Abstract
The endoplasmic reticulum (ER) stress response detects malfunctions in cellular physiology, and microbial pattern recognition receptors recognize external threats posed by infectious agents. This study has investigated whether proinflammatory cytokine expression by monocyte-derived dendritic cells is affected by the induction of ER stress. Activation of ER stress, in combination with Toll-like receptor (TLR) agonists, markedly enhanced expression of mRNA of the unique p19 subunit of IL-23, and also significantly augmented secretion of IL-23 protein. These effects were not seen for IL-12 secretion. The IL-23 gene was found to be a target of the ER stress-induced transcription factor C/EBP homologous protein (CHOP), which exhibited enhanced binding in the context of both ER stress and TLR stimulation. Knockdown of CHOP in U937 cells significantly reduced the synergistic effects of TLR and ER stress on IL-23p19 expression, but did not affect expression of other LPS-responsive genes. The integration of ER stress signals and the requirement for CHOP in the induction of IL-23 responses was also investigated in a physiological setting: infection of myeloid cells with Chlamydia trachomatis resulted in the expression of CHOP mRNA and induced the binding of CHOP to the IL-23 promoter. Furthermore, knockdown of CHOP significantly reduced the expression of IL-23 in response to this intracellular bacterium. Therefore, the effects of pathogens and other environmental factors on ER stress can profoundly affect the nature of innate and adaptive immune responses.
The mammalian innate immune system detects external threats by identifying components of microorganisms, using pattern recognition receptors (PRR) expressed on macrophages and dendritic cells (DC). Following PRR engagement, DCs link the innate and adaptive immune responses by producing cytokines that modulate T-cell responses.
Threats to the internal homeostasis of the cell can be detected within the endoplasmic reticulum (ER) and the unfolded protein response (UPR) is triggered when there is an imbalance between protein synthesis and the folding capacity of the ER (e.g., when protein secretion is rapidly up-regulated) (1). Three sentinel ER proteins, ATF6, PERK, and IRE-1, initiate independent signaling pathways that elicit the UPR; these pathways have both unique and shared gene targets.
PERK activation leads to decreased efficiency of protein translation via phosphorylation of eIF2α, but this translation “shut down” also potentiates ATF4 gene expression, resulting in activation of downstream ER stress-response pathways, including expression of C/EBP homologous protein (CHOP) and growth-arrest and DNA damage inducible protein GADD34. If homeostatic processes fail to resolve ER stress, CHOP activates apoptosis (2), whereas GADD34 dephosphorylates eIF2α to allow resumption of protein synthesis and survival (3). Activated IRE-1 acquires RNase activity and splices XBP-1 mRNA, which is then translated into a transcription factor that activates several UPR response genes (4). The UPR also activates “alarm” genes, including NF-κB (5).
In the immune system, an intact UPR is essential for development of plasma cells and classic DCs, but constitutive activation of IRE-1 has been demonstrated in plasmacytoid DCs (4, 6). ER stress signals are integral to the proinflammatory response to LPS in mice, and play a role in septic shock (7), but ER stress and the UPR have been implicated in human autoimmune or inflammatory diseases, such as myositis, type I diabetes, and inflammatory bowel disease (8–10).
Given the pivotal role of DC-produced IL-12 and IL-23 in controlling immune responses by T cells, we have examined the ability of ER stress to modify the production of these cytokines in response to PRR activation. We show that the ER stress-induced transcription factor, CHOP, plays an essential role in modulating IL-23, but not IL-12, production. Furthermore, we show that these signals can be provided in a physiological context because intracellular bacterial infection with Chlamydia trachomatis (CT), delivered both Toll-like receptor (TLR) and stress signals. Most importantly, CHOP expression induced by CT infection was shown to contribute toward optimal IL-23 responses.
Results
ER Stress Selectively Enhances Cytokine Gene Transcription and Secretion in Response to PRR Engagement.
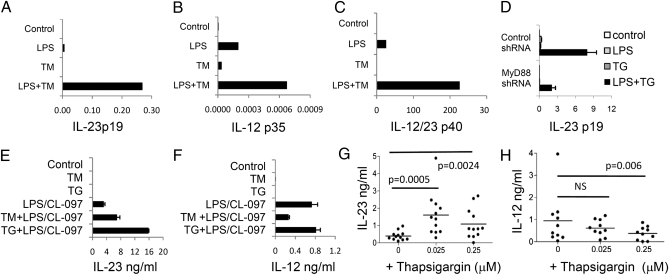
The effect of UPR activation on expression of proinflammatory cytokines by immature monocyte-derived dendritic cells (mDC) stimulated with PRR agonists was determined. Cells were stimulated with TLR agonists with or without the UPR inducing agent tunicamycin (TM) and expression of IL-23p19, IL-12p35, and the common IL-12/23 p40 subunit was analyzed by qRT-PCR. LPS induced detectable IL-23p19 mRNA, but this was markedly increased by TM (typically 40- to 65-fold the LPS response); in contrast, expression of IL-12p35 (typically 3- to 4-fold the LPS response) and IL-12/23 p40 (typically 3- to 9-fold the LPS response) was not enhanced to the same degree by the combination of ER stress and TLR stimuli. TM alone had little effect on expression of either cytokine at this time point (Fig. 1 A–C). Thapsigargin (TG), which induces the UPR by an independent mechanism (inhibiting the function of the ER ATPase, SERCA2B), also enhanced IL-23p19 expression in response to LPS (Fig. S1A). These findings in mDCs were reproduced in the human monocyte cell line U937, differentiated with phorbol myristate acetate to acquire LPS responsiveness (11). In U937, knockdown of MyD88 expression significantly inhibited IL-23p19 expression in response to LPS + TG, suggesting that the synergistic response required conventional TLR signaling (Fig. 1D). ER stress acted in synergy with other PPR agonists, including curdlan (dectin-1), peptidoglycan (TLR2 and NOD), Poly I:C (TLR3), and CL-097 (TLR8) to enhance IL-23p19—but not IL-12p35 —expression (Fig. S1 B and C). The lack of IL-12p35 responses to curdlan and peptidoglycan confirm previous reports that these agonists are ineffective in stimulating IL-12p35 transcription.
Fig. 1.
Activation of the UPR enhances expression of IL-23p19 mRNA and IL-23 secretion induced by TLR agonists. The mDC cells were stimulated with LPS in the presence or absence of 1 μg/mL TM for 7 h (A–C). Shown are qRT-PCR analysis of mRNA transcripts of (A) IL-23p19, (B) IL-12p35, and (C) IL-12/IL-23p40. (D) U937 cells expressing a control or MyD88 shRNA were stimulated with LPS and TG and analyzed for the expression of IL-23p19 mRNA. All mRNA values were normalized to the expression of HPRT mRNA and are representative of three independent experiments. (E–H) The mDCs were stimulated with agonists for TLR4 (ultrapure LPS) and TLR8 (CL-097) in the presence of or absence of TM or TG for 48 h and cell culture supernatants analyzed by ELISA for (E) IL-23 or (F) IL-12p70. The mDCs derived from 12 healthy donors (G and H) were stimulated with TLR4 and TLR8 agonists plus or minus TG at the indicated concentrations. Data (E and F) are expressed as the mean concentrations (with 95% confidence intervals) of cytokines secreted by replicate wells from the same donor, and G and H show median IL-12 and IL-23 concentrations.
Because ER stress can induce apoptosis and decrease mRNA translation through activation of PERK and eIF2α phosphorylation, it was critical to determine whether enhanced IL-23p19 transcription was mirrored by an increased IL-23 secretion. Because ultrapure LPS does not consistently induce detectable quantities of IL-12 and IL-23 from human mDCs (12), we used combinations of TLR agonists known to robustly induce the secretion of these cytokines. As shown in Fig. 1 E to H, treatment with TG or TM resulted in significant increases in the amounts of IL-23 secreted by mDCs in response to optimal combinations of TLR ligands (e.g., LPS + CL-097), but did not enhance IL-12 secretion irrespective of the TLR agonists used (Fig. 1E and Fig. S2). It has previously been shown that N-glycosylation of the IL-12 signal peptide is required for IL-12 secretion (13); therefore, we used TG rather than TM to compare the effect of ER stress on IL-12 and IL-23 secretion induced by TLR stimulation. Using mDCs derived from 12 healthy human donors, TG significantly enhanced IL-23 secretion in response to TLR agonists (Fig. 1 G and H). The higher concentration of TG tested was less effective than the lower in enhancing IL-23 secretion, consistent with TG causing some global translational inhibition by inducing the UPR. In contrast, IL-12 secretion was not enhanced by TG.
ER Stress Signaling Enhances only a Subset of Chemokine and Cytokine Genes.
To determine whether ER stress modulated the expression of other immune-related genes, we analyzed the global gene expression of mDCs stimulated by TLR4 and TLR8 agonists (LPS + CL097) in the presence or absence of TG at a concentration that consistently enhanced the secretion of IL-23 (0.025 μM). Data on a panel of cytokine and chemokine genes revealed that expression of only a small subset of these genes (IL-23p19, TNFα, thymic stromal lymphopoietin, osteoprotegerin) was significantly enhanced by ER stress signaling. All other genes were unchanged or reduced by ER stress induced by this concentration of TP (Fig. S3).
Inhibition of the ER Stress Signaling Through IRE-1 Inhibits Expression of LPS Responsive Genes.
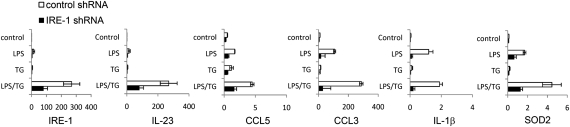
To investigate whether the effects of TG on IL-23p19 transcription were a result of induction of ER stress signaling, we analyzed IL-23p19 mRNA in U937 cells expressing silencing shRNAs specific for IRE-1. Significant and specific knockdown of this gene was achieved and resulted in marked inhibition of expression of IL-23p19 in response to LPS + TG (Fig. 2). Knockdown of IRE-1 mRNA also significantly reduced expression of IL-23p19 in response to LPS alone. However, other LPS responsive genes, CCL-3, CCL-5, CXCL8, and SOD2—whose expression was not notably enhanced by TG—were affected by IRE-1 knockdown to the same extent as IL-23p19. Thus, the specific effect of ER stress induction on IL-23p19 transcription could not be explained by the effects of the IRE-1 pathway.
Fig. 2.
Knockdown of IRE-1 mRNA in U937 cells decreases cytokine mRNA expression in response to LPS with or without TG. U937 cells transduced with shRNAs specific for IRE-1 or a nonsilencing control shRN, were stimulated with LPS and TG alone or in combination for 7 h. Gene expression was analyzed as described in Fig. 1. Data are expressed as means and 95% confidence intervals derived from at least three independently derived U937 lines transduced with lentiviral particles.
ER Stress-Induced Transcription Factor, CHOP, Binds the IL-23p19 Promoter.
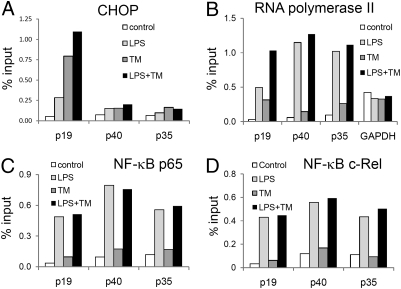
To further elucidate the specific effect of ER stress on IL-23p19 transcription, we examined the IL-23p19 promoter region for potential binding sites for ER stress-induced transcription factors, using University of California Santa Cruz Genome browser (http://genome.ucsc.edu/) (14), and identified a potential binding site for CHOP, (coordinates chr12:55017953–55017965, Human genome assembly version hg18), which is predicted to bind to this region as a heterodimer with C/EBPα. To determine whether CHOP bound the IL-23p19 promoter following ER stress or TLR stimulation, we performed ChIP assays using a CHOP-specific monoclonal antibody, and analyzed the presence of IL-23p19 promoter using real-time PCR with oligos spanning the putative CHOP binding site. A significant enrichment of the IL-23p19 promoter in mDCs stimulated with LPS or TM was detected, and the greatest enrichment was observed when these stimuli were combined (Fig. 3A). Although we detected no putative CHOP binding sites in the promoters of IL-12p40 or IL-12p35, we performed PCR using oligos spanning regions proximal to the previously reported NF-κB binding site in the IL-12p40 gene, or the GAPDH promoter region proximal to the RNA polymerase II binding site. These analyses were to control for possible nonspecific enrichment of DNA by using anti-CHOP antibodies, but we did not detect any consistent enrichment of IL-12p40 or GAPDH promoters in responses to LPS and TM. Specific enrichment of the IL-23p19 promoter was also seen with antibodies to RNA polymerase II in response to LPS + TM, further confirming that ER stress enhanced IL-23p19 transcription (Fig. 3B). Identical ChIP results were obtained in U937 cells using LPS and TG (Fig. S4A).
Fig. 3.
ER stress enhances binding of CHOP, but not NF-κB p65 or c-Rel, to the IL-23p19 promoter. The mDCs were stimulated with LPS (1 μg/mL), TM (1 μg/mL), or LPS plus TM for 5 h. Data show abundance of specified promoters following ChIP using antibodies specific for (A) CHOP, (B) RNA polymerase II, (C) NF-κB p65, and (D) NF-κB c-Rel. Data are expressed as a percentage of the quantity of same promoter DNA in the nuclear lysate before immunoprecipitation (% input). The quantity of promoter in the immunoprecipitate was determined by real-time qPCR using oligonucleotide primers specific for the IL-23p19 (p19), IL-12p35 (p35), IL-12/23 p40 (p40), and GAPDH promoters. The data are representative of two independent experiments.
NF-κB binding to the murine IL-23p19 promoter has been previously described (15) and we confirmed this using ChIP with anti-p65 and anti-c-Rel antibodies. NF-κB p65 and c-Rel binding was significantly enhanced by LPS, but was not induced or enhanced by ER stress (Fig. 3 C and D). Identical effects on NF-κB p65 and c-Rel binding to the IL-23p19 promoter were observed using U937 cells (Fig. S4 B and C). Addition of isohelenin, which specifically prevents NF-κB activation by inhibiting degradation of Iκ-Bα, abrogated IL-23p19 expression in response to either LPS alone or LPS plus TG (Fig. S4D). This finding suggests that although NF-κB binding is critical for IL-23p19 transcription, it is not the factor that mediates the effect of ER stress.
CHOP Expression Is Necessary for IL-23p19 Expression.
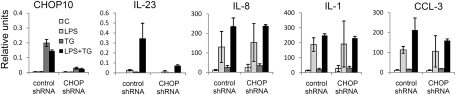
To determine whether CHOP is necessary for IL-23p19 gene expression, we analyzed IL-23p19 expression in U937 cells expressing CHOP-specific or control shRNAs. CHOP-specific shRNA expression significantly reduced the low level of CHOP mRNA in unstimulated U937 cells, and had very marked effects on up-regulation of CHOP following ER stress (Fig. 4). The synergistic stimulation of IL-23p19 gene expression by LPS and TG was also substantially reduced by CHOP knockdown, pointing to an important role for CHOP in IL-23 expression (Fig. 4). This effect was observed using three independent CHOP shRNA targets (Fig. S5). Furthermore, CHOP shRNA expression also substantially decreased the low level of IL-23p19 gene expression induced by LPS alone. In contrast to the global effects of IRE-1 knockdown on expression of LPS-responsive genes, knockdown of CHOP mRNA expression had no effect on mRNA levels of three other LPS-responsive genes, IL-1β, IL-8, and CCL3.
Fig. 4.
Knockdown of CHOP expression specifically reduces IL-23p19 expression. U937 cells transduced with shRNAs specific for CHOP, or a nonsilencing control, were stimulated with LPS (1 μg/mL), and TG (0.25 μM) for 7 h. Gene expression was analyzed by qRT-PCR for target genes as described in Fig. 1. The data show mean RNA expression and 95% confidence intervals derived from four independently derived U937 lines transduced with lentiviral particles.
CHOP Expression Is Induced by CT Infection.
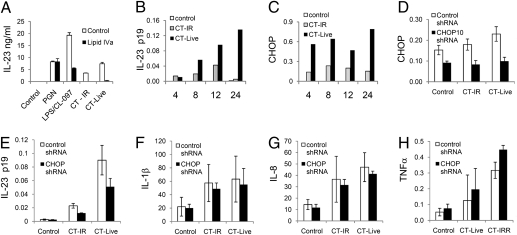
To determine the relevance of the synergy between ER stress and TLR signaling in a more physiologic setting, we examined whether intracellular bacterial infection by CT could induce the expression of CHOP, and whether this regulated IL-23p19 expression. CT infection of mDCs potently induced IL-23 secretion to a greater extent than addition of irradiated CT, and the inhibition of this response by Lipid IVa, an inhibitor of TLR4 activation in humans (16), suggests that TLR4 signaling is an important component of this response (Fig. 5A). CT infection induced both the expression of IL-23p19 and CHOP (Fig. 5 B and C); likewise, CT-infected U937 cells also express IL-23p19 (Fig. S6A). We therefore used U937 cells to determine whether CHOP was required for IL-23p19 transcription following CT infection. ChIP analysis revealed that CT infection induced CHOP binding to the IL-23p19 promoter along with NF-κB p65, c-Jun, and RNA polymerase II, the last being a general indicator of gene activation. In contrast, CT infection did not enhance RNA polymerase II binding to the GAPDH promoter (Fig. S6 C and D). Induction of CHOP expression in U937 in response to CT infection was inhibited by CHOP-specific shRNA, compared with control shRNA (Fig. 5D), and this also inhibited transcription of IL-23p19 in response to CT yet had no effect on transcription of IL-1β, IL-8, or TNFα. These data clearly demonstrate that CHOP expression is essential for IL-23p19 expression induced by CT infection.
Fig. 5.
CHOP expression is induced by CT infection and is required for optimal IL-23p19 expression. Either mDCs (A–C) or U937 cells expressing a control or CHOP shRNA (D–H) were infected with live (CT-live) or γ-irradiated (CT-IR) CT for 24 h. (A) The mDCs were infected with CT in the presence or absence of lipid IVa (1 μg/mL) and IL-23 secretion analyzed by ELISA. Then, mDCs were harvested over a 24-h time-course following no additional treatment or infection with live or γ-irradiated CT, and gene expression of (B) IL-23p19 and (C) CHOP was analyzed by qRT-PCR. The data are representative of three replicate experiments. U937 cells transduced with shRNAs specific for CHOP, or a nonsilencing control, were infected with CT for 24 h and examined for (D) CHOP, (E) IL-23p19, (F) IL-1β, (G) IL-8, and (H) TNFα. Data show mean mRNA expression and 95% confidence intervals derived from four independently derived U937 lines transduced with lentiviral particles.
Discussion
We have shown that ER stress combines with signals from TLRs and other PRRs to markedly enhance the induction of IL-23p19 expression and IL-23 secretion. In contrast, IL-12p35 expression was less affected by ER stress and IL-12 secretion not enhanced at all. The ability of ER stress induced by TG to enhance IL-23 secretion was recently described in murine and rat myeloid cells, but the mechanisms underlying this effect were not identified (17, 18). By investigating the signaling pathways known to be activated by ER stress, we initially showed that the IRE-1 pathway was used by all of the LPS responsive genes, but this included genes whose expression in response to LPS was not significantly enhanced by ER stress. This involvement of the IRE-1 pathway in the response to LPS is in agreement with recent data showing that XBP-1, a transcription factor dependent on IRE-1 activation, is required for optimal responsiveness of macrophages to TLR agonists (19).
Although we could not explain the sensitivity of IL-23p19 expression to ER stress on the basis of the involvement of IRE-1/XBP-1, the identification of a binding site in the IL-23p19 promoter for the ER stress-induced transcription factor, CHOP, provided a potential mechanism whereby IL-23 may be responsive to ER stress-induced transcription factors. This theory was supported by data that showed that knockdown of CHOP had a substantial effect on IL-23p19 expression in response to TG or TM combined with TLR agonists. Furthermore, CHOP knockdown did not affect other cytokine genes whose transcription was not enhanced by TG and TM. Interestingly, even the comparatively low induction of IL-23p19 expression by LPS alone was significantly inhibited by CHOP knockdown, suggesting that LPS alone engages the ER stress pathways leading to CHOP induction, albeit to a lesser extent than TG. The fact that LPS stimulation was sufficient to induce CHOP binding is not unexpected, as we and others have shown that TLR stimulation alone can activate the ER stress response (7, 20).
TLR agonists are known to activate NF-κB, and an inhibitor that blocks I-κBα degradation markedly reduced IL-23p19 expression, confirming that IL-23p19 expression is dependent on NF-κB activation. Expression of IL-23p19 is regulated at the transcriptional level by two members of the NF-κB family, p65 and c-Rel (15, 21, 22), but ER stress induced NF-κB p65 and c-Rel binding to the IL-23p19 promoter comparatively weakly, and no enhanced binding was detected when ER stress and TLR agonists were combined. Thus, although p65 and c-Rel are required for IL-23p19 expression, the specificity of the interaction between TG and TLR agonists for IL-23 production is not accounted for by an effect on these transcription factors. We suggest that collaboration between CHOP and NF-κB family members on the IL-23p19 promoter occurs to enhance IL-23p19 expression, and this hypothesis is supported by the finding that RNA polymerase II binding to IL-23p19 promoter is optimal in the presence of ER stress and TLR stimulation.
Two previous reports have described a role for CHOP in the regulation of cytokine expression. First, CHOP has been shown to regulate IL-6 transcription via regulation of an inhibitory isoform of NF-IL-6, rather than by binding to the IL-6 promoter (23). Second, CHOP binds the IL-8 promoter following prostaglandin stimulation of T cells (24). However, our results are unique in reporting ER stress inducing CHOP binding to a cytokine promoter, and thereby enhancing the effects of TLR stimulation.
Most importantly, we identified a physiological scenario where ER stress and TLR signals are delivered together to enhance cellular IL-23 expression. In addition to engaging TLR4, infection with the obligate intracellular bacterium, CT, also induced CHOP. Critically, CHOP expression was necessary for optimal induction of IL-23p19 expression by CT infection, suggesting that an interaction between PRR signaling and the ER stress response is a normal component of the response to intracellular bacteria, and that CHOP is a critical transcription factor in this process.
Why should the effects of ER stress influence innate immune responses mediated by PRR? McKenzie et al. have suggested that both extracellular microbial products and “danger” signals produced by stressed or damaged tissues, (e.g., PGE2, ATP, and urate), regulate the balance between production of IL-23 or IL-12 (25). Similarly, activation of the UPR may be an intracellular counterpart to the extracellular release of tissue-derived danger signals. Thus, viral infection of cells has been reported to induce the UPR (26), as does intracellular bacterial infection (i.e., CT), as previously noted. In addition to effects on DCs, CT infection strongly stimulates the expression of CHOP in monocytes and epithelial cells. By activating the UPR, the cell is able to sense that it harbors an infectious agent, which is also detected through PRRs, and that this agent may impose physiological stress as a result of its effect on ER homeostasis. Therefore, it would be teleologically appropriate to integrate signals from the UPR with those from PRR activation to obtain appropriate cytokine outputs. Another example of cross-talk between the UPR and PRR has recently been reported; TRIF-dependent TLR signaling has recently been shown to inhibit expression of CHOP (27). We have confirmed that LPS reduces CHOP expression induced by TG in U937 cells (Fig. 5A). At first sight these data appear paradoxical when considered together with our finding that CHOP is a critical transcription factor for the synergistic response to ER stress and TLR ligands. However, excessive amounts of CHOP would lead to apoptosis of the cell, and thence failure of appropriate cytokine responses, so it is not surprising that CHOP expression needs to be carefully regulated, even though it is an important transcription factor favoring IL-23 secretion.
Why should ER stress signals particularly favor IL-23 over IL-12 secretion? Again, McKenzie et al. (25) have suggested that IL-23 is an important driver of the early immune response to pathogens. In particular, it has the ability to rapidly induce IL-17 production from local T cells, thereby allowing early recruitment of neutrophils and an effective, immediate, innate immune response to pathogen invasion. At later stages of the immune response, the induction of Th1 cells via IL-12 production may be a more important mechanism for dealing with the threat posed by intracellular pathogens and effecting their clearance.
Although integration of ER stress signals and those from TLRs may be appropriate in response to infection, it may also be involved in pathologic inflammatory responses. ER stress has been implicated in a number of human disorders, including myositis and gut inflammation (8, 9). Whether the ability of ER stress signals to potentiate proinflammatory cytokine secretion contributes to the pathology of these diseases remains to be determined. In the HLA-B27 transgenic rat, an animal model that recapitulates many features of human spondyloarthritis, very high levels of HLA-B27 are expressed, with consequent misfolding of the B27 heavy chain in the ER. Cells taken from sites of inflammation in these animals can be shown to be mounting a UPR, and up-regulation of IL-23 production has also been shown (18, 28). The effect of unfolded HLA-B27 in this arthritis model may be exacerbated by mechanical factors, as tensional forces, such as those experienced by cells in joints and tendons, have been shown to be able to induce ER stress and CHOP expression in a PERK-dependent manner, at least when applied to fibroblasts (29). Arthritis in the B27-trangenic rat also requires the presence of gut bacteria, suggesting that engagement of PRR may play a part in pathogenesis when combined with the synergistic effect of CHOP activation on proinflammatory cytokine production. The effect of CHOP expression in this model cannot be addressed directly because of the inability to obtain gene-targeted rats. However, CHOP-null mice have been produced, but it has yet to be determined whether they are protected from inflammatory diseases, such as collagen-induced arthritis and experimental allergic encephalomyelitis, in which IL-23 is known to be a critical cytokine (30, 31).
In summary, our findings that ER stress modulates the responses to PRR in such a way as to favor production of certain proinflammatory cytokines, particularly IL-23, have important implications for our understanding of the pathogenesis of inflammatory diseases, such as arthritis, in which IL-23 is implicated.
Methods
Primary Cell Culture.
CD14+ monocytes were isolated from peripheral blood mononuclear cells of healthy volunteers by positive selection with CD14-specific microbeads (Miltenyi Biotech) and differentiated into mDCs with IL-4 and GM-CSF, as described previously (32).
Stimulation of Cells with PRR Agonists.
U937 cells were differentiated with 10 ng/mL phorbol 12-myristate 13-acetate (Sigma) for 24 h before stimulation with PRR agonists and TG. Monocytes and mDCs were cultured in 24-well plates (106 per well) for RNA analysis or in 0.2-mL U wells (10,000 per well) for analysis of IL-12 and IL-23 cytokine secretion by ELISA (all from Insight). PRR agonists were titrated and used at concentrations that gave optimal responses: PAM3CSK4, 1 μg/mL; CL-097, 5 μg/mL (Invitrogen), and Ac-muramyl-Ala-D-Glu-NH2, MDP, 10 μg/mL (Bachem). Peptidyoglycan (10 μg/mL) was derived from Bacillus subtillis and ultrapure LPS (1 μg/mL) from Escherichia coli, 026:B6 (both from Sigma). Stimulated cells were incubated for 8 h before RNA isolation, or for 48 h for analysis of cytokine secretion.
Real-Time qPCR.
Total RNA was extracted from cells using RNeasy columns (Qiagen) as described by the manufacturers. The expression of each test gene was normalized to the expression of HPRT mRNA and expressed as relative units to the expression of HPRT, or further manipulated to obtain a value expressed as fold-change compared with the unstimulated control cells. All qRT-PCR probe primer sets were obtained from Applied Biosystems and are listed in Table S1.
Lentivirus-Mediated Delivery of shRNA to U937 Cells.
The 293T packaging cells were cotransfected with the lentiviral vector pLK0.1 (nonsilencing control) or CHOP shRNA (TRCN0000007263, TRCN0000007264, and TRCN0000007267), MyD88 shRNA (TRCN0000011223), or IRE-1 (TRC0000000530) in conjunction with packaging plasmids pCMV8.91 and pMDG using TransIT (Cambridge Biosciences).
Microarray Analysis.
RNA was extracted from mDCs that were stimulated with LPS (1 μg/mL) and CL-097 (5 μg/mL) for 4 h in the presence or absence of TP (0.25 μM). RNA was labeled and hybridized on to Affymetrix Human Gene 1.0 ST arrays according to the manufacturer's instructions. Arrays were scanned on an Affymetrix Genechip Scanner and raw data extracted using Affymetrix GeneChip Command Console. Downstream data analysis was performed using GenespringGX 10 (Agilent).
Chromatin Immunoprecipitation.
Assays were performed following the manufacturer's instructions (Active Motif) with some modifications. RNA Polymerase II (Active Motif), CHOP (Abcam), c-Rel, and p65 antibodies were all purchased from Santa Cruz (Insight). In brief, 1 to 2.0 × 106 cells were used in each ChIP assay. Fragmented DNA following enzyme digest was analyzed by gel electrophoresis to ensure that 200- to 500-bp sized fragments were obtained before proceeding with ChIP with 3 to 5 μg of antibody using ChIP kits from Upstate Biotechnology (Millipore). DNA pellets were resuspended in DNase-free water, and analyzed by real-time PCR amplification using KAPA SYBR FAST qPCR reagents, (KAPABIOSYSTEMS), and the following cycling program: a first cycle of 5 min, a second cycle for 20 s at 95 °C, and 30 s at 60 °C, which was repeated 50 times. Finally, one cycle of dissociation of 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s, and 60 °C for 15 s was performed to confirm that the melting curve indicated the presence of one PCR amplified DNA species. Oliognucleotide sequences for PCR are listed in Table S2.
Statistical Analysis.
All values are expressed as the mean and the error bars show 95% confidence intervals for the dataset. Nonoverlapping error bars between datasets indicate P ≤ 0.01 (33). Statistical analysis was performed using Graph Pad Prism software (GraphPad Prism). The analysis of larger datasets was performed using the Friedman test for nonparametric data and post hoc testing using Wilcoxin signed rank test to assign significance between groups of datasets.
Supplementary Material
Acknowledgments
This work was supported by supported by grants from Arthritis Research UK and the National Institute for Health Research Cambridge Biomedical Research Centre.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.J.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011736107/-/DCSupplemental.
References
- 1.Schröder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 2.Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novoa I, et al. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22:1180–1187. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J Exp Med. 2007;204:2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem. 2005;138:501–507. doi: 10.1093/jb/mvi143. [DOI] [PubMed] [Google Scholar]
- 8.Vattemi G, Engel WK, McFerrin J, Askanas V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol. 2004;164:1–7. doi: 10.1016/S0002-9440(10)63089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heazlewood CK, et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007;8:1. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type I - polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy FJ, Hayes MP, Burd PR. Disparate intracellular processing of human IL-12 preprotein subunits: atypical processing of the P35 signal peptide. J Immunol. 2000;164:839–847. doi: 10.4049/jimmunol.164.2.839. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn RM, et al. The UCSC Genome Browser Database: Update 2009. Nucleic Acids Res. 2009;37(Database issue):D755–D761. doi: 10.1093/nar/gkn875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mise-Omata S, et al. A proximal kappaB site in the IL-23 p19 promoter is responsible for RelA- and c-Rel-dependent transcription. J Immunol. 2007;179:6596–6603. doi: 10.4049/jimmunol.179.10.6596. [DOI] [PubMed] [Google Scholar]
- 16.Walsh C, et al. Elucidation of the MD-2/TLR4 interface required for signaling by lipid IVa. J Immunol. 2008;181:1245–1254. doi: 10.4049/jimmunol.181.2.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler MC, et al. KDEL-retained antigen in B lymphocytes induces a proinflammatory response: a possible role for endoplasmic reticulum stress in adaptive T cell immunity. J Immunol. 2008;181:256–264. doi: 10.4049/jimmunol.181.1.256. [DOI] [PubMed] [Google Scholar]
- 18.DeLay ML, et al. HLA-B27 misfolding and the unfolded protein response augment interleukin-23 production and are associated with Th17 activation in transgenic rats. Arthritis Rheum. 2009;60:2633–2643. doi: 10.1002/art.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodall JC, Ellis L, Yeo GS, Gaston JS. Does HLA-B27 influence the monocyte inflammatory response to lipopolysaccharide? Rheumatology (Oxford) 2007;46:232–237. doi: 10.1093/rheumatology/kel226. [DOI] [PubMed] [Google Scholar]
- 21.Carmody RJ, Ruan Q, Liou HC, Chen YH. Essential roles of c-Rel in TLR-induced IL-23 p19 gene expression in dendritic cells. J Immunol. 2007;178:186–191. doi: 10.4049/jimmunol.178.1.186. [DOI] [PubMed] [Google Scholar]
- 22.El Mezayen R, El Gazzar M, Myer R, High KP. Aging-dependent upregulation of IL-23p19 gene expression in dendritic cells is associated with differential transcription factor binding and histone modifications. Aging Cell. 2009;8:553–565. doi: 10.1111/j.1474-9726.2009.00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattori T, Ohoka N, Hayashi H, Onozaki K. C/EBP homologous protein (CHOP) up-regulates IL-6 transcription by trapping negative regulating NF-IL6 isoform. FEBS Lett. 2003;541:33–39. doi: 10.1016/s0014-5793(03)00283-7. [DOI] [PubMed] [Google Scholar]
- 24.Cucinotta M, et al. Regulation of interleukin-8 gene at a distinct site of its promoter by CCAAT enhancer-binding protein homologous protein in prostaglandin E2-treated human T cells. J Biol Chem. 2008;283:29760–29769. doi: 10.1074/jbc.M803145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27:17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 26.He B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006;13:393–403. doi: 10.1038/sj.cdd.4401833. [DOI] [PubMed] [Google Scholar]
- 27.Woo CW, et al. Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner MJ, et al. HLA-B27 misfolding in transgenic rats is associated with activation of the unfolded protein response. J Immunol. 2005;175:2438–2448. doi: 10.4049/jimmunol.175.4.2438. [DOI] [PubMed] [Google Scholar]
- 29.Mak BC, et al. Novel function of PERK as a mediator of force-induced apoptosis. J Biol Chem. 2008;283:23462–23472. doi: 10.1074/jbc.M803194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarvis LB, et al. Autoreactive human peripheral blood CD8+ T cells with a regulatory phenotype and function. Eur J Immunol. 2005;35:2896–2908. doi: 10.1002/eji.200526162. [DOI] [PubMed] [Google Scholar]
- 33.Cumming G, Fidler F, Vaux DL. Error bars in experimental biology. J Cell Biol. 2007;177:7–11. doi: 10.1083/jcb.200611141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.