Abstract
The failing heart is subject to elevated metabolic demands, adverse remodeling, chronic apoptosis, and ventricular dysfunction. The interplay among such pathologic changes is largely unknown. Several laboratories have identified a unique posttranslational modification that may have significant effects on cardiovascular function. The O-linked β-N-acetylglucosamine (O-GlcNAc) posttranslational modification (O-GlcNAcylation) integrates glucose metabolism with intracellular protein activity and localization. Because O-GlcNAc is derived from glucose, we hypothesized that altered O-GlcNAcylation would occur during heart failure and figure prominently in its pathophysiology. After 5 d of coronary ligation in WT mice, cardiac O-GlcNAc transferase (OGT; which adds O-GlcNAc to proteins) and levels of O-GlcNAcylation were significantly (P < 0.05) elevated in the surviving remote myocardium. We used inducible, cardiac myocyte-specific Cre recombinase transgenic mice crossed with loxP-flanked OGT mice to genetically delete cardiomyocyte OGT (cmOGT KO) and ascertain its role in the failing heart. After tamoxifen induction, cardiac O-GlcNAcylation of proteins and OGT levels were significantly reduced compared with WT, but not in other tissues. WT and cardiomyocyte OGT KO mice underwent nonreperfused coronary ligation and were followed for 4 wk. Although OGT deletion caused no functional change in sham-operated mice, OGT deletion in infarcted mice significantly exacerbated cardiac dysfunction compared with WT. These data provide keen insights into the pathophysiology of the failing heart and illuminate a previously unrecognized point of integration between metabolism and cardiac function in the failing heart.
Keywords: heart failure, metabolism, O-GlcNAc, remodeling, infarct
Heart failure is a multifactorial process manifested as an inability to maintain cardiac output. Although various pharmacologic interventions manage the symptoms, no cure exists. Pathologic remodeling of the remote myocardium represents an attractive target for treatment of heart disease. Such remodeling is physically and temporally remote from the acute infarct and is characterized by apoptotic myocyte loss and reorganization of the ECM with collagen secreted from the cardiac fibroblasts to fill in the space left by these dead cells. This addition of collagen results in a more fibrotic, stiffer ventricle. Despite our appreciation of such pathologic events, few viable treatments exist.
Profound metabolic derangements and metabolic dysfunction typify bioenergetic changes in the failing heart (1). Others have documented down-regulation of essential transcription factors, such as peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) (2). PGC-1α is a transcriptional coactivator that, along with the peroxisome proliferator-activated receptor family of transcription factors, regulates several genes required for energy generation, and in particular, fatty acid oxidation (3, 4). Moreover, PGC-1α has also been shown to be essential in the failing myocardium (5).
Although the widely appreciated shift to the use of glucose during heart failure may be beneficial (6) because glycolytic ATP production spares oxygen, it is not necessarily linked to an increase in glucose oxidation (7). Excess glucose flux may be shunted into the accessory pathways that diverge off of glycolysis (8), which may include the hexosamine biosynthetic pathway (HBP). Upon entering the cell, 95% to 99% of glucose contributes to energy production or storage as glycogen. The remainder (<5%) is funneled into one of several accessory pathways of glycolysis, such as the polyol pathway, pentose phosphate pathway, and HBP pathway. The HBP begins with the transamination of fructose-6-phosphate, via the rate-limiting enzyme glutamine:fructose amidotransferase (GFAT), and culminates in the production of the high energy molecule uridine diphosphate N-acetylglucosamine (UDP-GlcNAc). This pathway has been shown to be important in the cardiovascular system (9, 10) and has been implicated in several chronic diseases (11).
UDP-GlcNAc serves as the monosaccharide donor for the dynamic posttranslational modification of O-linked β-N-acetylglucosamine (O-GlcNAc) (12). This modification (known as O-GlcNAcylation) is attached to proteins at serine and threonine residues akin to phosphorylation and occurs both in the nucleus and cytosol of all cell types. Although O-GlcNAcylation and phosphorylation are similar, and sometimes reciprocal, an important difference must be noted: although there are hundreds of kinases and phosphatases that regulate the phosphate signal, only two enzymes regulate O-GlcNAcylation. One enzyme, O-GlcNAc transferase (OGT), adds the modification, where it remains until removed by O-GlcNAcase (OGA). Residing on the X chromosome, OGT is a singly coded enzyme that is highly conserved across multicellular eukaryotes. Although only one gene has been identified, there are three potential splice variants, including nuclear/cytoplasmic, mitochondrial, and a “short OGT.” Successful embryonic development requires OGT supporting its biological importance (13). The protein itself has two domains, the first of which contains a N-terminal tetratricopeptide repeat domain, used for substrate recognition and binding, and a C-terminal catalytic domain. We focused on the potential role of OGT in the present study.
Because the O-GlcNAc posttranslational modification is derived from glucose, is a metabolic sensor, and is implicated in several chronic diseases, it follows that the levels of this posttranslational modification might change in the failing heart. The current study was devised to determine if such change occurred and what role it played in the pathogenesis of heart failure. Here, we provide seminal evidence that there is altered O-GlcNAcylation in the failing heart. Moreover, tissue-specific genetic deletion of O-GlcNAc transferase reduces O-GlcNAcylation and exacerbates cardiac dysfunction and mortality during infarct induced heart failure. We also provide potential insight into the mechanism of such deterioration, which suggests exacerbated pathological remodeling with an increase in apoptosis and fibrosis in the absence of O-GlcNAc transferase in the failing heart. This study provides unique insights into remodeling within the failing heart and implicates an essential signaling system.
Results
Characterization of Infarct-Induced Heart Failure.
Male WT C57/BL6 mice (Jackson Laboratory) were subjected to left coronary artery ligation or sham surgery. After 5 d, mice were anesthetized with 1.5% isoflurane, and hearts were removed and stored for subsequent analysis. Following coronary ligation, animals exhibited heart failure-like symptoms such as the initiation of a large scar as seen in the representative Masson trichrome section (Fig. S1A), an elevated heart weight to tibia length ratio (P < 0.05; Fig. S1B), cardiac hypertrophy as determined by wheat germ agglutinin staining (Fig. S1C, P < 0.05; representative image in Fig. S1D), pulmonary edema (P < 0.05; Fig. S1E), and up-regulation of atrial natriuretic peptide and brain natriuretic peptide mRNA compared with WT sham.
In these same hearts, levels of glycolytic and HBP enzyme mRNAs were also determined. Hexokinase (HEK) 1 was significantly (P < 0.05) elevated, whereas HEK2, GAPDH, and phospho-fructokinase-1 (PFK-1) remained unchanged (Fig. S2A). This increase in HEK1 was accompanied by a significant (P < 0.05) increase in GFAT1 but not GFAT2 (Fig. S2A), the rate-limiting enzyme of the HBP. Although GFAT1 was elevated, no change in the steady-state levels of UDP-HexNAc occurred. While this increase in glycolytic transcripts occurred, levels of key metabolic transcriptional regulators PGC-1α and PGC-1β were markedly (P < 0.05) reduced (Fig. S2A). With this reduction, genes controlled by PGC-1 such as carnitine palmitoyltransferase 1 (CPT-1), carnitine palmitoyltransferase 2 (CPT-2), medium chain acyl-CoA dehydrogenase (MCAD), ATP synthase subunit 5 (ATP-5O) and cytochrome oxidase subunit 5B (COXIV 5B; P < 0.05; Fig. S2A) were also reduced. Along with this decrease in genes required for fatty acid metabolism, the levels of glucose transporters (GLUT) 1 and 4 were also determined. Interestingly, although GLUT1 levels did not change, there was a significant reduction in GLUT4 (P < 0.05; Fig. S2A), also a PGC-dependent product. Hearts were also harvested and quantitative RT-PCR (qRT-PCR) analysis was performed 28 d following surgery (Fig. S2B).
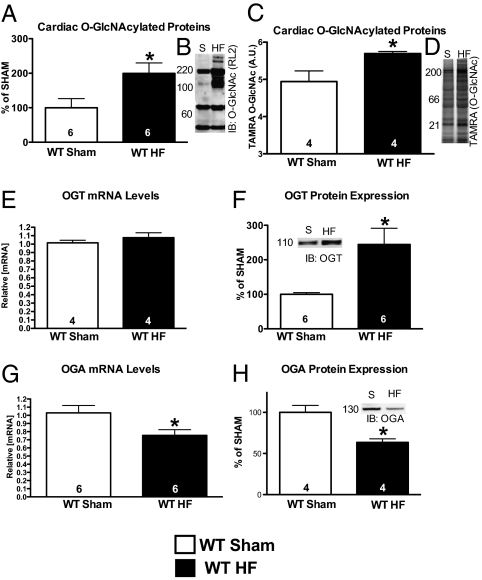
Interestingly, global O-GlcNAcylation was elevated at 5 d as evidenced by Western blot (Fig. 1A, P < 0.05; representative image in shown in Fig. 1B) and a nonantibody technique using Click chemistry (Fig. 1C, P < 0.05; representative image shown in Fig. 1D). Whereas the mRNA levels of OGT, the enzyme that adds the modification to proteins, were unchanged (Fig. 1E), the protein levels were increased 5 d after infarction (Fig. 1F, P < 0.05; representative image shown in Fig. 1F, Inset). The opposing enzyme, OGA, was significantly repressed at the mRNA level (Fig. 1G, P < 0.05) and the protein level (Fig. 1H, P < 0.05; representative image shown in Fig. 1H, Inset). Taken together, the repression of OGA and the enhanced OGT levels give a possible explanation for the increase in global O-GlcNAcylation 5 d after infarction. This increased level of O-GlcNAcylation was maintained throughout the 4-wk course of our study (Fig. S3A, P < 0.05; representative image shown in Fig. S3B). As the elevation in O-GlcNAcylation appeared to be a result of increased levels of OGT protein, we focused our efforts on determining the role that OGT plays in the failing heart by creating a cardiomyocyte-specific OGT KO.
Fig. 1.
Cardiac O-GlcNAcylation changes following 5 d of coronary artery ligation. Hearts from the same animals as in Figs. S1 and S2 were used to determine the changes in cardiac O-GlcNAcylation. (A) Coronary ligation for 5 d produced significantly elevated O-GlcNAcylation according to immunoblot (representative image shown in B). (C) Secondarily, a nonantibody method (Click-iT method) also revealed a similar significant increase (representative image shown in D). (E) OGT mRNA levels were unchanged in the failing heart. (F) OGT protein was significantly elevated in the failing heart (representative image shown in Inset). (G) OGA mRNA levels were significantly decreased in the failing heart. (H) OGA protein levels were reduced (representative image shown in Inset); *P < 0.05 versus WT sham.
Cardiomyocyte-Specific Deletion of OGT.
To study the role that O-GlcNAcylation plays in heart failure, an inducible, cardiomyocyte-specific KO of OGT was made. Homozygous OGT floxed (14) mice were crossed with an α-myosin heavy chain-driven mutated estrogen receptor flanked cre recombinase Mer-Cre-Mer (MCM) transgenic mice (15). Mice were injected with tamoxifen (20 mg/kg/d) for 5 d, followed by a 5-d “washout” period. Tissues were harvested and snap-frozen until needed for analysis or saved for immunohistochemical stains. In cardiomyocyte-specific OGT KO (cmOGT KO) mouse hearts, OGT mRNA levels (Fig. 2A), OGT protein levels (Fig. 2B), and levels of O-GlcNAcylated proteins (Fig. 2C) were all significantly reduced (P < 0.05) compared with WT mice. Cardiac restriction of the transgene was confirmed when no changes in OGT levels were noted in the skeletal muscle (Fig. 2B) or lungs (Fig. 2B). This cardiomyocyte-specific loss of OGT was further confirmed when isolated cardiomyocytes were subjected to qRT-PCR for OGT mRNA (P < 0.05; Fig. S4A). This can clearly be seen in the representative immunofluorescent sections stained for OGT (Fig. 2D) or for O-GlcNAcylated proteins (Fig. 2E). Although loss of OGT (green, Fig. 2D) or O-GlcNAcylated protein (red, Fig. 2E) signal is evident in the vast majority of myocytes, a mosaicism does exist at the same extent as seen in the isolated myocyte data (Fig. S4A). The opposing enzyme to OGT, OGA, was also studied in the cmOGTKO heart. Following OGT ablation, OGA mRNA levels were also significantly reduced (P < 0.05; Fig. S4B), although no change in OGA protein was witnessed (Fig. S4C). We also determined the mRNA levels of several glycolytic enzymes in the cmOGTKO group. HEK1, HEK2, and PFK-1 were unchanged whereas GAPDH was significantly (P < 0.05) elevated (Fig. S4D). The levels of the rate-limiting enzymes GFAT1 and GFAT2 were also unchanged (Fig. S4E). Finally a histological assessment was performed on the cmOGT KO mice, with no sign of increased hypertrophy, apoptosis, or collagen accumulation (Fig. S5 A, C, and E; representative images shown in Fig. S5 B, C, and F, respectively).
Fig. 2.
Cardiac-specific deletion of OGT. To determine the efficacy of targeted OGT deletion, MCM Tg mice were bred onto a homozygous OGT floxed mouse line. All mice in Figs. 2–4 are homozygous OGT floxed mice. The cmOGT KO or WT designations refer only to the presence or absence of the MCM transgene. Following induction of the transgene via tamoxifen injections, OGT mRNA levels (A), OGT protein levels (B), and levels of O-GlcNAcylated proteins (C) were significantly reduced in the cmOGT KO hearts. To confirm tissue specificity of OGT ablation, skeletal muscle and lungs were removed and subjected to immunoblot for OGT; no change was observed (B). Cardiac myocyte specific ablation of OGT was confirmed via immunofluorescence. Using antibodies specific for OGT and cardiac troponin, a mosaic loss of green (OGT) signal occurs in the cmOGT KO myocardium compared with the WT, in which the signal remains largely uniform throughout the myocardium (D). In addition, cmOGT KO hearts were stained for O-GlcNAcylated proteins and a similar mosaic pattern emerged (E); *P < 0.05 versus WT.
Cardiac-Specific Deletion of OGT Does Not Cause Cardiac Dysfunction.
To determine the effects of OGT ablation in naive animals over a longer time frame, WT and cmOGT KO mice were injected with tamoxifen, subjected to sham surgery, and followed for 4 wk via echocardiography. The cardiac ablation of OGT, and reduction in total O-GlcNAcylation, persisted through 4 wk as determined by click chemistry (Fig. S6A, P < 0.05; representative image shown in Fig. S6B). Surprisingly, the OGT-deficient mice showed no significant changes in cardiac function as measured by left ventricular inner diameter in diastole (Fig. S6C) or systole (Fig. S6D), nor was there any increase in cardiac hypertrophy (Fig. S6E; representative image shown in Fig. S6F), cardiac apoptosis (Fig. S6G; representative image shown in Fig. S6H), or fibrosis (Fig. S6I; representative image shown in Fig. S6J). These findings were surprising, as standard whole-body KO of OGT is embryonic-lethal (13). These results indicate that a reduction of cardiac OGT and reduced O-GlcNAcylation do not affect cardiac function in the otherwise unstressed heart.
Cardiac-Specific Deletion of OGT Exacerbates Heart Failure.
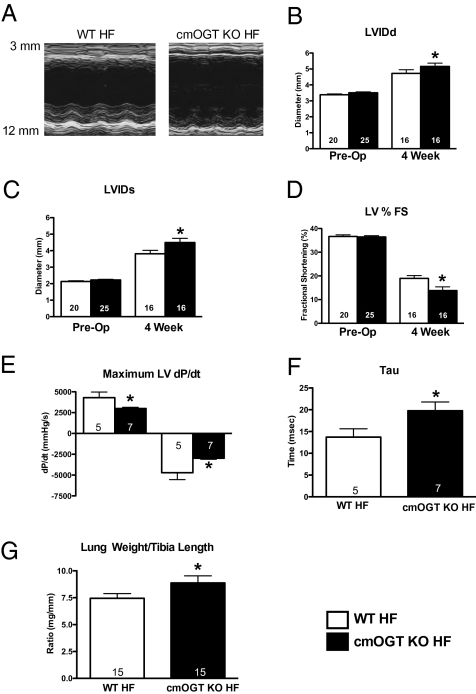
To determine the role that OGT ablation plays in heart failure, WT and cmOGT KO mice were injected with tamoxifen, subjected to infarction, and harvested at 24 h for infarct determination or followed for 4 wk using echocardiography. Cardiac-specific ablation of OGT had no affect on infarct size at 24 h as determined by Evans blue and triphenyl tetrazolium chloride staining (Fig. S7A; representative images shown in Fig. S7B and Fig. S7C). Representative M-mode echocardiographs are shown in Fig. 3A. Over the course of the study, cmOGT KO mice suffered exaggerated left ventricular dilation in diastole (P < 0.05; Fig. 3B) and systole (P < 0.05; Fig. 3C). Fractional shortening was worse in the cmOGT KO mice compared with WT mice (P < 0.05; Fig. 3D). Along with these changes in left ventricular geometry, there was a significant decline in left ventricular function. The left ventricular dP/dt, an index of contractility, was markedly (P < 0.05) reduced in the cmOGT KO group (Fig. 3E), whereas an elevation in tau (P < 0.05; Fig. 3F) provided evidence of impaired relaxation. Failed cmOGT KO mice also suffered pulmonary edema, indicated by an elevated lung weight-to-tibia length ratio (P < 0.05; Fig. 3G). Although cmOGT ablation exacerbated postinfarct ventricular dysfunction and more of the cmOGT KO mice died during the 4-wk protocol, there was no significant difference in survival; 80% of WT mice survived and 64% of cmOGT KO mice were alive after 4 wk. Here, we have shown that, although no baseline abnormalities exist, OGT ablation significantly reduces the heart's compensatory capacity during infarct-induced heart failure.
Fig. 3.
OGT ablation exacerbates heart failure. Following cardiac ablation of OGT, mice were subjected to permanent coronary ligation and followed for 4 wk via echocardiography, with representative M-modes shown (A). cmOGT KO mice subjected to coronary ligation showed a significant increase in diastolic (B) and systolic (C) diameters and a significant reduction in fractional shortening by 4 wk (D). Left ventricular dP/dt, an index of cardiac function, was also reduced in the cmOGT KO HF group (E), whereas an elevation in tau provides evidence of impaired relaxation (F). Finally, an increased lung weight-to-tibia length ratio (G) suggests pulmonary edema caused by a slightly elevated LVEDP; *P < 0.05 versus WT HF.
OGT Ablation Exacerbates Postinfarct Remodeling.
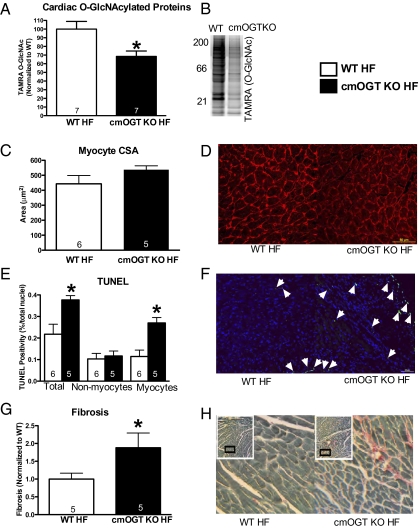
To provide an explanation for the exacerbated dysfunction in the cmOGT KO heart failure (HF) group, we first determined if the reduction in O-GlcNAcylation was maintained throughout the course of the study. Using click chemistry, levels of O-GlcNAcylation were significantly lower in the cmOGT KO HF group, proving that ablation of OGT is persistent up to 4 wk (P < 0.05; Fig. 4A; representative image shown in Fig. 4B). We also determined that the reduction of O-GlcNAcylated proteins remained reduced compared with WT shams at 4 wk (Fig. S8A, P < 0.05; representative image shown in Fig. S8B). Next we began to assess the pathological remodeling occurring within the surviving myocardium. Although there was no difference in myocyte hypertrophy according to wheat germ agglutinin staining (Fig. 4C; representative image shown in Fig. 4D), there was a significant (P < 0.05) increase in the total number of TUNEL-positive nuclei (Fig. 4E; representative image shown in Fig. 4F) in the remote, noninfarcted myocardium. This increased rate of apoptosis was detected in only the myocytes (based on morphology) and there was no apparent difference in the nonmyocytes. Potentially related to the higher rate of myocyte apoptosis, fibrosis (assessed by fast green/Sirius red staining) was elevated in the remote, noninfarcted myocardium (P < 0.05; Fig. 4G; representative image shown in Fig. 4H).
Fig. 4.
OGT ablation increases pathological remodeling of the left ventricle. Following 4 wk of coronary ligation, hearts were removed and subjected to biochemical and histological assessments. (A) Reduction in O-GlcNAcylation persisted in the cmOGT KO HF group as determined by Click-it; a representative image is shown in B. Ablation of OGT had no effect on cardiomyocyte size (C) in the failing heart; representative image shown in D. There was an increase in the total number of TUNEL-positive nuclei, and more specifically the number of TUNEL-positive myocytes, in the cmOGT KO group compared with WT (E). A representative image is shown (F) with arrowheads denoting positive nuclei. Following this elevated rate of apoptosis, total fibrotic area was also exacerbated in the cmOGT KO failing hearts (G); representative image is also shown (H). Both TUNEL and fibrotic measurements were made in a section remote from the infarct; *P < 0.05 versus WT HF.
OGT Deletion Exacerbated PGC-1α Suppression Early After Myocardial Infarction.
To provide a possible link between OGT and a mechanism of heart failure, additional WT and cmOGT KO mice were injected with tamoxifen and then subjected to infarction. Tissues were harvested at 5 d after surgery. This time point was chosen because it immediately precedes the separation in functional parameters, and is the same time point shown in Fig. S2A, which indicates suppression in PGC-dependent transcriptional activity in WT failing versus sham-treated hearts. Five d after infarction, there was an increase in HEK1 mRNA levels (P < 0.05; Fig. S2A), which correlates with previous reports of increased glycolysis. This increase was not exacerbated when cmOGT KO mice were subjected to 5 d of coronary ligation. Likewise, no other glycolytic enzymes examined changed (Fig. S9A). The rate limiting enzyme in the HBP, GFAT, was unchanged between the two ligated groups. OGA mRNA levels, however, were decreased in these same animals (P < 0.05), and protein expression of OGA remained unchanged. Surprisingly, PGC-1α and -β were further suppressed in the cmOGT KO HF hearts compared with WT HF hearts (P < 0.05; Fig. S9A). Such reduction is even more impressive considering that, 5 d after ligation, PGC-1α is reduced compared with sham (Fig. S2A). Associated with suppression of PGC-1α, dependent transcripts, such as CPT-1, CPT-2, MCAD, ATP-5O, COXIV-5B (P < 0.05; Fig. S9A), GLUT1, and GLUT4 (P < 0.05; Fig. S9A), were also reduced.
To determine if this apparent metabolic derangement persisted, WT and cmOGTKO mice were subjected to 4 wk of coronary ligation and harvested for qRT-PCR analysis. Although no significant differences were detected, the data did trend toward maintaining the metabolic phenotype of PGC-1 repression (Fig. S9B). These findings imply a veritable metabolic collapse in the absence of OGT in the postinfarct heart. Although it is possible that such changes in PGC transcript are related to direct modulation of PGC transcription by O-GlcNAcylation, we cannot rule out the likely possibility that such changes simply reflect the exacerbation of heart failure in the cmOGT KO mice.
Discussion
The mechanical demands of the failing heart outpace the endogenous metabolic capacity. Following myocardial infarction, a large akinetic area develops in the ventricular wall, which places undue wall stress on the remote, albeit surviving, myocardium. The heart continues to dilate, thereby perpetuating wall stress on the surviving and hypertrophying myocardium. Thus, understanding homeostatic metabolic regulation and elucidating the pathologic changes operative during heart failure represent essential approaches to developing new and more effective therapies for the failing heart. Accordingly, we were intrigued by the potential interaction between a unique glucose-derived posttranslational modification (via O-GlcNAc) and the failing myocardium. Because of the varied indirect metabolic contributions to the O-GlcNAc modification, it should not necessarily be thought of as a simple glycolytic readout. Indeed, emerging evidence supports a much more complicated role for O-GlcNAcylation in primary cells and intact tissue.
OGT has been shown to be required for cell division and embryogenesis (13). We initially hypothesized that cardiomyocyte ablation of OGT would induce heart failure and were surprised that the mice thrived during the 4-wk course of the study. Although it does not appear to be necessary for normal cardiac function, as measured in anesthetized animals, OGT is certainly required as part of the endogenous compensatory response to infarct-induced heart failure. Nevertheless, it is possible that chronic (e.g., several months) deficiency of OGT may create a phenotype in the absence of infarction.
Again, these data clearly demonstrate that OGT expression is necessary for at least some of the multifarious compensatory changes during heart failure. This leads to the proverbial question regarding mechanism. The exaggerated remodeling and apoptosis in the post-myocardial infarction cmOGT KO mice are prognostic of poor outcomes during heart failure. It is important to realize that such changes occurred in the remote, surviving myocardium and were not directly related to differences in infarct size because the infarct size soon after surgery was not different between the two groups. This opens up new areas of investigation and leads one to ponder whether O-GlcNAylation represents a novel, important, and direct regulator of fibrotic changes in the failing heart, or whether such changes merely reflected enhanced apoptosis. These histologic changes did not occur in isolation, as cardiac dysfunction was also exacerbated in the failing cmOGT KO hearts. Thus, relevant physiological changes corroborate our biochemical and pathological readouts.
One unanswered question is what is responsible for the augmentation of O-GlcNAcylation in the failing heart. Here, we show OGT protein expression appeared elevated in the failing heart, but this did not seem to result from a concomitant elevation in OGT mRNA levels. At the same time, the opposing enzyme (OGA) was down-regulated at the mRNA and protein levels. In addition, GFAT1 mRNA was up-regulated in the failing WT heart. One possible explanation of the elevation in OGT protein might relate to relative suppression of the ubiquitin–proteasome system (UPS). During heart failure, UPS activity is reduced (16), and such reduction could allow an apparent increase in protein half-life via reduced degradation. Other studies of O-GlcNAcylation have indicated that O-GlcNAc modification could directly limit UPS activity (17). Whether O-GlcNAcylation mediated restriction of UPS occurs in failing hearts is unknown, but such events are possible and worthy of exploration.
Finally, we evaluated UDP-HexNAc levels to determine a potential change in the levels of the sugar donor (UDP-GlcNAc) for the O-GlcNAc modification. The measurement of UDP-HexNAc assumes a consistent ratio of UDP-GlcNAc to UDP-GalNAc, in which UDP-GlcNAc would predominate by as much as 4:1. The HPLC technique we used cannot make a differentiation between the two hexosamines. Moreover, measuring the “snapshot” levels of UDP-GlcNAc (specifically) tells us nothing about flux per se. Ultimately, our data showing no difference in UDP-HexNAc levels do not exclude the possibility of enhanced HBP flux as a result of enhanced glucose uptake early in heart failure. This is an important, albeit technically demanding, issue that we are attempting to address in other studies by using a metabolomics approach.
This invokes a large issue related to metabolism in the failing heart. Some have compared metabolism in the failing heart to that in the diabetic heart. That is, there is an inability to properly use metabolic substrate, particularly fatty acids. Given the recent results from Dillmann and coworkers regarding the potential involvement of O-GlcNAcylation in diabetic cardiomyopathy (18), and our determination that O-GlcNAcylation was elevated early in the failing heart, we initially assumed such enhanced O-GlcNAcylation would be maladaptive and that ablation of OGT would at least partially rescue cardiac function during heart failure. Such hypotheses were clearly incorrect, likely reflecting the complexities of chronic metabolic regulation.
The present work provides seminal insights into a recently discovered regulator of cardiovascular function (9). The present data clearly and directly demonstrate that cardiac OGT expression is essential in the failing heart, although apparently dispensable for acute maintenance of cardiac function. Based on such a direct demonstration, future studies will be necessary to identify the specific targets that are O-GlcNAcylated in the failing myocardium and how such targets figure in the pathophysiology of heart failure. Interestingly, Murphy and coworkers (19) recently identified several contractile proteins that are O-GlcNAcylated in the myocyte. It would be interesting to identify what, if any, changes occurred at the contractile protein level in the failing heart. Such endeavors would provide valuable insight into the targets and mechanisms of O-GlcNAcylation in the failing heart. Clearly, our understanding of metabolism and metabolic regulation in the heart will continue to change, particularly in light of the important influence of O-GlcNAcylation in the cardiovascular system.
Materials and Methods
In Vivo Heart Failure Studies.
Adult (3–4 mo old) mice were subjected to in vivo coronary ligation to induce heart failure, as described previously (20–22). Using sterile technique, mice were subjected to a thoracotomy and the left coronary artery visualized and permanently occluded with the aid of a dissecting microscope. Upon recovery of spontaneous respiration, the intubation tube was removed and mice were allowed to recover in a temperature-controlled area supplemented with 100% oxygen. All animal procedures were performed in accordance with National Institutes of Health guidelines and approved by the University of Louisville Animal Care and Use Committee.
Echocardiographic Assessment of Cardiac Function.
Transthoracic echocardiography of the left ventricle using a 15-MHz linear array transducer (15L8) interfaced with a Sequoia C512 system (Acuson) was performed as previously described (20–22). At the indicated times, mice were anesthetized with 2% isoflurane, maintained under anesthesia with 1.25% isoflurane, and examined. Ventricular parameters were measured as recommended by the American Society of Echocardiography (23). All data were calculated from 10 independent cardiac cycles per experiment.
Invasive Hemodynamic Measurement.
Mice were anesthetized and intubated and a 1.0-F Millar catheter was inserted into the right carotid artery and advanced to the left ventricle. Measurements were taken at 1 kHz using the MPVS 400 system (Millar Instruments). Data were analyzed using PVAN software (Millar Instruments).
Protein Isolation, Immunoblotting, Histology, and Immunofluorescence.
Protein isolation, immunoblotting, histology, and immunofluorescence techniques were performed using standard protocols.
RT-PCR and Quantitative Real-Time PCR.
Total RNA from snap-frozen noninfarcted left ventricular sections or isolated cardiomyocytes was extracted with TRIzol (Invitrogen). Total RNA levels were quantified using the ratio of absorbance at 260–280 nm (A 260:A 280 ratio) with the NanoDrop 1000 Spectrophotometer (Thermo Scientific). To verify purity of the sample, the absorbance ratio from 260 to 230 nm (A260:A230) was used. We limited the use of RNA with 260:230 ratio >1.8. Total RNA (1 μg) then was subjected to reverse transcriptase (20 μL final volume) reaction for 30 min to synthesize cDNA using an IScript cDNA synthesis kit (BioRad). The relative level of mRNA transcripts was quantified by real-time PCR using SYBR Green (BioRad). The data generated was normalized to 18S ribosomal RNA or β-actin threshold cycle (CT) values by using the ΔΔCT comparative method (24). The sequences for all primers used can be found in Table S1.
Enzymatic Labeling of O-GlcNAc–Modified Proteins.
O-GlcNAcylated proteins were labeled by using a Click-iT enzymatic labeling kit (Invitrogen) according to the manufacturer's instructions. The dried-labeled protein sample was resuspended in SDS/PAGE buffer for electrophoresis.
Measurement of UDP-GlcNAc.
Measurement of UDP-GlcNAc in mouse heart tissues was determined with published methods (25, 26). The content of UDP-GlcNAc was quantified by UV detection (A254) after calibration with UDP-GlcNAc standard.
Isolation of Cardiomyocytes from Adult Hearts.
Cardiomyocytes from adult mouse hearts were isolated according to the Alliance for Cellular Signaling procedure protocol PP00000125 with modification. Briefly, after anesthesia with isoflurane, hearts were quickly removed and perfused through the aorta with perfusion buffer containing (in mmol/L): 135 NaCl, 4.0 KCl, 1.0 MgCl2, 0.33 NaH2PO4, 10 Hepes, 10 glucose, and 0.1% 2,3-butanedione monoxime, pH 6.95. Then, the heart was perfused again with perfusion buffer supplemented with collagenase II for 11–20 min. The left ventricle was removed after perfusion and teased into small pieces with fine forceps in stopping buffer (perfusion buffer with 10% bovine calf serum and 12.5 μmol/L calcium added to stop digestion). The cell suspension was transferred to a 15-mL conical tube. The heart tissues were further dissociated using plastic transfer pipettes with different sized openings. Myocytes were allowed to sediment by gravity for 8–10 min. After removal of the supernatant, the pellet was carefully suspended in stopping buffer. Calcium was reintroduced into cells by the addition of 10, 20, 30, 40, and 100 μL of 30 mmol/L-calcium to reach a final calcium concentration of 1.25 mmol/L.
Statistical Analysis.
Where appropriate, a two-tailed Student t test was performed for group comparisons. Survival was determined by log-rank test of a Kaplan–Meier survival curve. P < 0.05 was assumed to be significant in all cases.
Supplementary Material
Acknowledgments
We thank Allison Aird and Linda Harrison for expert technical assistance during the course of these studies. We also would like to thank Dr. Natasha Zachara (Johns Hopkins University) for providing the OGA antibody. This study was supported by National Institutes of Health Grants HL083320, HL094419, RR024489 (to S.P.J.), HL078825, HL099014, RR024489 (to S.D.P.), and R01 HL065660 (to Y.-T.X.); American Heart Association (AHA) National Center Scientist Development Grant 0535270N (to S.P.J.); Kentucky Science and Engineering Foundation Grant KSEF-1677-RDE-011 (to S.P.J.); a Veterans Administration Merit Award (to S.D.P.); AHA Predoctoral Fellowships (Great Rivers Affiliate) 0815502D (to L.J.W.) and 0715493B (to G.A.N.); and AHA Postdoctoral Fellowship (Great Rivers Affiliate) 08245643D (to H.T.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.W.H. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1001907107/-/DCSupplemental.
References
- 1.Razeghi P, et al. Metabolic gene expression in fetal and failing human heart. Circulation. 2001;104:2923–2931. doi: 10.1161/hc4901.100526. [DOI] [PubMed] [Google Scholar]
- 2.Garnier A, et al. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arany Z, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Cha SH, Rodgers JT, Puigserver P, Chohnan S, Lane MD. Hypothalamic malonyl-CoA triggers mitochondrial biogenesis and oxidative gene expression in skeletal muscle: Role of PGC-1alpha. Proc Natl Acad Sci USA. 2006;103:15410–15415. doi: 10.1073/pnas.0607334103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arany Z, et al. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao R, et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 7.Allard MF, Schönekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–H750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 8.Yang X, et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 9.Ngoh GA, Jones SP. New insights into metabolic signaling and cell survival: the role of beta-O-linkage of N-acetylglucosamine. J Pharmacol Exp Ther. 2008;327:602–609. doi: 10.1124/jpet.108.143263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 12.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: Signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 13.Shafi R, et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA. 2000;97:5735–5739. doi: 10.1073/pnas.100471497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Donnell N, Zachara NE, Hart GW, Marth JD. Ogt-dependent X-chromosome-linked protein glycosylation is a requisite modification in somatic cell function and embryo viability. Mol Cell Biol. 2004;24:1680–1690. doi: 10.1128/MCB.24.4.1680-1690.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohal DS, et al. Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein. Circ Res. 2001;89:20–25. doi: 10.1161/hh1301.092687. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–1328. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, et al. O-GlcNAc modification is an endogenous inhibitor of the proteasome. Cell. 2003;115:715–725. doi: 10.1016/s0092-8674(03)00974-7. [DOI] [PubMed] [Google Scholar]
- 18.Clark RJ, et al. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–44237. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez-Correa GA, et al. O-linked GlcNAc modification of cardiac myofilament proteins. A novel regulator of myocardial contractile function. Circ Res. 2008;103:1354–1358. doi: 10.1161/CIRCRESAHA.108.184978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer JJ, et al. Low-dose simvastatin improves survival and ventricular function via eNOS in congestive heart failure. Am J Physiol Heart Circ Physiol. 2006;291:H2743–H2751. doi: 10.1152/ajpheart.00347.2006. [DOI] [PubMed] [Google Scholar]
- 21.Jones SP, et al. Deficiency of iNOS does not attenuate severe congestive heart failure in mice. Am J Physiol Heart Circ Physiol. 2005;288:H365–H370. doi: 10.1152/ajpheart.00245.2004. [DOI] [PubMed] [Google Scholar]
- 22.Jones SP, et al. Endothelial nitric oxide synthase overexpression attenuates congestive heart failure in mice. Proc Natl Acad Sci USA. 2003;100:4891–4896. doi: 10.1073/pnas.0837428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiller NB, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Wice BM, et al. The intracellular accumulation of UDP-N-acetylhexosamines is concomitant with the inability of human colon cancer cells to differentiate. J Biol Chem. 1985;260:139–146. [PubMed] [Google Scholar]
- 26.Robinson KA, Weinstein ML, Lindenmayer GE, Buse MG. Effects of diabetes and hyperglycemia on the hexosamine synthesis pathway in rat muscle and liver. Diabetes. 1995;44:1438–1446. doi: 10.2337/diab.44.12.1438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.