Abstract
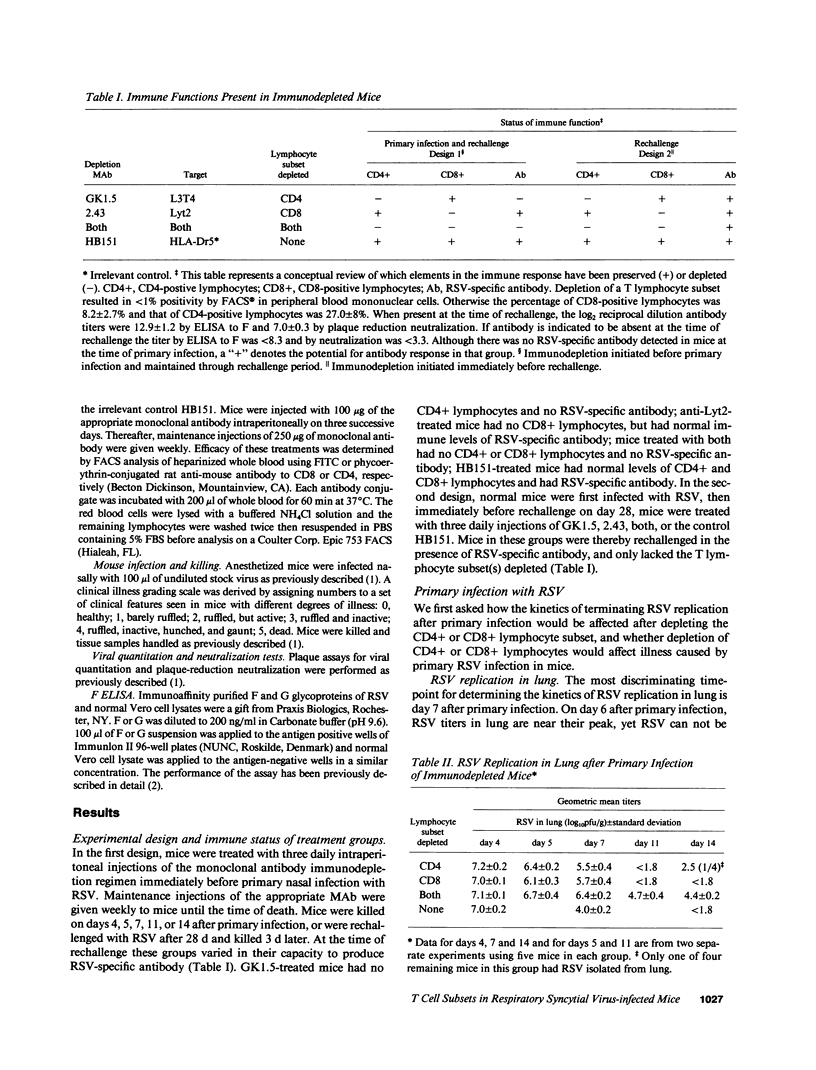
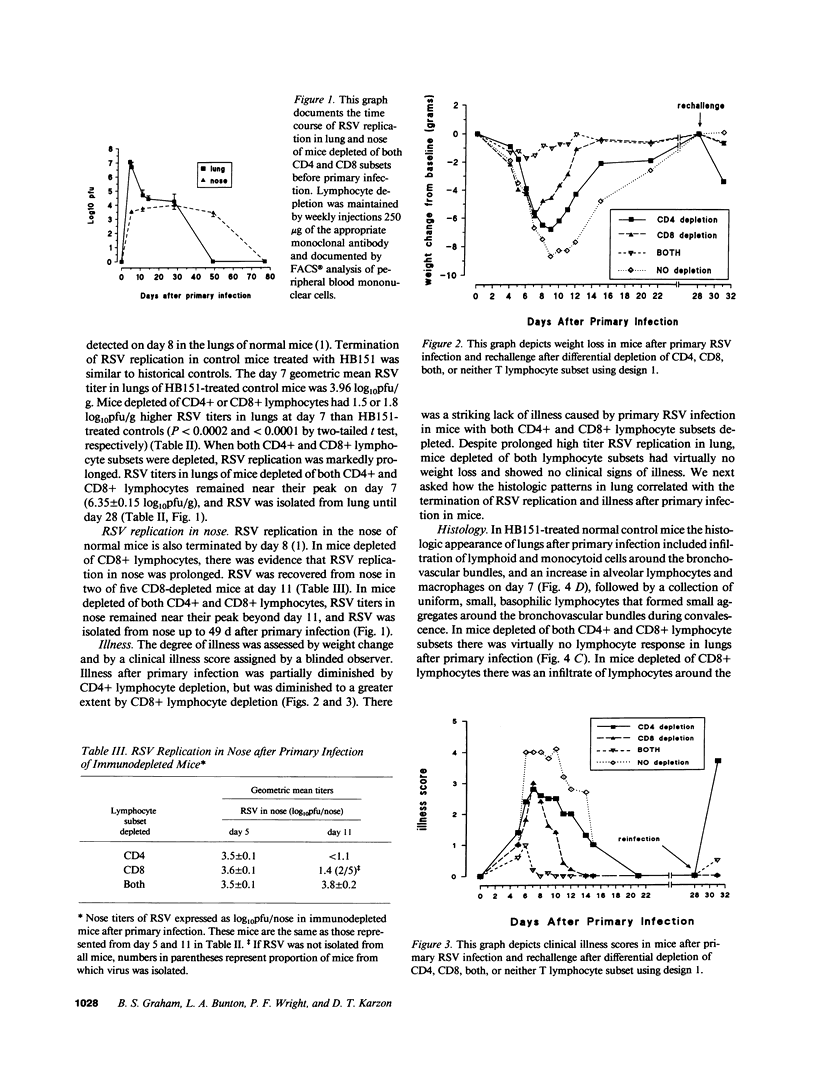
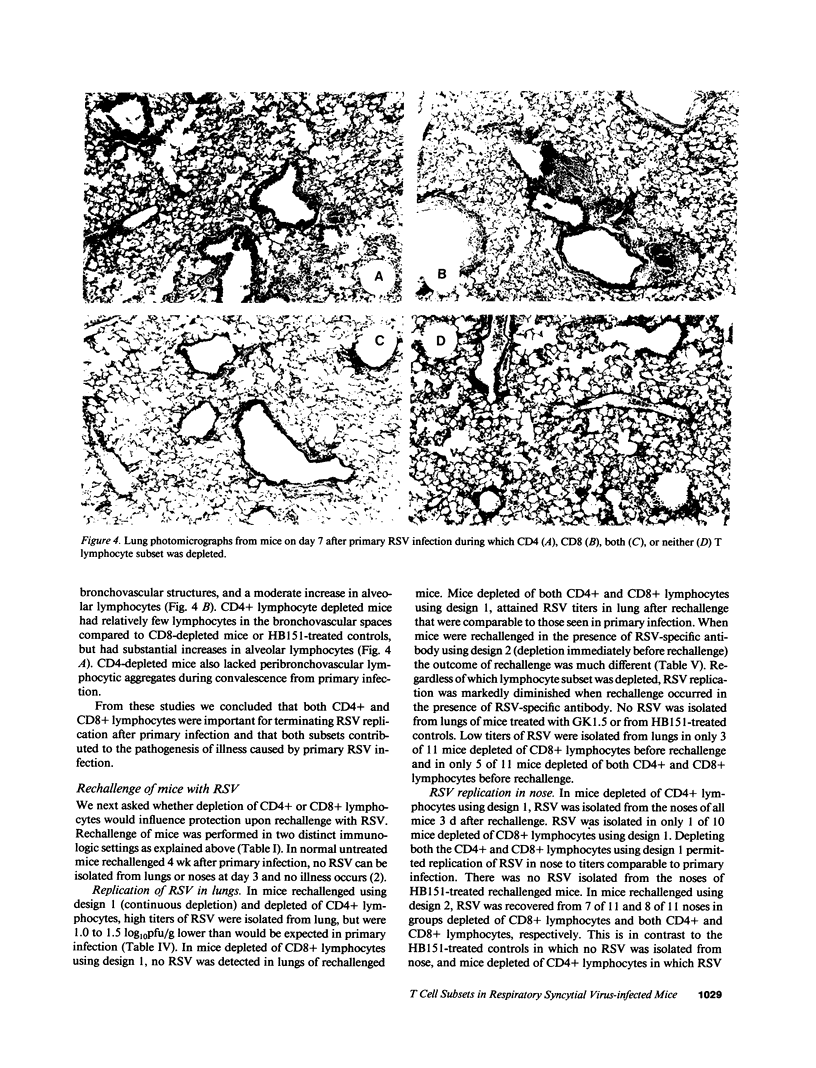
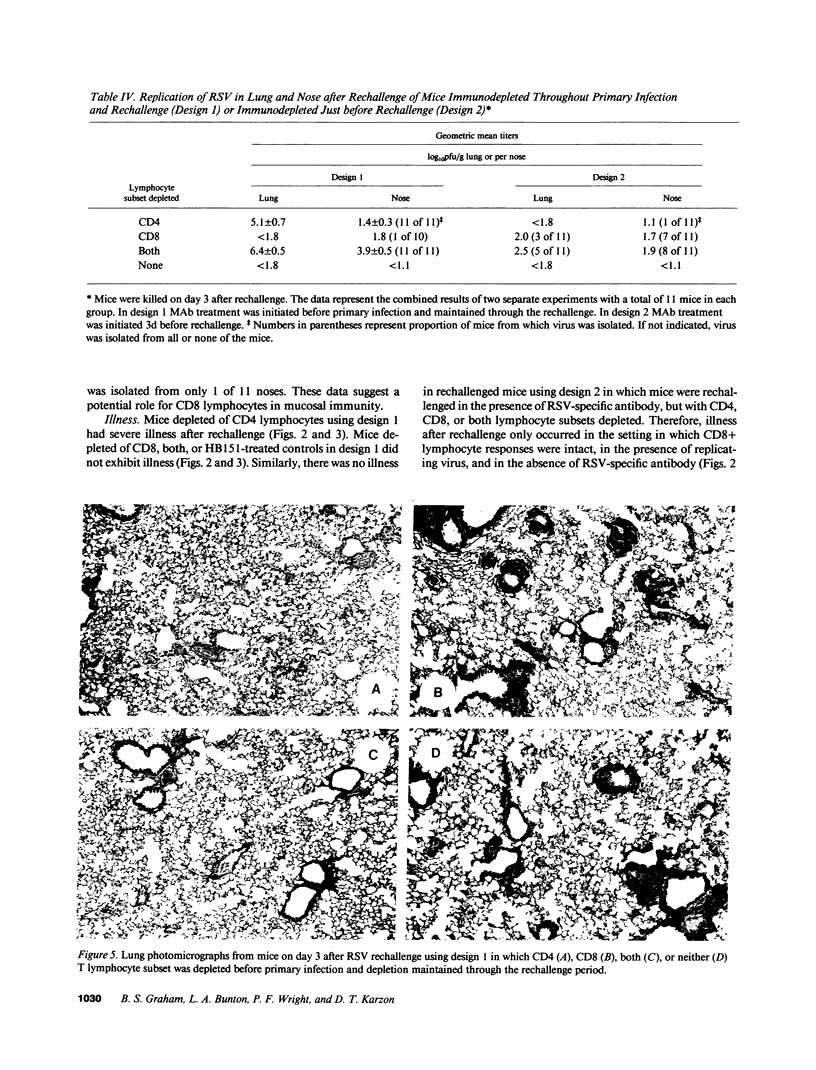
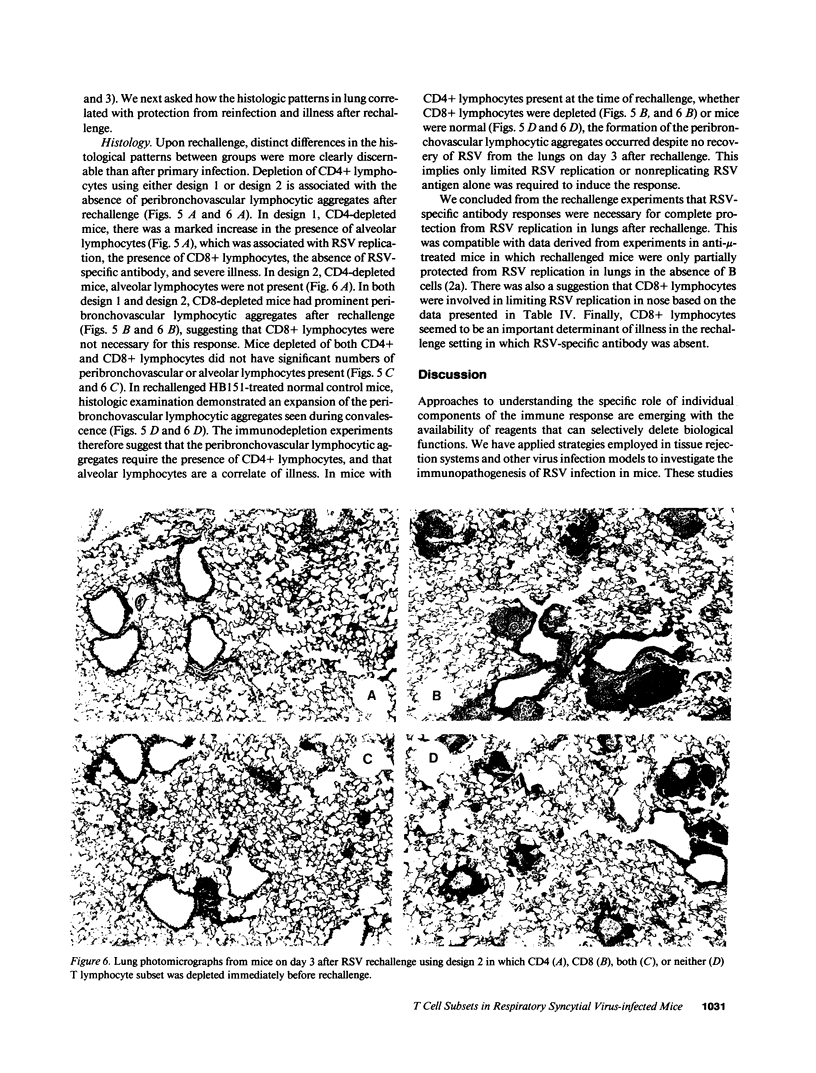
The role of CD4+ and CD8+ T lymphocytes in terminating respiratory syncytial virus (RSV) replication, causing disease, and protecting from reinfection was investigated using a BALB/c mouse model in which CD4+ or CD8+ lymphocytes or both were depleted by injections of Mab directed against the respective mouse lymphocyte determinants. Kinetics of RSV replication, illness, and pathology were assessed after primary infection and rechallenge. Both CD4+ and CD8+ lymphocyte subsets were involved in terminating RSV replication after primary infection. When both T lymphocyte subsets were depleted RSV replication was markedly prolonged, yet no illness was evident, suggesting that host immune response rather than viral cytocidal effect was the primary determinant of disease in mice. Both CD4+ and CD8+ lymphocytes contributed to illness, although CD8+ lymphocytes appeared to play the dominant role in this particular system. Analysis of histological responses suggested that CD4+ lymphocytes were required for the appearance of peribronchovascular lymphocytic aggregates seen in normal mice after rechallenge, and that the presence of alveolar lymphocytes was correlated with illness. It is postulated that antibody is an illness-sparing mechanism for protecting mice from RSV infection, and that T lymphocytes are an important determinant of illness. Further delineation of RSV-induced immunopathogenesis in primary infection and reinfection will provide important information for the development of vaccine strategies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. E., Doherty P. C. Immune T cells can protect or induce fatal neurological disease in murine lymphocytic choriomeningitis. Cell Immunol. 1985 Feb;90(2):401–407. doi: 10.1016/0008-8749(85)90204-7. [DOI] [PubMed] [Google Scholar]
- Anderson J. J., Norden J., Saunders D., Toms G. L., Scott R. Analysis of the local and systemic immune responses induced in BALB/c mice by experimental respiratory syncytial virus infection. J Gen Virol. 1990 Jul;71(Pt 7):1561–1570. doi: 10.1099/0022-1317-71-7-1561. [DOI] [PubMed] [Google Scholar]
- Baenziger J., Hengartner H., Zinkernagel R. M., Cole G. A. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. Eur J Immunol. 1986 Apr;16(4):387–393. doi: 10.1002/eji.1830160413. [DOI] [PubMed] [Google Scholar]
- Bangham C. R., Cannon M. J., Karzon D. T., Askonas B. A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985 Oct;56(1):55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., McMichael A. J. Specific human cytotoxic T cells recognize B-cell lines persistently infected with respiratory syncytial virus. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9183–9187. doi: 10.1073/pnas.83.23.9183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., Openshaw P. J., Ball L. A., King A. M., Wertz G. W., Askonas B. A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986 Dec 15;137(12):3973–3977. [PubMed] [Google Scholar]
- Cannon M. J., Openshaw P. J., Askonas B. A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988 Sep 1;168(3):1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M. J., Stott E. J., Taylor G., Askonas B. A. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology. 1987 Sep;62(1):133–138. [PMC free article] [PubMed] [Google Scholar]
- Chiba Y., Higashidate Y., Suga K., Honjo K., Tsutsumi H., Ogra P. L. Development of cell-mediated cytotoxic immunity to respiratory syncytial virus in human infants following naturally acquired infection. J Med Virol. 1989 Jul;28(3):133–139. doi: 10.1002/jmv.1890280304. [DOI] [PubMed] [Google Scholar]
- Cobbold S. P., Martin G., Qin S., Waldmann H. Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature. 1986 Sep 11;323(6084):164–166. doi: 10.1038/323164a0. [DOI] [PubMed] [Google Scholar]
- Cobbold S., Waldmann H. Skin allograft rejection by L3/T4+ and Lyt-2+ T cell subsets. Transplantation. 1986 May;41(5):634–639. doi: 10.1097/00007890-198605000-00016. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Fishaut M., Tubergen D., McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980 Feb;96(2):179–186. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- García-Monzón C., Moreno-Otero R., Pajares J. M., García-Sánchez A., López-Botet M., de Landázuri M. O., Sánchez-Madrid F. Expression of a novel activation antigen on intrahepatic CD8+ T lymphocytes in viral chronic active hepatitis. Gastroenterology. 1990 Apr;98(4):1029–1035. doi: 10.1016/0016-5085(90)90030-5. [DOI] [PubMed] [Google Scholar]
- Graham B. S., Bunton L. A., Wright P. F., Karzon D. T. Reinfection of mice with respiratory syncytial virus. J Med Virol. 1991 May;34(1):7–13. doi: 10.1002/jmv.1890340103. [DOI] [PubMed] [Google Scholar]
- Graham B. S., Perkins M. D., Wright P. F., Karzon D. T. Primary respiratory syncytial virus infection in mice. J Med Virol. 1988 Oct;26(2):153–162. doi: 10.1002/jmv.1890260207. [DOI] [PubMed] [Google Scholar]
- Isaacs D., Bangham C. R., McMichael A. J. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987 Oct 3;2(8562):769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- Jamieson B. D., Butler L. D., Ahmed R. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J Virol. 1987 Dec;61(12):3930–3937. doi: 10.1128/jvi.61.12.3930-3937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I. M., Urban J. F., Jr, Finkelman F. D. The role of L3T4+ and Lyt-2+ T cells in the IgE response and immunity to Nippostrongylus brasiliensis. J Immunol. 1988 May 1;140(9):3206–3211. [PubMed] [Google Scholar]
- Kelly E. A., Colley D. G. In vivo effects of monoclonal anti-L3T4 antibody on immune responsiveness of mice infected with Schistosoma mansoni. Reduction of irradiated cercariae-induced resistance. J Immunol. 1988 Apr 15;140(8):2737–2745. [PubMed] [Google Scholar]
- Kishimoto C., Abelmann W. H. In vivo significance of T cells in the development of Coxsackievirus B3 myocarditis in mice. Immature but antigen-specific T cells aggravate cardiac injury. Circ Res. 1990 Sep;67(3):589–598. doi: 10.1161/01.res.67.3.589. [DOI] [PubMed] [Google Scholar]
- Leist T. P., Cobbold S. P., Waldmann H., Aguet M., Zinkernagel R. M. Functional analysis of T lymphocyte subsets in antiviral host defense. J Immunol. 1987 Apr 1;138(7):2278–2281. [PubMed] [Google Scholar]
- Lightman S., Cobbold S., Waldmann H., Askonas B. A. Do L3T4+ T cells act as effector cells in protection against influenza virus infection. Immunology. 1987 Sep;62(1):139–144. [PMC free article] [PubMed] [Google Scholar]
- Milner M. E., de la Monte S. M., Hutchins G. M. Fatal respiratory syncytial virus infection in severe combined immunodeficiency syndrome. Am J Dis Child. 1985 Nov;139(11):1111–1114. doi: 10.1001/archpedi.1985.02140130049028. [DOI] [PubMed] [Google Scholar]
- Moskophidis D., Cobbold S. P., Waldmann H., Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987 Jun;61(6):1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. A., Rubino K. L., Levely M. E., Adams E. G., Collins P. L. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J Virol. 1990 Sep;64(9):4232–4241. doi: 10.1128/jvi.64.9.4232-4241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Openshaw P. J., Anderson K., Wertz G. W., Askonas B. A. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990 Apr;64(4):1683–1689. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton R. M., Cannon M. J., Openshaw P. J., Ball L. A., Wertz G. W., Askonas B. A. Cytotoxic T cell specificity for respiratory syncytial virus proteins: fusion protein is an important target antigen. J Gen Virol. 1987 Aug;68(Pt 8):2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Sriram S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J Immunol. 1988 May 1;140(9):2950–2955. [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Scott R., Pullan C. R., Scott M., McQuillin J. Cell-mediated immunity in respiratory syncytial virus disease. J Med Virol. 1984;13(1):105–114. doi: 10.1002/jmv.1890130112. [DOI] [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Hayle A. J. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J Gen Virol. 1985 Dec;66(Pt 12):2533–2538. doi: 10.1099/0022-1317-66-12-2533. [DOI] [PubMed] [Google Scholar]
- Vallbracht A., Maier K., Stierhof Y. D., Wiedmann K. H., Flehmig B., Fleischer B. Liver-derived cytotoxic T cells in hepatitis A virus infection. J Infect Dis. 1989 Aug;160(2):209–217. doi: 10.1093/infdis/160.2.209. [DOI] [PubMed] [Google Scholar]
- Welliver R. C., Kaul A., Ogra P. L. Cell-mediated immune response to respiratory syncytial virus infection: relationship to the development of reactive airway disease. J Pediatr. 1979 Mar;94(3):370–375. doi: 10.1016/s0022-3476(79)80573-9. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]