot Analysis and Immunoprecipitation.
Western blot analyses and immunoprecipitation experiments were performed as described (6, 31). Cytosolic and nuclear fractions from primary hepatocytes were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce Biotechnology).
Q-PCR.
Total RNA from either primary hepatocytes or liver tissue was extracted using an RNeasy minikit (Qiagen). cDNAs generated by SuperScript II enzyme (Invitrogen) were analyzed by Q-PCR using a SYBR green PCR kit and TP800, Thermal Cycler DICE Real Time System (TAKARA). All data were normalized to ribosomal L32 expression.
In Vivo imaging.
For imaging, 18-h fasted mice were i.p. injected with 100 mg/kg of sterile firefly d-luciferin. After 10 min, mice were anesthetized and imaged on an IVIS 200 Imaging System (Xenogen) as described (9, 34).
ChIP.
Nuclear isolation, cross-linking, and ChIP assays from primary mouse hepatocytes were performed as described previously (7).
In Vitro Phosphatase Assay.
Induction and purification of GST fusion proteins [pGEX5X-1, pGEX5X-1 161–181 CRTC2 WT, and pGEX5X-1 161–181 CRTC2 S171A mutant (7)] in Escherichia coli were performed according to the manufacturer's protocol (Amersham Pharmacia Biotech). GST-fused substrates (GST alone, GST-WT CRTC2 amino acids 161–181, and GST-S171A CRTC2 amino acids 161–181) were phosphorylated for 30 min using active AMP-dependent protein kinase (Millipore). The phosphatase assay was performed using phosphorylated substrate with the purified phosphatase complex for 2–6 h at 30 °C. Results were assessed by Western blot analysis with anti-pS171 CRTC2 antibodies as described (35).
In Situ Glucose Production Assay in Perfused Liver.
Eight-week-old C57BL/6 mice were infected with either Ad-GFP or Ad-SMEK2 adenovirus for 5 d before the experiment. The 24-h fasted mice were anesthetized, and the inferior vena cava and portal vein were intubated as described previously, with a slight modification (36). The livers were preperfused using Krebs’ Ringer buffer (KRB) at 37 °C for 30 min before the start of the perfusion experiment. After the initial perfusion with KRB (10 min), KRB with gluconeogenic substrate (20 mM lactate and 2 mM pyruvate) was then infused during a 10- to 30-min period, followed by a period of basal KRB infusion (30–45 min). At 5-min time points, the outflow was collected for 2 min and glucose concentrations were measured using a Glucose Assay Kit (Cayman Chemical).
Hyperinsulinemic-Euglycemic Clamp Study.
Seven days after indwelling catheters were placed into the right internal jugular vein in mice, a hyperinsulinemic-euglycemic clamp study was performed as described previously (37).
Statistical Analyses.
Results are represented as either the mean ± SEM (for metabolites) or the mean ± SD (for Q-PCR and luciferase assays). The comparison of different groups was carried out using a two-tailed unpaired Student's t test as described (9). Differences were considered statistically significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Andrew Dillin (Salk institute, La Jolla, CA) for critical review of this manuscript, Dr. Jianxin Xie (Cell Signaling Technology, Boston, MA) for providing P-TORC2 antibody, Dr. Hong-Duk Youn (Seoul National University, Seoul, Korea) for providing PP2B expression plasmid, and Dr. Seong-Tae Kim (Sungkyunkwan University, Suwon, Korea) for providing γ-H2AX antibody. We also thank Sun-Myong Park and Bo-Kyoung Kim for technical assistance. This work was supported by Grant A084651 from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea. T.H. is a Frank and Else Schilling American Cancer Society Professor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012665107/-/DCSupplemental.
References
- 1.Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- 2.Herzig S, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 3.Arias J, et al. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. doi: 10.1038/370226a0. [DOI] [PubMed] [Google Scholar]
- 4.Chrivia JC, et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 5.Conkright MD, et al. TORCs: Transducers of regulated CREB activity. Mol Cell. 2003;12:413–423. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Koo SH, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 7.Screaton RA, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, et al. Targeted disruption of the CREB coactivator Crtc2 increases insulin sensitivity. Proc Natl Acad Sci USA. 2010;107:3087–3092. doi: 10.1073/pnas.0914897107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dentin R, et al. Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature. 2007;449:366–369. doi: 10.1038/nature06128. [DOI] [PubMed] [Google Scholar]
- 10.Mendoza MC, et al. Loss of SMEK, a novel, conserved protein, suppresses MEK1 null cell polarity, chemotaxis, and gene expression defects. Mol Cell Biol. 2005;25:7839–7853. doi: 10.1128/MCB.25.17.7839-7853.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolff S, et al. SMK-1, an essential regulator of DAF-16-mediated longevity. Cell. 2006;124:1039–1053. doi: 10.1016/j.cell.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Hastie CJ, Vázquez-Martin C, Philp A, Stark MJ, Cohen PT. The Saccharomyces cerevisiae orthologue of the human protein phosphatase 4 core regulatory subunit R2 confers resistance to the anticancer drug cisplatin. FEBS J. 2006;273:3322–3334. doi: 10.1111/j.1742-4658.2006.05336.x. [DOI] [PubMed] [Google Scholar]
- 13.Huang X, Cheng A, Honkanen RE. Genomic organization of the human PP4 gene encoding a serine/threonine protein phosphatase (PP4) suggests a common ancestry with PP2A. Genomics. 1997;44:336–343. doi: 10.1006/geno.1997.4891. [DOI] [PubMed] [Google Scholar]
- 14.Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- 15.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 16.Puigserver P, et al. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 17.Yoon JC, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 20.Koo SH, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 21.Leone TC, et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: Muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmoll D, et al. Identification of a cAMP response element within the glucose-6-phosphatase hydrolytic subunit gene promoter which is involved in the transcriptional regulation by cAMP and glucocorticoids in H4IIE hepatoma cells. Biochem J. 1999;338:457–463. [PMC free article] [PubMed] [Google Scholar]
- 23.Streeper RS, et al. A multicomponent insulin response sequence mediates a strong repression of mouse glucose-6-phosphatase gene transcription by insulin. J Biol Chem. 1997;272:11698–11701. doi: 10.1074/jbc.272.18.11698. [DOI] [PubMed] [Google Scholar]
- 24.Gingras AC, et al. A novel, evolutionarily conserved protein phosphatase complex involved in cisplatin sensitivity. Mol Cell Proteomics. 2005;4:1725–1740. doi: 10.1074/mcp.M500231-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci USA. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou G, Boomer JS, Tan TH. Protein phosphatase 4 is a positive regulator of hematopoietic progenitor kinase 1. J Biol Chem. 2004;279:49551–49561. doi: 10.1074/jbc.M410317200. [DOI] [PubMed] [Google Scholar]
- 27.Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, et al. Histone deacetylase 3 (HDAC3) activity is regulated by interaction with protein serine/threonine phosphatase 4. Genes Dev. 2005;19:827–839. doi: 10.1101/gad.1286005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury D, et al. A PP4-phosphatase complex dephosphorylates gamma-H2AX generated during DNA replication. Mol Cell. 2008;31:33–46. doi: 10.1016/j.molcel.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakada S, Chen GI, Gingras AC, Durocher D. PP4 is a gamma H2AX phosphatase required for recovery from the DNA damage checkpoint. EMBO Rep. 2008;9:1019–1026. doi: 10.1038/embor.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen GI, et al. PP4R4/KIAA1622 forms a novel stable cytosolic complex with phosphoprotein phosphatase 4. J Biol Chem. 2008;283:29273–29284. doi: 10.1074/jbc.M803443200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon YS, Seo WY, Lee MW, Kim ST, Koo SH. Salt-inducible kinase regulates hepatic lipogenesis by controlling SREBP-1c phosphorylation. J Biol Chem. 2009;284:10446–10452. doi: 10.1074/jbc.M900096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki M, Singh RN, Crystal RG. Regulatable promoters for use in gene therapy applications: modification of the 5′-flanking region of the CFTR gene with multiple cAMP response elements to support basal, low-level gene expression that can be upregulated by exogenous agents that raise intracellular levels of cAMP. Hum Gene Ther. 1996;7:1883–1893. doi: 10.1089/hum.1996.7.15-1883. [DOI] [PubMed] [Google Scholar]
- 34.Lee MW, et al. Regulation of hepatic gluconeogenesis by an ER-bound transcription factor, CREBH. Cell Metab. 2010;11:331–339. doi: 10.1016/j.cmet.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Yoon YS, Ryu D, Lee MW, Hong S, Koo SH. Adiponectin and thiazolidinedione targets CRTC2 to regulate hepatic gluconeogenesis. Exp Mol Med. 2009;41:577–583. doi: 10.3858/emm.2009.41.8.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Canettieri G, et al. Attenuation of a phosphorylation-dependent activator by an HDAC-PP1 complex. Nat Struct Biol. 2003;10:175–181. doi: 10.1038/nsb895. [DOI] [PubMed] [Google Scholar]
- 37.Albuquerque GG, et al. Gluconeogenesis and ketogenesis in perfused liver of rats submitted to short-term insulin-induced hypoglycaemia. Cell Biochem Funct. 2008;26:228–232. doi: 10.1002/cbf.1440. [DOI] [PubMed] [Google Scholar]
- 38.Ryu D, et al. TORC2 regulates hepatic insulin signaling via a mammalian phosphatidic acid phosphatase, LIPIN1. Cell Metab. 2009;9:240–251. doi: 10.1016/j.cmet.2009.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.