Abstract
Neonicotinoid insecticides control crop pests based on their action as agonists at the insect nicotinic acetylcholine receptor, which accepts chloropyridinyl- and chlorothiazolyl-analogs almost equally well. In some cases, these compounds have also been reported to enhance plant vigor and (a)biotic stress tolerance, independent of their insecticidal function. However, this mode of action has not been defined. Using Arabidopsis thaliana, we show that the neonicotinoid compounds, imidacloprid (IMI) and clothianidin (CLO), via their 6-chloropyridinyl-3-carboxylic acid and 2-chlorothiazolyl-5-carboxylic acid metabolites, respectively, induce salicylic acid (SA)-associated plant responses. SA is a phytohormone best known for its role in plant defense against pathogens and as an inducer of systemic acquired resistance; however, it can also modulate abiotic stress responses. These neonicotinoids effect a similar global transcriptional response to that of SA, including genes involved in (a)biotic stress response. Furthermore, similar to SA, IMI and CLO induce systemic acquired resistance, resulting in reduced growth of a powdery mildew pathogen. The action of CLO induces the endogenous synthesis of SA via the SA biosynthetic enzyme ICS1, with ICS1 required for CLO-induced accumulation of SA, expression of the SA marker PR1, and fully enhanced resistance to powdery mildew. In contrast, the action of IMI does not induce endogenous synthesis of SA. Instead, IMI is further bioactivated to 6-chloro-2-hydroxypyridinyl-3-carboxylic acid, which is shown here to be a potent inducer of PR1 and inhibitor of SA-sensitive enzymes. Thus, via different mechanisms, these chloropyridinyl- and chlorothiazolyl-neonicotinoids induce SA responses associated with enhanced stress tolerance.
Neonicotinoids are the newest of the three major classes of insecticides, which also include the organophosphorus compounds and pyrethroids. Imidacloprid (IMI), with a chloropyridinyl (Cl-pyr) substituent, is the most important neonicotinoid, used primarily as a systemic compound absorbed and translocated by plants to control sucking insect pests (1). The neonicotinoids clothianidin (2) (CLO) and a metabolic precursor, the oxadiazine compound thiamethoxam (3, 4), which have chlorothiazolyl (Cl-thia) substituents, are also extensively used as systemic insecticides in plants. The neonicotinoids IMI and CLO are oxidatively cleaved in planta to 6-chloropyridinyl-3-carboxylic acid (CPA) and 2-chlorothiazolyl-5-carboxylic acid (CTA), respectively, among other metabolites (5). In studying metabolism of neonicotinoids in spinach (5) under insect-free conditions, we sometimes observed enhancement of foliage growth, plant vigor, and drought-tolerance. These remarkable effects of neonicotinoids directly on plants, independent of controlling insect pests, have also been noted by many researchers and farmers and documented in both research publications and patent disclosures, especially for IMI (6–8) and the CLO precursor, thiamethoxam (9). In addition, treatment with IMI and its carboxylic acid metabolite CPA has been associated with enhanced resistance against microbial pathogens (6, 7), although their mode of action has not been defined. Therefore, these neonicotinoids have been cited as inducing a “stress shield” (e.g., ref. 6).
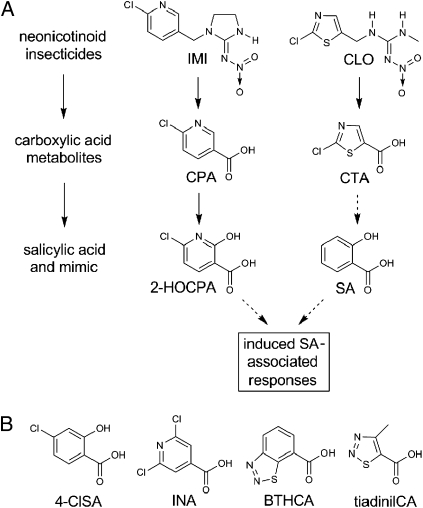
This study characterizes the mechanisms by which IMI, CLO, and their carboxylic acid metabolites induce a stress shield in Arabidopsis thaliana, a model plant species with extensive genetic and genomic resources. We find that the effects of IMI and CLO are attributable to their carboxylic acid metabolites CPA and CTA, respectively, and are similar to those of salicylic acid (SA) (Fig. 1A), an established local and systemic activator of a broad spectrum of plant defense responses resulting in systemic acquired resistance (SAR) (10, 11). Importantly, although both IMI and CLO activate SA-associated plant defense responses, we find that they differ in their mode of activation, with CLO inducing endogenous biosynthesis of SA in the plant and IMI undergoing metabolism to a highly potent analog of SA (Fig. 1A). This finding could explain the greater transcriptional impact we observed for IMI compared with CLO (and SA) on plant responses.
Fig. 1.
(A) Neonicotinoids IMI and CLO, via their carboxylic acid metabolites CPA and CTA, respectively, induce responses in Arabidopsis similar to those of SA. IMI and CPA are proposed to act via their metabolite 2-HOCPA, an SA mimic, whereas CLO and CTA induce endogenous SA biosynthesis. Solid arrows indicate formation of a metabolite and dashed arrows induction of a pathway. (B) Arylcarboxylic acids that are known functional analogs of SA or inducers of SA-associated markers or responses. Note that BTH and tiadinil are bioactivated to the carboxylic acid (CA) metabolites shown.
Results
Neonicotinoids Induce Global Transcriptional Response Similar to That of SA.
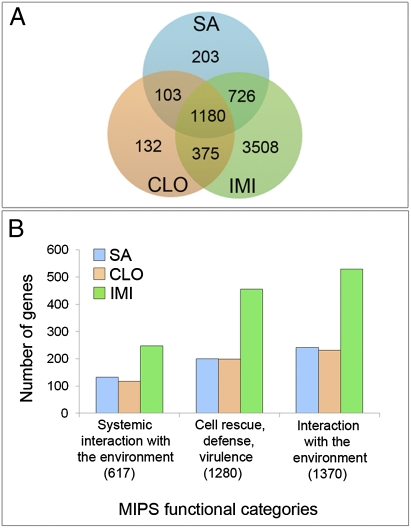
A global view of the transcriptional response was obtained by comparing expression profiles using the Affymetrix ATH1 GeneChip of fully expanded mature leaves harvested from A. thaliana Col-0 (wild-type) plants following soil application with 4 mM IMI, CLO, or SA, compared with leaves obtained from control plants (Dataset 1). Ninety-one percent of the 2,212 genes with significant differences in expression in response to SA versus the control were also altered by treatment with IMI or CLO (Fig. 2A). Although almost all CLO-impacted genes (93%) were also affected by SA or IMI, IMI treatment exclusively affected 3,508 genes under these conditions. It is known that the extent of SA-responsive transcriptional changes varies depending upon the concentration of SA or SA analog, the timeframe for analyzing the response, and the assay system (10, 12). Therefore, to determine whether the IMI-exclusive gene set of 3,508 is also associated with SA-dependent responses not identified in the parallel SA treatment, we compared our findings with those characterizing the early response to SA (13), obtained after treatment with the functional SA-analog S-methyl benzo[1,2,3]thiadiazole-7-carbothioate (BTH) (14), or identified as SA-dependent in response to infection with powdery mildew using the Arabidopsis SA biosynthetic mutant ics1 (15). Thirty percent of the IMI-exclusive set were previously associated with SA-dependent responses as ascertained by these studies.
Fig. 2.
(A) Venn diagrams depicting transcriptional response in Arabidopsis leaves 4 d after soil treatment with 4 mM SA, IMI, or CLO vs. the control. Genes with at least twofold expression change and false discovery rate <0.05 were considered significant. (B). Arabidopsis MIPS parent functional process categories most impacted by treatment with SA, CLO, and IMI (P values <1 × 10−10) are shown with the number of genes in each category (y axis) impacted by chemical treatment compared with the total genes on the ATH1 array in each functional category (x axis, in parentheses). Analyses were performed using BIOMAPs with P values calculated using a binomial hypergeometric function.
The functional processes impacted by IMI, CLO, and SA were determined using MapMan (16) and BioMaps in Virtual Plant (17). Results with the three compounds were qualitatively similar, but the magnitude of the response (number of genes associated with a given process) was consistently greater with IMI treatment compared with SA and CLO. Parent functional process categories defined by the Munich Institute for Protein Sequences (MIPS) (18) that were most strongly impacted by SA are associated with Systemic Interaction with the Environment; Cell Rescue, Defense, and Virulence; and Interaction with the Environment (Fig. 2B). This finding was also the case for treatment with IMI and CLO. IMI treatment affected approximately twofold more genes associated with these process categories. Furthermore, the magnitude of the change in expression compared with the control was usually heightened with IMI compared with CLO and SA. For example, a set of 94 SA-dependent genes defined by Wang et al. (19) shows universally elevated expression in response to IMI compared with CLO and SA (SI Appendix). This set includes the pathogenesis-related protein 1 (PR1), the most robust marker of SA-dependent gene expression, which was induced 534-fold by treatment with IMI compared with 163- and 51-fold increases with SA and CLO, respectively. Taken together, our analysis indicates that the majority of genes induced exclusively by IMI reflects its increased potency. However, as treatment with IMI also had a more profound impact on carbohydrate, nitrogen, and specialized product metabolism than treatment with SA or CLO (SI Appendix), it is also possible that IMI or a metabolite of IMI alter a small subset of responses not impacted by SA or CLO.
Neonicotinoids Induce SAR Similar to SA.
The microarray analysis above indicates that treatments with IMI and CLO result in a similar transcriptional response to that of SA, including the enhanced expression of genes involved in plant defense. Exogenously applied SA is an effective inducer of SAR and results in enhanced resistance to a variety of pathogens, including powdery mildews (10, 11). Therefore, we assessed whether SAR was induced by soil treatment with IMI and CLO, in addition to SA. We found Arabidopsis plants treated with soil application of 4 mM IMI, CLO, or SA exhibited enhanced resistance to the powdery mildew Golovinomyces orontii compared with control plants (Table 1).
Table 1.
Neonicotinoid-induced enhanced resistance of Arabidopsis to powdery mildew
| Percent of total plants with ≥35% mildew coverage | |||
| Treatment | Exp. 1 | Exp. 2 | P value |
| Control | 53 | 84 | |
| SA | 19 | 30 | ≤0.02 |
| CLO | 0 | 0 | ≤0.0001 |
| IMI | 4 | 26 | ≤0.0002 |
Four days after soil treatment with 4 mM chemical or control, boxes of Arabidopsis Col-0 plants were infected with powdery mildew conidia. Powdery mildew growth and reproduction was assessed at 10 d postinfection using a modified standard scoring system (20) to describe the visible percent-coverage on fully expanded leaves of similar age per plant (SI Appendix). Exp. 1 (n ≥ 21) and 2 (n ≥ 10) were performed 1 mo apart. P values shown are valid for each experiment.
IMI and CLO Differ in Their Requirement for an Intact Induced SA Biosynthetic Pathway.
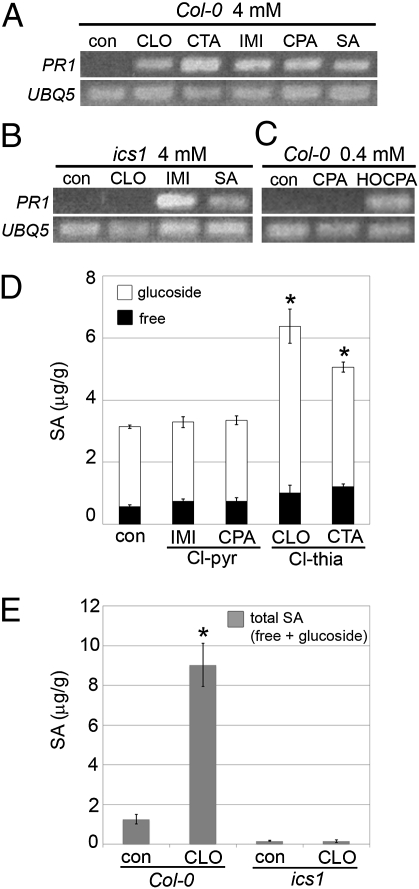
The mechanism of action of IMI and CLO in inducing SA-associated responses was investigated by assessing the expression of PR1, an established marker for this pathway. To determine whether endogenous production of SA via isochorismate synthase 1 (ICS1) was required, an ics1 (also known as sid2 or eds16) null mutant (21) was used. Not only IMI and CLO, but also their carboxylic acid metabolites CPA and CTA, respectively, induced robust PR1 expression in wild-type plants, as assessed by RT-PCR (Fig. 3A). Surprisingly, IMI and CLO differed in their requirement for endogenous biosynthesis of SA in the plant. IMI did not require SA synthesis via ICS1 for PR1 induction, whereas CLO did (Fig. 3B). This finding suggests that CLO (and its metabolite CTA) induce endogenous synthesis of SA, resulting in SA-associated responses. In contrast, as IMI induction of PR1 does not require SA synthesis via ICS1, this suggests that IMI, or a metabolite of IMI, may act as a functional SA analog. Known functional SA analogs BTH and 2,6-dichloroisonicotinic acid (INA) (Fig. 1B) do not require ICS1 for PR1 induction (22).
Fig. 3.
Induction of the SA marker PR1 and SA accumulation in Arabidopsis. RT-PCR (A–C) was performed for PR1 and the housekeeping gene UBQ5 for Col-0 and ics1 SA biosynthetic mutant 4 d after chemical treatment compared with control (con). (D) SA accumulation at 4 d after 4-mM chemical treatment compared with control. Treatment with SA in the same experiment resulted in: free SA = 8.4 ± 0.9 and SA glucoside = 27 ± 4 μg/g. Data are mean ± SD (n = 3) with *P < 0.001. (E) Total SA accumulation (free plus SA-glucoside) in response to 4 mM CLO for Col-0 or ics1 plants 4 d posttreatment. Independent experiments gave similar results (SI Appendix).
Neonicotinoid Induction of SA Biosynthesis.
Concentrations of free and conjugated SA were determined 4 d after soil treatment with neonicotinoids to establish whether they induce endogenous synthesis of SA (Fig. 3D). The Cl-thia compounds CLO and CTA induced total SA accumulation, attaining concentrations associated with the induction of PR1 and SAR (23), whereas treatment with the Cl-pyr compounds IMI and CPA did not result in a significant elevation in concentration of total SA compared with control plants.
ICS1-Dependence of CLO-Induced SA Synthesis and Enhanced Disease Resistance.
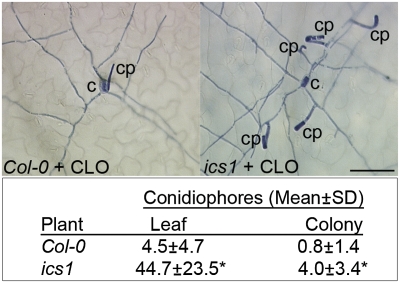
As induction of the SA marker gene PR1 by CLO required ICS1, we assessed whether SA accumulation following treatment with CLO also requires ICS1. SA accumulation in response to CLO was abrogated in the ics1 mutant (Fig. 3E). Furthermore, visual disease scoring (SI Appendix) and microscopic assessment (Fig. 4) of powdery mildew growth and reproduction showed that CLO-induced enhanced resistance to powdery mildew was compromised in ics1 compared with wild-type plants.
Fig. 4.
CLO-induced enhanced resistance of Arabidopsis to powdery mildew is mediated by ICS1. Representative microscopic images at the leaf surface of a powdery mildew colony at 5 d postinfection, shown for Col-0 and ics1 plants treated with 4 mM CLO and infected 4 d later with powdery mildew conidia. c, germinated conidia; cp, conidiophores (reproductive structures). (Scale bar, 100 μm.) Quantitative data (n = 6 leaves) for total conidiophores per leaf and per colony on a leaf for above experiment. *P ≤ 0.002. An independent experiment (n = 6) gave similar results.
Chloropyridinyl Neonicotinoid Metabolite Is a Putative SA Analog.
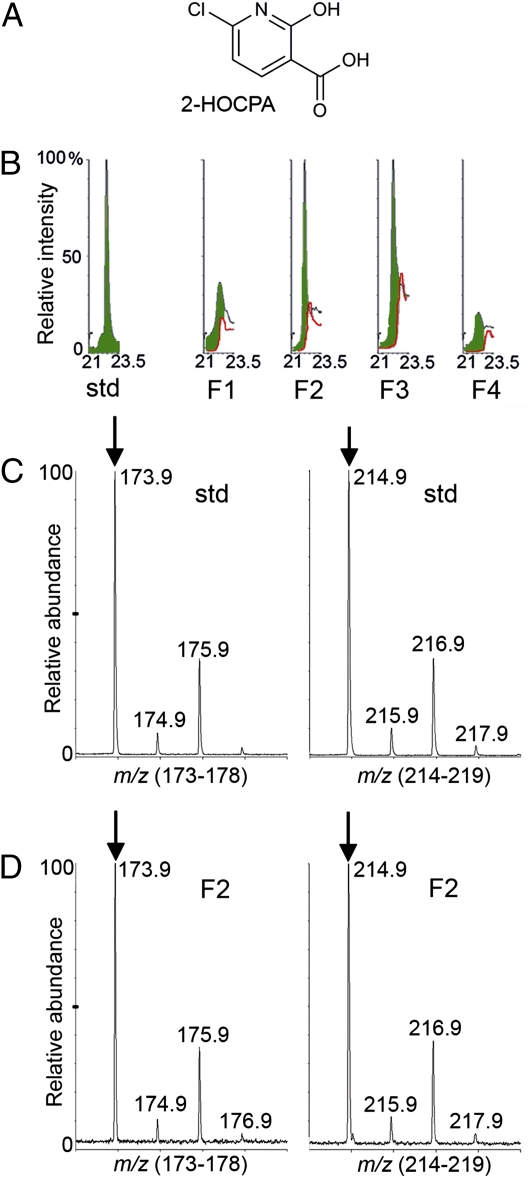
Because IMI and CPA induce PR1 in Arabidopsis in an ICS1-independent manner and they do not induce significant accumulation of SA, we proposed that CPA or a metabolite of CPA acts directly as an analog of SA. The acid 6-chloro-2-hydroxypyridinyl-3-carboxylic acid (2-HOCPA) was considered to be the most interesting candidate because it is structurally similar to SA (2-hydroxybenzoic acid), with even closer similarity to 4-chlorosalicylic acid (4-ClSA), an active SA derivative (24) (Fig. 1B). In addition, the bacterial conversion of CPA to 2-HOCPA has been established (25), and a similar enzymatic conversion (the 2-hydroxylation of benzoic acid to SA) has been reported in plants (26). To test the hypothesis that 2-HOCPA is the active CPA metabolite and SA analog, the required authentic standard was prepared by an improved version of an earlier synthesis (27) (SI Appendix). LC/MS analyses on HPLC-fractionated extracts from CPA-treated Arabidopsis served to identify 2-HOCPA in CPA-treated leaves but not in control leaves (Fig. 5 and SI Appendix). The possibility that 2-HOCPA could act as an SA analog was examined by its ability to induce PR1 expression. Not only did 2-HOCPA induce PR1 expression, but it was considerably more potent than CPA, as PR1 expression was induced by treatment with 0.4 mM 2-HOCPA but not CPA (Fig. 3C). Taken together, these data suggest that 2-HOCPA is a bioactivated metabolite acting similarly to SA.
Fig. 5.
Identification of 2-HOCPA (A) as a metabolite of CPA in Arabidopsis. (B) LC/MS chromatograms for m/z = 174 and tR = 21.0 to 23.5 min for the synthetic 2-HOCPA standard (std) and HPLC-fractionated leaf extracts (fractions F1–F4) from CPA-treated (4 mM, 4 d posttreatment; black) and control (red) Arabidopsis leaf extracts. The green region in the overlay composite chromatograms can be largely attributed to 2-HOCPA. MS profile (tR = 22 min) of (C) 2-HOCPA standard and (D) HPLC fraction F2 from CPA treated plants. Arrows point to [M+1]+ = 173.9 and [M + 1 + acetonitrile]+ = 214.9 with 35Cl. Note the characteristic 35Cl:37Cl ratio of 3:1 for both ions. The MS profile for the parallel HPLC fraction F2 from control plants did not exhibit any of the MS ions shown (SI Appendix).
The hydroxy-CPA 2-HOCPA Binds to SA-Sensitive Proteins.
Because 2-HOCPA has obvious structural similarities to SA, we determined whether it functions similarly to SA at the biochemical level. An SA receptor has not been identified (10); however, there are two known SA-sensitive proteins, PBS3 and SABP2, which promote the induction of PR1 in systemic tissue and SAR (28, 29) and are inhibited by SA with a Ki of 8 to 16 μM (29–31). Here, we establish that in addition to SA, 4-ClSA and 2-HOCPA but not CPA inhibit PBS3 activity at physiologically relevant concentrations (30) (Table 2). The inhibition of PBS3 activity by 2-HOCPA is similar to that observed for the SA analog INA, which results in 9% inhibition of PBS3 activity at 30 μM and 70% inhibition at 300 μM INA (30). Inhibition of PBS3 activity by 2-HOCPA and not CPA highlights the importance of hydroxylation at the 2' position for inhibitory activity, as reported (30).
Table 2.
Inhibitory activity of 2-HOCPA to SA-binding protein PBS3
| Inhibition (%) at indicated concentration* | ||
| Compound | 30 μM | 300 μM |
| SA | 32 ± 5 | 73 ± 1 |
| CPA | 0 ± 3 | 3 ± 1 |
| 2-HOCPA | 5 ± 1 | 61 ± 1 |
| 4-ClSA | 11 ± 6 | 83 ± 5 |
Data are mean ± SD (n = 3). An independent experiment (n = 3) gave similar results.
*Percent inhibition compares activity with the addition of test compound to control reaction.
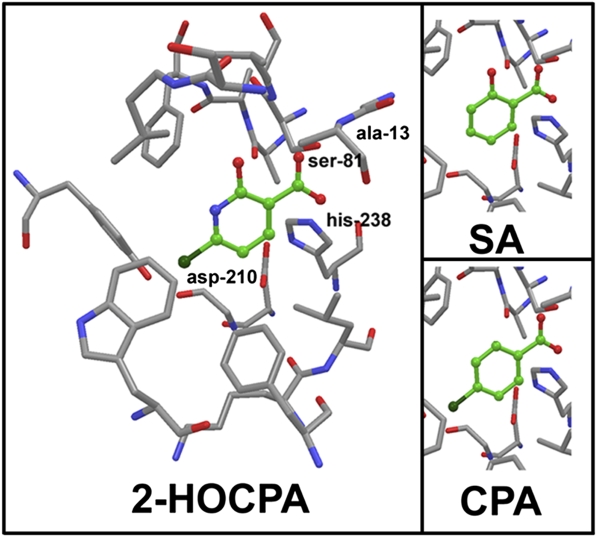
The binding of 2-HOCPA and CPA in the active site of SABP2 was modeled in comparison with SA (Fig. 6), 4-ClSA, and several related compounds (SI Appendix). The chloro and hydroxyl groups of 2-HOCPA play important roles in the binding; 2-HOCPA is calculated to bind the active site of SABP2 with similar affinity to SA and 4-ClSA, and with higher affinity than CPA and the isomers 4-HOCPA and 5-HOCPA, further supporting 2-HOCPA as the bioactive metabolite and functional analog of SA. With each compound, the interaction lengths for Ser O (from OH) to C of the ligand carboxylate are 2.9 to 3.0 Å, and for Ala 13N to the nearest O of the ligand carboxylate are 2.7 Å. Furthermore, the carboxylic acid metabolite of BTH is predicted to bind SABP2 with higher affinity than INA, consistent with SABP2 inhibition studies (33).
Fig. 6.
The active site of SABP2 based on Forouhar et al. (32) and the binding modes of 2-HOCPA and CPA compared with SA. The catalytic triad residues are Ser-81, His-238, and Asp-210.
Discussion
Neonicotinoids Induce SA-Associated Plant-Defense Responses.
This study establishes that IMI and CLO induce SA-associated plant responses in structurally-dependent ways with the Cl-pyr and Cl-thia moieties as the pharmacophores (Fig. 1). This conclusion is based on three distinct lines of evidence: global expression profiles highly similar to that of SA with greatly enhanced expression of SA-associated genes including the marker PR1; enhanced resistance to powdery mildew; and either elevated endogenous biosynthesis of SA (by CLO and CTA) or metabolic conversion to an active SA-mimic (for the IMI metabolite, CPA).
The Cl-thia compound CLO and its metabolite CTA induce endogenous synthesis of SA via ICS1 that is required for SA-associated gene expression (e.g., PR1), and fully enhanced resistance to powdery mildew. This result differs from previous findings with the thiadiazolyl compounds BTH and tiadinil (Fig. 1B), both of which induce PR1 expression and SAR, as BTH does not require endogenous accumulation of SA for these induced responses (22, 34) and the fungicide tiadinil does not induce endogenous synthesis of SA (35). Further study is needed to confirm tiadinil does not require endogenous SA accumulation for SA-associated gene expression and SAR, as the tiadinil experiments did not use Arabidopsis (35) and species-specificity may be important (36). However, long-established inducers of SAR, such as BTH and INA, do act on a wide variety of species and the beneficial effects of IMI and CLO on plant vigor and stress tolerance have been reported or claimed for a similarly diverse group of plants (7, 9).
In contrast to the Cl-thia compounds, IMI and its metabolite CPA elicit SA-associated responses in Arabidopsis, but do not induce significant accumulation of SA nor require ICS1 to induce PR1 expression. Therefore, the hypothesis was tested that CPA, the common metabolite of all Cl-pyr neonicotinoids (5), is hydroxylated to the isostere of SA (and 4-ClSA). Analyses of CPA-treated Arabidopsis showed the presence of a hydroxy-CPA, tentatively identified as 2-HOCPA. The very high PR1-inducing potency of 2-HOCPA and its tested or modeled ability to inhibit PBS3 and SABP2, respectively, support 2-HOCPA as an active mimic of SA, similar to the well established Cl-pyr SA analog, INA (Fig. 1B).
Action of Neonicotinoids in Insects and Plants.
Cl-pyr and Cl-thia neonicotinoids, with combined annual world-wide sales of over 1.5 billion dollars, have in common outstanding insecticidal activity, often with independent enhancement of plant vigor and stress tolerance. Control of pest insects by neonicotinoids is based on their action as nicotinic agonists, and the nicotinic acetylcholine receptor site for insecticidal activity accepts the Cl-pyr and Cl-thia neonicotinoids almost equally well (37). In contrast to insecticidal action, which requires an intact neonicotinoid molecule, the activation of SA-associated responses by IMI and CLO requires only the carboxylic acid cleavage product. This activation of SA-associated responses may have an unanticipated negative impact on systemic insect defense, as robust activation of SA responses can down-regulate jasmonic acid responses important for defense against insects (10, 12). In our study, only a small subset of insect-induced, jasmonic acid-associated responses were repressed by treatment with SA, CLO, or IMI (Dataset 2).
It has been proposed that neonicotinoids promote stress tolerance of plants (e.g., to drought) by increasing NAD(P) to compensate for a stress-induced decrease in NAD(P) levels (7), presumably with a neonicotinoid metabolite functioning as a nicotinamide analog that feeds into the NAD salvage pathway. However, three lines of evidence argue against this being the dominant mode of action of CLO and IMI. First, such a nicotinamide analog could only be formed from Cl-pyr neonicotinoids, such as IMI and CPA, not Cl-thia neonicotinoids, including CLO and CTA (5). Second, neither genes involved in the four-step NAD salvage pathway nor genes thought to be rate-limiting steps in NAD or NADP biosynthesis (i.e., NMNAT and NAD kinases, respectively) exhibited enhanced expression in our microarray studies (SI Appendix). Third, although exogenous application of NAD(P)(H) to Arabidopsis can result in expression of the SA marker PR1 and SAR, PR1 expression requires ICS1 (38), which is not the case for IMI. Instead, we present multiple lines of evidence showing Cl-pyr and Cl-thia nenonicotinoids induce SA-associated responses, including biotic-stress tolerance.
Fitness Cost or Benefit Associated with Activation of SA Responses via Neonicotinoids.
Tens of millions of pounds of Cl-pyr and Cl-thia neonicotinoids are currently applied each year for crop protection. These large amounts of neonicotinoids used to protect crops against insect pests make them coincidentally and unintentionally the major inducers of SAR used in agricultural production. Importantly, reports of the neonicotinoid-induced stress shield have not been associated with a concomitant decrease in yield, but with enhanced growth. Mutants exhibiting constitutively enhanced biosynthesis of SA and PR gene expression often exhibit an associated fitness cost (i.e., reduced plant size) (11). However, this fitness cost may not be the result of enhanced accumulation of SA per se, as the overproduction of SA in tobacco using constitutively expressed bacterial transgenes did not noticeably alter its development or appearance (39). With chemical SAR-inducers, fitness cost or benefit is dependent upon the chemical dosage, the age, and exposure of the plants to (a)biotic stress (40–42). The exogenous application of SAR-inducers tends to benefit plant fitness and yield when it primes the plant for a rapid response to subsequent stress that is then encountered.
The phytohormone SA is best known for its role as a mediator of plant defense, but it can also play a significant, although complicated, role in response to abiotic stress (10, 43) and stress-induced developmental transitions, including flowering time (44). Therefore, although not specifically examined here, the activation of SA-associated responses by these neonicotinoids might also explain the observed phenotypes of enhanced abiotic stress tolerance (e.g., to drought) and earlier flowering (6). Under changing climatic conditions, reliable methods of improving stress tolerance (e.g., to pathogens, drought, or heat) become even more critical, as is the need for a mechanistic underpinning for any treatment employed. Here, we provide the building blocks for future mechanistic studies of neonicotinoid action via SA and its mimics in promoting a stress shield.
Methods
Chemicals.
Sources for the neonicotinoids and metabolites were previously reported (5). Other chemicals were obtained from Aldrich and biochemicals from Sigma, except for 2-HOCPA (SI Appendix).
Plant Lines, Growth Conditions, Chemical Treatments, and Infection with Powdery Mildew.
A. thaliana ecotype Columbia-0 (Col-0) and ics1-2 (eds16-1) mutant (21) in the Col-0 background were grown evenly spaced in 16.6 × 12.4 × 5.8-cm boxes containing Metro mix 200 (Scotts Sierra Horticultural Products) with a 12-h photoperiod (photosynthetically active radiation = 100 μmol·m−2·s−1). Flats were bottom-watered weekly and fertilized with 0.25× Hoagland's solution at 3 wk. Boxes were treated with 100 mL chemical solution or water (control) via soil application at 4 wk. Initial experiments examined PR1 expression by RT-PCR (method described in SI Appendix) in response to 0.4 and 4 mM chemical at 2 and 4 d after soil treatment. PR1 expression was robust and consistent in response to 4 mM SA, IMI, and CLO at 4 d after soil treatment (standard protocol). Cell death was sometimes observed, particularly on older leaves, following application with IMI or CLO. Mature, fully expanded leaves with minimal visible cell death were harvested and immediately frozen on dry ice or liquid nitrogen and stored at −80 °C.
For the powdery mildew experiments, 4 d after chemical soil application, plants were infected with G. orontii MGH isolate conidia from two fully-infected leaves per box using a settling tower and mesh screen, as previously reported (15), to maximize reproducibility. G. orontii-infected and uninfected plants were maintained under the same conditions (above). Assessment of powdery mildew growth and reproduction was previously described (15).
Analysis of SA and SA-Glucoside.
Frozen Arabidopsis leaves (400–500 mg fresh weight) were extracted with cold methanol, the methanol-soluble fraction was treated with β-glucosidase or no hydrolase then trichloroacetic acid, and the supernatants from centrifugation were extracted with ethyl acetate-cyclohexane [1:1] (Fig. 3D) or ethyl acetate-cyclopentane [1:1] (Fig. 3E) to recover free SA (without β-glucosidase) or free SA plus SA-glucoside (with β-glucosidase treatment) for LC/MS/MS analysis (Fig. 3D; method described in SI Appendix) or HPLC analysis with fluorescence detection (Fig. 3E) [method previously described (45)]. Statistical analysis of treatments compared with the control used an unpaired Student's t test.
Detection and Characterization of 2-HOCPA.
Frozen CPA-treated or control Arabidopsis leaves (∼500 mg fresh weight) were extracted as for SA above (45). CPA-treated and control plant extracts were HPLC-fractionated with four 1-mL fractions (F1–F4) encompassing the elution time of synthesized 2-HOCPA analyzed by LC/MS as detailed in SI Appendix.
Inhibition of SA-Binding Protein PBS3.
Inhibitory activity was assessed using the coupled adenylation assay with 150 μM p-aminobenzoic acid as the acyl substrate and 30 or 300 μM of the test compound relative to the control, as in our earlier study (30).
Docking Model for SABP2 Active Site.
Potential ligands were docked to the SABP2 crystal structure 1Y7I (32) after addition of hydrogen atoms and removal of nonprotein moieties using Glide 5.5, as implemented in Maestro 9.0 (46) and detailed in SI Appendix.
Supplementary Material
Acknowledgments
We thank our University of California Berkeley colleagues Tami Clark, Darmood Wei, Hai Liang Huang, and Gajanth Sanmuganatha for experimental assistance. This work was supported by the William Muriece Hoskins Chair in Chemical and Molecular Entomology (K.A.F., J.E.C., and A.G.G.), the Winkler Family Foundation (M.C.W. and D.C.), the William Carroll Smith Fellowship in Plant Pathology (to R.A.O.), National Science Foundation Grant CHE-0840505 (to K.A.D.), a Dupont Young Professor Award (to R.S.), and a Chevron Fellowship (to E.M.B.).
Footnotes
The authors declare no conflict of interest.
Data deposition: Arabidopsis microarray data are deposited in the Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo) with accession no. GSE20188.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013020107/-/DCSupplemental.
References
- 1.Kagabu S. Molecular design of neonicotinoids: Past present and future. In: Voss A, Ramos G, editors. Chemistry of Crop Protection. Weinheim, Germany: Wiley-VCH; 2003. pp. 193–212. [Google Scholar]
- 2.Jeschke P, et al. Clothianidin (TI-435): The third-member of the chloronicotinyl insecticide (CNI) family. Pfanzenschutz-Nachrichten Bayer. 2003;56:5–24. [Google Scholar]
- 3.Nauen R, Ebbinghaus-Kintscher U, Salgado VL, Kaussmann M. Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pestic Biochem Physiol. 2003;76(2):55–69. [Google Scholar]
- 4.Maienfisch P, Brandl F, Kobel W, Rindlisbacher A, Senn R. CGA 293'343: A novel, broad-spectrum neonicotinoid insecticide. In: Yamamoto I, Casida JE, editors. Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor. Tokyo, Japan: Springer-Verlag; 1999. pp. 177–209. [Google Scholar]
- 5.Ford KA, Casida JE. Comparative metabolism and pharmacokinetics of seven neonicotinoid insecticides in spinach. J Agric Food Chem. 2008;56:10168–10175. doi: 10.1021/jf8020909. [DOI] [PubMed] [Google Scholar]
- 6.Thielert W. A unique product: The story of the imidacloprid stress shield. Pflanzenschutz-Nachrichten Bayer. 2006;59:73–86. [Google Scholar]
- 7.Thielert W, Metzlaff M, De Block M. Increase of stress tolerance by application of neonicotinoids on plants engineered to be stress tolerant. 2009 US Patent Appl 0270254 A1. [Google Scholar]
- 8.Gonias ED, Oosterhuis DM, Bibi AC. Physiologic response of cotton to the insecticide imidacloprid under high temperature stress. J Plant Growth Regul. 2008;27:77–82. [Google Scholar]
- 9.Senn R, Hofer D, Thieme T, Lange L. Method for improving plant growth. 2004 US Patent 6,753,296 B1. [Google Scholar]
- 10.Vlot AC, Dempsey DA, Klessig DF. Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol. 2009;47:177–206. doi: 10.1146/annurev.phyto.050908.135202. [DOI] [PubMed] [Google Scholar]
- 11.Durrant WE, Dong X. Systemic acquired resistance. Annu Rev Phytopathol. 2004;42:185–209. doi: 10.1146/annurev.phyto.42.040803.140421. [DOI] [PubMed] [Google Scholar]
- 12.Pieterse CMJ, Leon-Reyes A, Van der Ent S, Van Wees SCM. Networking by small-molecule hormones in plant immunity. Nat Chem Biol. 2009;5:308–316. doi: 10.1038/nchembio.164. [DOI] [PubMed] [Google Scholar]
- 13.Blanco F, et al. Early genomic responses to salicylic acid in Arabidopsis. Plant Mol Biol. 2009;70:79–102. doi: 10.1007/s11103-009-9458-1. [DOI] [PubMed] [Google Scholar]
- 14.Wang D, Amornsiripanitch N, Dong X. A genomic approach to identify regulatory nodes in the transcriptional network of systemic acquired resistance in plants. PLoS Pathog. 2006 doi: 10.1371/journal.ppat.0020123. 10.1371/journal.ppat.0020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandran D, et al. Temporal global expression data reveal known and novel salicylate-impacted processes and regulators mediating powdery mildew growth and reproduction on Arabidopsis. Plant Physiol. 2009;149:1435–1451. doi: 10.1104/pp.108.132985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thimm O, et al. MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J. 2004;37:914–939. doi: 10.1111/j.1365-313x.2004.02016.x. [DOI] [PubMed] [Google Scholar]
- 17.Katari MS, et al. VirtualPlant: A software platform to support systems biology research. Plant Physiol. 2010;152:500–515. doi: 10.1104/pp.109.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoof H, et al. MIPS Arabidopsis thaliana Database (MAtDB): An integrated biological knowledge resource for plant genomics. Nucleic Acids Res. 2004;32(Database issue):D373–D376. doi: 10.1093/nar/gkh068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, et al. The genetic network controlling the Arabidopsis transcriptional response to Pseudomonas syringae pv. maculicola: Roles of major regulators and the phytotoxin coronatine. Mol Plant Microbe Interact. 2008;21:1408–1420. doi: 10.1094/MPMI-21-11-1408. [DOI] [PubMed] [Google Scholar]
- 20.Reuber TL, et al. Correlation of defense gene induction defects with powdery mildew susceptibility in Arabidopsis enhanced disease susceptibility mutants. Plant J. 1998;16:473–485. doi: 10.1046/j.1365-313x.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- 21.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–565. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 22.Nawrath C, Métraux JP. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell. 1999;11:1393–1404. doi: 10.1105/tpc.11.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strawn MA, et al. Arabidopsis isochorismate synthase functional in pathogen-induced salicylate biosynthesis exhibits properties consistent with a role in diverse stress responses. J Biol Chem. 2007;282:5919–5933. doi: 10.1074/jbc.M605193200. [DOI] [PubMed] [Google Scholar]
- 24.Conrath U, Chen Z, Ricigliano JR, Klessig DF. Two inducers of plant defense responses, 2,6-dichloroisonicotinec acid and salicylic acid, inhibit catalase activity in tobacco. Proc Natl Acad Sci USA. 1995;92:7143–7147. doi: 10.1073/pnas.92.16.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tinschert A, Tschech A, Heinzmann K, Kiener A. Novel regioselective hydroxylations of pyridine carboxylic acids at position C2 and pyrazine carboxylic acids at position C3. Appl Microbiol Biotechnol. 2000;53:185–195. doi: 10.1007/s002530050007. [DOI] [PubMed] [Google Scholar]
- 26.Leon J, Yalpani N, Raskin I, Lawton MA. Induction of benzoic acid 2-hydroxylase in virus-inoculated tobacco. Plant Physiol. 1993;103:323–328. doi: 10.1104/pp.103.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beaman AG, Miller ON. Nicotinsäurederivate, German Patent 2,157,334. 1972 [Google Scholar]
- 28.Lee MW, Lu H, Jung HW, Greenberg JT. A key role for the Arabidopsis WIN3 protein in disease resistance triggered by Pseudomonas syringae that secrete AvrRpt2. Mol Plant Microbe Interact. 2007;20:1192–1200. doi: 10.1094/MPMI-20-10-1192. [DOI] [PubMed] [Google Scholar]
- 29.Park SW, Kaimoyo E, Kumar D, Mosher S, Klessig DF. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science. 2007;318:113–116. doi: 10.1126/science.1147113. [DOI] [PubMed] [Google Scholar]
- 30.Okrent RA, Brooks MD, Wildermuth MC. Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J Biol Chem. 2009;284:9742–9754. doi: 10.1074/jbc.M806662200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SW, et al. Use of a synthetic salicylic acid analog to investigate the roles of methyl salicylate and its esterases in plant disease resistance. J Biol Chem. 2009;284:7307–7317. doi: 10.1074/jbc.M807968200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forouhar F, et al. Structural and biochemical studies identify tobacco SABP2 as a methyl salicylate esterase and implicate it in plant innate immunity. Proc Natl Acad Sci USA. 2005;102:1773–1778. doi: 10.1073/pnas.0409227102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du H, Klessig DF. Identification of a soluble, high-affinity salicylic acid-binding protein in tobacco. Plant Physiol. 1997;113:1319–1327. doi: 10.1104/pp.113.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawton KA, et al. Benzothiadiazole induces disease resistance in Arabidopsis by activation of the systemic acquired resistance signal transduction pathway. Plant J. 1996;10:71–82. doi: 10.1046/j.1365-313x.1996.10010071.x. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda M, et al. Thiadiazole carboxylic acid moiety of tiadinil, SV-03, induces systemic acquired resistance in tobacco without salicylic acid accumulation. J Pestic Sci. 2006;31:329–334. [Google Scholar]
- 36.Hatzios KK, Penner D. Metabolism of Herbicides in Higher Plants. Minneapolis, MN: Burgess Publishing Company; 1982. [Google Scholar]
- 37.Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol. 2003;48:339–364. doi: 10.1146/annurev.ento.48.091801.112731. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Mou Z. Extracellular pyridine nucleotides induce PR gene expression and disease resistance in Arabidopsis. Plant J. 2009;57:302–312. doi: 10.1111/j.1365-313X.2008.03687.x. [DOI] [PubMed] [Google Scholar]
- 39.Verberne MC, Verpoorte R, Bol JF, Mercado-Blanco J, Linthorst HJM. Overproduction of salicylic acid in plants by bacterial transgenes enhances pathogen resistance. Nat Biotechnol. 2000;18:779–783. doi: 10.1038/77347. [DOI] [PubMed] [Google Scholar]
- 40.Walters DR, Boyle C. Induced resistance and allocation costs: What is the impact of pathogen challenge? Physiol Mol Plant Pathol. 2005;66:40–44. [Google Scholar]
- 41.Dietrich R, Ploss K, Heil M. Growth responses and fitness costs after induction of pathogen resistance depend on environmental conditions. Plant Cell Environ. 2005;28:211–222. [Google Scholar]
- 42.van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J. Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:5602–5607. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan S, Lin H-H. Role of salicylic acid in plant abiotic stress. Z Naturforsch C. 2008;63:313–320. doi: 10.1515/znc-2008-5-601. [DOI] [PubMed] [Google Scholar]
- 44.Davis SJ. Integrating hormones into the floral-transition pathway of Arabidopsis thaliana. Plant Cell Environ. 2009;32:1201–1210. doi: 10.1111/j.1365-3040.2009.01968.x. [DOI] [PubMed] [Google Scholar]
- 45.Nobuta K, et al. The GH3 acyl adenylase family member PBS3 regulates salicylic acid-dependent defense responses in Arabidopsis. Plant Physiol. 2007;144:1144–1156. doi: 10.1104/pp.107.097691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friesner RA, et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.