Abstract
The Cys-loop family of receptors mediates synaptic neurotransmission in the central nervous system of vertebrates. These receptors share several structural characteristics and assemble in the plasma membrane as multimers with fivefold symmetry. Of these, the ionotropic GABA receptors are key players in the pathogenesis of diseases like epilepsy, anxiety, and schizophrenia. Different experimental approaches have shed some light on the mechanisms behind the function of these receptors; but little is known about their structure at high resolution. Sequence homology with the nicotinic acetylcholine receptor predicts that ionotropic GABA receptors possess four transmembrane segments (TM1–4) and that TM2 forms the wall of the ion channel. However, the role of the other three segments is unclear. The GABAρ1 receptor plays a fundamental role in the regulation of neurotransmission along the visual pathway, is highly sensitive to GABA, and exhibits little desensitization. In our recent investigations of the role of TM4 in receptor function, a key residue in this domain (W475) was found to be involved in activation of the receptor. Here we have generated a structural model of the GABAρ1 receptor in silico and assessed its validity by electrophysiologically testing nine amino acid substitutions of W475 and deletions of the neighboring residues (Y474 and S476). The results identify a critical linkage between the ligand-binding domain and the TM4 domain and provide a framework for more detailed structure-function analyses of ionotropic GABA receptors.
Keywords: receptor structure, two-microelectrode voltage clamp, Xenopus oocyte, in silico modeling
GABAρ receptors play a central role in the visual pathway, negatively modulating glutamatergic transmission in the retina (1, 2). These receptors are located at the axon terminals of rod bipolar cells, where GABAergic input is received from amacrine neurons (3). There are three known genes coding for GABAρ receptors, GABAρ1 to -3, all of which form functional homomeric receptors with pharmacological properties very different from those of other GABA receptors. For example, they are highly sensitive to GABA, desensitize very little, and are essentially bicuculline/baclofen insensitive in situ (4, 5) and when expressed in heterologous systems, such as the Xenopus laevis oocyte (6, 7).
The Cys-loop receptor superfamily, within which the GABAρ receptors are classified, includes cation-selective receptors, such as the nicotinic acetylcholine (nACh) and 5-hydroxytryptamine 3 (5-HT3) receptors, as well as anion-selective receptors, such as those for glycine and GABA (8). All these receptors share well-conserved amino acid sequences and presumably assemble in the plasma membrane as pentamers. Each subunit of the pentamer contains a large extracellular N-terminal ligand-binding domain (LBD), four transmembrane (TM1–4) helices, and a variable intracellular loop between TM3 and TM4 (Fig. 1 A and B). The TM2 segment lines the pore domain and has subdomains that selectively discriminate ions according to their electric charge. The remaining three helices (TM1, -3, and -4) function as a “hydrophobic shelter,” incorporating and protecting the pore in the plasma membrane (9–11).
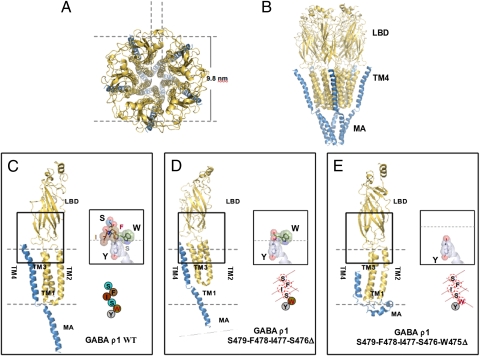
Fig. 1.
Structural model of the homopentameric GABAρ1 receptor. (A) View from the extracellular side revealing the ion-conducting pore. (B) Side view of the receptor embedded in the membrane showing its three largest domains: LBD, transmembrane domains (TM), and membrane-associated domain (MA). (C) Details of a single subunit of GABAρ1 WT anchored in the membrane. The box shows the interaction between the carboxyl-terminal TM4 domain and the Cys-loop at the extracellular domain; the inset shows the disposition of the residues at the helix. In this and following insets, the dashed lines indicate the plane of the plasma membrane. (D) The structure of the helix persists despite the elimination of the last four residues of TM4. (E) Structure and function of the receptor collapse by the serial deletion to W475 in the TM4 domain. Note the displacement of TM4 and MA. S476X and W475X were functionally characterized in ref. 16.
Despite their crucial role in neurotransmission and involvement in many neuronal processes and diseases, the fine structure of ionotropic GABA receptors remains largely unknown and no crystal structure information is available to date. Nevertheless, homology-based computer modeling provides sufficiently accurate structural details to test the potential role of functional domains of the receptor (12–15). We are interested in understanding the functional role of the carboxy-terminus of the TM4 segments of GABAρ receptors. In previous work, we found that deletion of one to four residues (S471 to S474) still gave rise to functional receptors. However, further deletions to and beyond residue W475 resulted in receptors that did not gate the GABAρ receptor ion channel (16). Molecular modeling of GABAρ1 (Fig. 1 A and B) suggests that this mutant loses a hydrophobic interaction between TM4 and the extracellular Cys-loop domain that is necessary for activation of the channel (Fig. 1 C–E).
Here we extend the structure-function studies of the TM4 domain of GABAρ1, focusing on residue W475 embedded close to the upper edge of the membrane and whose removal resulted in nonfunctional receptors. Molecular modeling was applied to predict the functional outcome of single-residue substitutions and deletions of the neighboring residues (Y474 and S476); and the mutant receptors were tested electrophysiologically after their expression in X. laevis oocytes. The structural impacts of the modifications were computer modeled, taking advantage of the refined structure of the nAChR (11), the X-ray crystal structures of the ACh binding protein (17), and prokaryotic homologs of the Cys-loop receptors ELIC from Erwinia chrysantemi (18) and GLIC from Gleobacter violaceus (19, 20).
Results
GABAρ1 and GABAρ1W475X.
Our previous experiments (16) showed that deletion of five residues from the carboxy-terminus of GABAρ1 blocked receptor function of the expressed protein. The stable architecture of the TM4 α-helix in GABAρ1 consists of a triangular elastic bracket-like structure in the extracellular leaflet of the membrane. W475 is oriented toward the large extracellular N terminal and interacts via an almost planar hydrophobic interaction with L207 in the Cys-loop domain (Fig. 2A). The side chains of Y474 and I477 are oriented toward the plasma membrane; S476 acts like a hinge. This structure is completely broken after deleting the last five residues of the receptor (mutant W475X) (Fig. 1E). Deletions before W475 produced receptors with different properties (Fig. 1D) (16), but once W475 was removed the integral structure of TM4 was unstable, leading to the disruption of the hydrophobic interaction with the Cys-loop required for proper gating of the ion-channel. The essential role of this residue was investigated further by modeling single amino acid substitutions and predicting their function. We then performed electrophysiological studies to determine whether or not the expressed receptor was functional (Table 1).
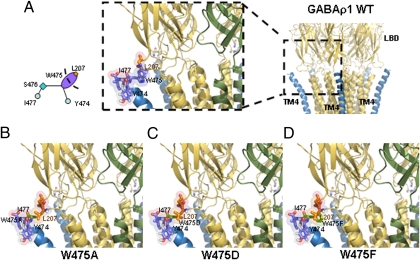
Fig. 2.
Expanded view of the interaction between TM4 and the Cys-loop. (A) Positions of the residues forming the elastic bracket-like structure exposing W475 to L207 by hydrophobic interactions. (B–D) Substitutions W475A, W475D, and W475F that were predicted to be stable. However, W475D did not form functional receptors.
Table 1.
Summary of the mutants, structural impact, and functional outcome
| Mutant | Outcome (+) active/(−) not active | Homology model prediction | Prediction accuracy |
| W475A | + | Increase in the GABA EC50 and a decrease in the number of ligand binding sites | Yes |
| W475L | + | High stability, decreased sensitivity | Yes |
| W475R | − | Unstable conformation | Yes |
| W475F | + | Keeps the structure similar to WT | Yes |
| W475G | + | Substitution is well tolerated | Yes |
| W475D | − | Stable interaction of peptide bonds | No |
| W475Δ | − | Deletion will disrupt the interaction of TM4 | Yes |
| Y474Δ | − | Decreased stability of TM4 | Yes |
| S476Δ | + | Mutation preserves the core architecture, conferring stability | Yes |
ρ1 W475A.
The model of W475A shows a subtle interaction between 475A and the hydrophobic side chain of L207, which forms part of the Cys-loop (Fig. 2B). This interaction promotes the stability of the receptor, although in the WT (W475) the structure is more flexible. The in silico prediction of the model was that this substitution increases the GABA EC50 concomitant with a decrease in the number of ligand binding sites available in the receptor, a result of the rigid hydrophobic interaction. In simple functional tests, injection of the cRNA of GABAρ1W475A into X. laevis oocytes gave rise to functional receptors.
ρ1 W475D.
Molecular modeling suggested that the W475D substitution stabilizes the interaction of the positive charge of the amine group of the peptide bond formed between P206 and L207, generating a salt bridge, maintaining the stability of both elements (Fig. 2C), and giving rise to functional receptors. However, no responses to GABA were detected after injecting two different cRNAs of this mutant into oocytes from three different donors. This result was unexpected. Although failure of transferring the protein to the membrane has not been excluded, it suggests that further refinements of the model are needed to improve the accuracy of the functional predictions.
ρ1 W475F.
In this mutant, the aromatic hydrophobic Phe in TM4 is oriented as in WT W475, and thus interacts with the hydrophobic side chain of L207 in the Cys-loop (Fig. 2D). This interaction gives rise to a structure similar to that of the WT receptor, implying that the substitution is stable in the membrane. Electrophysiological studies confirmed that this mutant formed functional GABA-gated receptors.
ρ1 W475G.
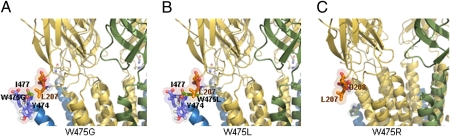
This substitution is well tolerated in the structure because of the unique nature of this residue among the proteinogenic amino acids, namely the absence of an untangled side chain (Fig. 3A). The predicted structure of this receptor is stable and, in fact, this mutant generated functional receptors in the oocytes.
Fig. 3.
(A) W475G and (B) W475L were predicted to be stable and functional and they generated GABA-gated ion-channels. (C) In contrast, W475R was predicted to be unstable and was nonfunctional.
ρ1 W475L.
The bulky hydrophobic nature of this residue was predicted to lead to a receptor with greater stability in the membrane, but with the risk of a decrease in sensitivity to the ligand caused by steric effects (Fig. 3B). However, this mutant formed functional GABA receptors.
ρ1 W475R.
The accepted hypotheses about the orientation of TM domains and their assembly suggest that positively charged residues in the sequences flanking the TM segments are asymmetrically distributed, and that an excess of positively charged residues defines a cytoplasmic domain (21). The W475R substitution was predicted to lead to an unstable conformation of the receptor, because of electrostatic repulsion between the positive interacting charges of W475R and the peptide bond formed by P206-L207 (Fig. 3C). The functionality of this conformation was anticipated to be very low, and expression of this mutant in oocytes consistently failed to yield responses to GABA.
ρ1 W475Δ.
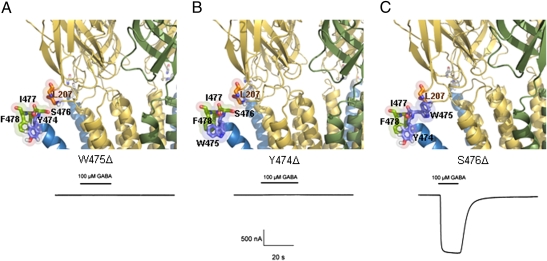
This deletion will disrupt the interaction of TM4 with the Cys-loop, exposing the polar side chain of S476, hiding the hydrophobic side chain of L207 in the Cys-loop, and exposing this region to the solvent. These effects will decrease conformational stability, which in turn will impair GABAρ1 receptor function (Fig. 4A). Electrophysiological experiments showed that this mutant does not produce functional receptors.
Fig. 4.
(A) W475Δ disrupted the hydrophobic interaction with L207, thus forming nonfunctional receptors. (B) In Y474Δ, W475 is displaced and does not form the hydrophobic interaction properly. The mutant did not form functional receptors when expressed in oocytes. (C) The S476Δ deletion did not alter the interaction between W475 and L207 and yielded functional receptors. Traces below the figures are sample records of membrane currents in response to 10−4 M GABA.
ρ1 Y474Δ.
The structure of this deletion mutant suggests a positional change in the TM4 domain, leading W475 to a tilted position toward the plasma membrane's hydrophobic region, similar to that occupied by Y474 (displaced ∼70° with respect to its original position in the GABAρ1 WT TM4 helix). It is expected to exhibit decreased stability because of the charged field conferred by the amine at the pyrrole end of the indole ring. However, the rest of the domain adopts a conformation similar to that of the W475Δ deletion, which decreases the probability of forming a functional receptor (Fig. 4B). In agreement with this prediction, the injection of this mutant construct into oocytes did not generate functional receptors.
ρ1 S476Δ.
This mutation preserves the core architecture of the triangular elastic bracket-like structure, but I477 takes the place of S476 and F478 becomes a third hydrophobic component, conferring the stability necessary to predict that this substitution will give rise to functional receptors. As in ρ1W475F, the aromatic ring of F478 would be stabilized in the polar side of the phosphate group of membrane phospholipids (Fig. 4C). This mutant gave rise to functional receptors when expressed in frog oocytes.
Discussion
GABAρ receptors play a fundamental role in neurotransmission along the visual pathway, but their high-resolution structure has not been determined. Understanding how the functional modules of the receptor interact with each other is important to complement the structural models of the receptor that have been generated computationally. This article describes an in silico-generated structural model of the GABAρ1 receptor, whose validity was tested by assaying electrophysiologically the impact of amino acid substitutions at W475 of the TM4 domain, a residue that is critical for the proper function of the receptor (16).
The TM1, TM2, and TM4 α-helices of the Cys-loop receptors are organized as a hydrophobic shelter that surrounds and embeds the ion channel formed by the TM2 segment in the plasma membrane (22). The W475 residue in TM4 plays a critical role in this structure by providing a hydrophobic interaction with L207 in the Cys-loop, which is required for proper gating of the ion-channel. Pons et al. (23) revealed the relevance of TM4 in the nACh α7 and 5HT3 receptors, suggesting a role in the process of transferring receptors to the plasma membrane. Residue W443 of TM4 of nACh α7 was proposed to interact with the Cys-loop, inducing a conformationally available state for the binding of agonist. Recently, Haeger et al. (24) demonstrated that the proper pentameric assembly of Cys-loop receptors depends on intersubunit interactions between extracellular domains and intrasubunit interactions between transmembrane segments. The authors successfully rescued the expression of truncated Gly and 5-HT3A receptors by coexpressing them with the TM4 domain in oocytes. In addition, alanine scanning along the four transmembrane domains of the GlyR led to the conclusion that an intramembranous aromatic network determines the correct assembly of this receptor. Similar conclusions were suggested by Butler et al. (25), indicating that the C terminus stabilizes the 5-HT3 receptor, allowing correct subunit folding and maturation.
The structural model provided here predicts a close interaction between the TM4 and the Cys-loop, consistent with the observations in nicotinic, glycine, and 5-HT3 receptors. In a previous study, serial deletions from the carboxy-terminus showed that residue W475, immersed in the TM4 domain of GABAρ1, is necessary to give rise to functional receptors, even though the truncated receptor reaches the plasma membrane (16). Deletion of this residue likely blocked formation of GABA-gated channels becaue of the disruption of the hydrophobic bridge with the extracellular domain, as predicted (Fig. 1 C–E), interrupting the geometry of interactions required for opening the ion channel. Several amino acid substitutions of W475 resulted in functional receptors: W475A, W475L, W475F, and W475G all gave rise to GABA responses when expressed in oocytes, and in all cases the structural model predicted the outcome of the mutation. More studies are necessary to determine fine functional details, such as the affinity for the ligands, stability of the receptor, and single-channel characteristics.
The substitution W475R was expected to interrupt the hydrophobic interaction with the Cys-loop and, accordingly, oocytes expressing that mutant did not generate currents when exposed to GABA. However, we still do not know if this mutant reaches the plasma membrane. W475D was predicted to generate functional receptors, but unexpectedly it did not generate GABA-currents. A possible explanation for the lack of function of the W475D mutant is that the model predicted its membrane stability because of the formation of a salt bridge between P206 and L207 in the Cys-loop and D475 in TM4, acting as an electron donor. This finding suggests that the structure may be stable but unable to trigger conformational changes linked to activation of the channels, because stability no longer depends on hydrophobic interactions present in the WT receptor.
Deletion of Y474, a highly conserved position in the TM4 domain of anion-selective GABA receptors, predicted disruption of the function of the receptor, which was confirmed in functional assays. This position is occupied by hydrophobic residues, such as Leu, Met, or Phe in other Cys-loop receptors (26, 27), and is located in the plasma membrane at the interface that forms a bridge with the extracellular domain. A structural conformation that involves Y474, S476, and I477 forms a “hinge” that exposes W475 to form a hydrophobic bridge with L207. Deletion of S476 still gives rise to functional receptors, because the geometry of the hinge is not disrupted, permitting generation of the hydrophobic bridge with the Cys-loop by keeping W475 exposed.
The structural and functional observations described here are in accord with our model for GABAρ1, as well as with previous structural models of the Cys-loop receptor family (11, 28). More refined models will improve their predictive accuracy. This initial study provides a framework for more detailed structure-function studies of ionotropic GABA receptors. In view of the involvement of GABA receptors in many brain functions and diseases, obtaining a high-resolution structure of ionotropic GABA receptors is imperative for the near future.
Materials and Methods
Molecular Modeling.
The pentameric model of human GABAρ1 was developed using ESyPred3D software based on MODELER (University of Namur, Belgium) and SWISS-MODEL (Swiss Institute of Bioinformatics, University of Basel, Switzerland) algorithms, using as a template the cryo-electron microscopy structure generated for Torpedo marmorata nAChR, PDB ID: 2BG9. The pentamer was reconstructed using ICM v3.5, MolSoft LLC software and energy minimization was calculated with GROMOS96 (part of DeepView/Swiss-Pdb Viewer v4.0.1 software). The conserved domains were confirmed from the consensus secondary structure prediction server Jpred3 (The Barton Group, University of Dundee, Scotland). The point mutants (W475A, W475D, W475F, W475G, W475L, W475R, W475Δ, Y474Δ, S476Δ) and rasterization were generated using the Mutagenesis and Ray tools of PyMOL v0.99 DeLano Scientific LLC software.
Molecular Biology and Electrophysiology.
The deletion GABAW475X was described in Reyes-Ruiz et al. (16). The following substitutions were generated via site-directed mutagenesis by means of PCR and subcloning: W475A, W475D, W475F, W475G, W475L, W475R, W475Δ, Y474Δ, S476Δ. Complementary RNA was synthesized using the mMessage Machine from Ambion and 50 nL were injected at 1 μg/μL in defolliculated oocytes. Each deletion was injected two or three times in oocytes from different frogs. A two-microelectrode voltage clamp (29) was used to determine simply whether or not expression of the mutant receptors in Xenopus oocytes yielded responses to GABA.
Acknowledgments
We thank A. Limón for help with the manuscript, Dr. A. Rojo (Metropolitan Autonomous University) for critical analysis of the paper, and Dr. Nicholas Spitzer for his significant review of the manuscript. This work was supported by Consejo Nacional de Ciencia y Technología (CONACyT) Grant 101851, Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica–Universidad Nacional Autónoma de México Grants IN202609 and IN205308 (to R.M. and A.M.-T.), the King Abdul Aziz City for Science and Technology Grant KACST-46749 (to R.M.); and the A & W Shedid Fund (A.E.-M. and A.M.-T.). A.E.-M. was a CONACyT fellow.
Footnotes
The authors declare no conflict of interest.
References
- 1.Polenzani L, Woodward RM, Miledi R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc Natl Acad Sci USA. 1991;88:4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50:923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher EL, Koulen P, Wässle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol. 1998;396:351–365. doi: 10.1002/(sici)1096-9861(19980706)396:3<351::aid-cne6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Woodward RM, Polenzani L, Miledi R. Characterization of bicuculline/baclofen-insensitive gamma-aminobutyric acid receptors expressed in Xenopus oocytes. I. Effects of Cl- channel inhibitors. Mol Pharmacol. 1992;42(1):165–173. [PubMed] [Google Scholar]
- 5.Woodward RM, Polenzani L, Miledi R. Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acidA and gamma-aminobutyric acidB receptor agonists and antagonists. Mol Pharmacol. 1993;43(4):609–625. [PubMed] [Google Scholar]
- 6.Cutting GR, et al. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: A GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci USA. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Torres A, Vazquez AE, Panicker MM, Miledi R. Cloning and functional expression of alternative spliced variants of the rho1 gamma-aminobutyrate receptor. Proc Natl Acad Sci USA. 1998;95:4019–4022. doi: 10.1073/pnas.95.7.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyazawa A, Fujiyoshi Y, Stowell M, Unwin N. Nicotinic acetylcholine receptor at 4.6 A resolution: Transverse tunnels in the channel wall. J Mol Biol. 1999;288:765–786. doi: 10.1006/jmbi.1999.2721. [DOI] [PubMed] [Google Scholar]
- 10.Paas Y, et al. Pore conformations and gating mechanism of a Cys-loop receptor. Proc Natl Acad Sci USA. 2005;102:15877–15882. doi: 10.1073/pnas.0507599102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Melis C, Lummis SC, Molteni C. Molecular dynamics simulations of GABA binding to the GABAC receptor: the role of Arg104. Biophys J. 2008;95:4115–4123. doi: 10.1529/biophysj.107.127589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dougherty DA. Cys-loop neuroreceptors: Structure to the rescue? Chem Rev. 2008;108:1642–1653. doi: 10.1021/cr078207z. [DOI] [PubMed] [Google Scholar]
- 14.Adamian L, et al. Structural model of rho1 GABAC receptor based on evolutionary analysis: Testing of predicted protein-protein interactions involved in receptor assembly and function. Protein Sci. 2009;18:2371–2383. doi: 10.1002/pro.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Xue F, Chang Y. Structural determinants for antagonist pharmacology that distinguish the rho1 GABAC receptor from GABAA receptors. Mol Pharmacol. 2008;74(4):941–951. doi: 10.1124/mol.108.048710. [DOI] [PubMed] [Google Scholar]
- 16.Reyes-Ruiz JM, Ochoa-de la Paz LD, Martínez-Torres A, Miledi R. Functional impact of serial deletions at the C-terminus of the human GABArho1 receptor. Biochim Biophys Acta. 2010;1798:1002–1007. doi: 10.1016/j.bbamem.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Brejc K, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 18.Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 19.Bocquet N, et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 20.Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–118. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 21.von Heijne G, Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988;174:671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]
- 22.Unwin N. Structure of the acetylcholine-gated channel. Novartis Found Symp. 2002;245:5–15. [PubMed] [Google Scholar]
- 23.Pons S, et al. Critical role of the C-terminal segment in the maturation and export to the cell surface of the homopentameric alpha 7-5HT3A receptor. Eur J Neurosci. 2004;20:2022–2030. doi: 10.1111/j.1460-9568.2004.03673.x. [DOI] [PubMed] [Google Scholar]
- 24.Haeger S, et al. An intramembrane aromatic network determines pentameric assembly of Cys-loop receptors. Nat Struct Mol Biol. 2010;17(1):90–98. doi: 10.1038/nsmb.1721. [DOI] [PubMed] [Google Scholar]
- 25.Butler AS, et al. Importance of the C-terminus of the human 5-HT3A receptor subunit. Neuropharmacology. 2009;56:292–302. doi: 10.1016/j.neuropharm.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Lee WY, Free CR, Sine SM. Binding to gating transduction in nicotinic receptors: Cys-loop energetically couples to pre-M1 and M2-M3 regions. J Neurosci. 2009;29:3189–3199. doi: 10.1523/JNEUROSCI.6185-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortiz-Miranda SI, Lasalde JA, Pappone PA, McNamee MG. Mutations in the M4 domain of the Torpedo californica nicotinic acetylcholine receptor alter channel opening and closing. J Membr Biol. 1997;158(1):17–30. doi: 10.1007/s002329900240. [DOI] [PubMed] [Google Scholar]
- 28.Pless SA, Lynch JW. Illuminating the structure and function of Cys-loop receptors. Clin Exp Pharmacol Physiol. 2008;35:1137–1142. doi: 10.1111/j.1440-1681.2008.04954.x. [DOI] [PubMed] [Google Scholar]
- 29.Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]