Abstract
Glatiramer acetate (GA), an immunomodulator used in multiple sclerosis (MS) therapy, induces the production of secreted IL-1 receptor antagonist (sIL-1Ra), a natural inhibitor of IL-1β, in human monocytes, and in turn enhances sIL-1Ra circulating levels in MS patients. GA is a mixture of peptides with random Glu, Lys, Ala, and Tyr sequences of high polarity and hydrophilic nature that is unlikely to cross the blood–brain barrier. In contrast, sIL-1Ra crosses the blood–brain barrier and, in turn, may mediate GA anti-inflammatory activities within the CNS by counteracting IL-1β activities. Here we identify intracellular signaling pathways induced by GA that control sIL-1Ra expression in human monocytes. By using kinase knockdown and specific inhibitors, we demonstrate that GA induces sIL-1Ra production via the activation of PI3Kδ, Akt, MEK1/2, and ERK1/2, demonstrating that both PI3Kδ/Akt and MEK/ERK pathways rule sIL-1Ra expression in human monocytes. The pathways act in parallel upstream glycogen synthase kinase-3α/β (GSK3α/β), the knockdown of which enhances sIL-1Ra production. Together, our findings demonstrate the existence of signal transduction triggered by GA, further highlighting the mechanisms of action of this drug in MS.
Keywords: multiple sclerosis, immunomodulation, inflammation, Copaxone, signal transduction
Secreted IL-1 receptor antagonist (sIL-1Ra) is a natural IL-1 inhibitor that competes with the agonist for the binding to IL-1 type I receptor to which it binds without inducing signal transduction (1, 2). Because it potently inhibits the various effects of IL-1, sIL-1Ra is considered an important regulator of the inflammatory and overall immune response mediated by IL-1 (3, 4). The importance of sIL-1Ra in the maintenance of cytokine homeostasis in humans was recently demonstrated (5). IL-1β is a pleiotropic proinflammatory cytokine the production of which is tightly controlled (6). Because IL-1β is expressed by microglial cells and infiltrating monocyte/macrophages throughout the white matter in and around the lesions in acute multiple sclerosis (MS) (7), it is likely to play an important part in MS pathogenesis. This assertion was confirmed in multiple studies performed in experimental autoimmune encephalomyelitis (EAE), i.e., an MS animal model. Indeed, rats treated with sIL-1Ra develop milder signs of EAE (8). sIL-1Ra delivered by nonreplicative HSV-1 vectors in EAE C57BL/6 mice delays disease onset and decreases disease severity (9). In addition, IL-1α/β double deficient mice exhibit significant resistance to EAE induction with reduction in disease severity, although IL-1Ra−/− mice are highly susceptible to EAE induction in the absence of pertussis toxin administration (10). These observations demonstrate that the IL-1/IL-1Ra system is crucial for autoantigen-specific T-cell induction in mice, and that sIL-1Ra can efficiently block IL-1β effects and can ameliorate EAE disease course (8–11). Moreover, IL-1β is essential for the differentiation of human and mouse IL-17–producing T helper cells (12, 13), corroborating the reduced production of IL-17 in IL-1−/− EAE mice and its increase in IL-1Ra−/− EAE mice (10). In both IL-1−/− and IL-1Ra−/− mice, the production of IFNγ by T cells followed that of IL-17, suggesting the involvement of the IL-1 system in the polarization of both Th1 and Th17 subsets (10). Overall, these studies suggest that alterations of the IL-1/IL-1Ra system are implicated in the development of autoimmunity within the CNS.
Glatiramer acetate (GA; Copolymer-1, Copaxone) is a mixture of synthetic peptides of 50–90 amino acid randomly composed of Glu, Lys, Ala, and Tyr. GA is an approved drug for treatment of relapsing remitting MS (14). However, the mechanisms of GA actions are still elusive. Mainly based on studies in EAE, GA activity was attributed to perturbations in T-cell reactivity to antigen, thus focusing on its effects on the adaptive immune response. Nevertheless, increasing number of reports indicate that GA treatment also exerts immunomodulatory activity on cells of the myelo-monocytic lineage, i.e., monocytes/macrophages and dendritic cells (15–23). These observations suggest that GA might be useful in autoimmune diseases other than MS, as suggested by its beneficial effect in animal models of uveoretinitis (24), inflammatory bowel disease (25), and graft rejection (26).
Like IFNβ, another immunomodulator used in MS therapy (27, 28), GA induces sIL-1Ra production in isolated human monocytes, an activity reflected by the enhancement of sIL-1Ra levels in the blood of GA-treated MS patients and EAE mice (29). Thus, both GA and IFNβ, which display comparable therapeutic effects (30), act on sIL-1Ra levels that might be a common mode of action in MS treatment. The ability of circulating sIL-1Ra to cross the blood–brain barrier (31) indicates that it may inhibit the proinflammatory activities of IL-1β into the CNS, a mechanism particularly important in regard to GA, the high polarity and hydrophilic nature of which are likely to impede CNS penetration (32). Therefore, sIL-1Ra might mediate part of the beneficial antiinflammatory effects of GA into the CNS. Increasing evidence of the direct effects of GA on monocytes strengthens the hypothesis that it exerts immunomodulatory effects mainly in the periphery and not directly in the CNS (32).
The induction of sIL-1Ra by GA in human monocytes suggests the triggering of intracellular pathways leading to gene transcription. In monocytes activated under acute/infectious or chronic/sterile inflammatory conditions, the production of sIL-1Ra depends on the activation of PI3Kδ (33), highlighting an important role of this kinase independently of the stimulus. In addition, both MAPK and glycogen-synthase kinase 3 (GSK3) were shown to play a part in the control of sIL-1Ra production (34, 35). Here, we address the question of GA-induced signaling pathways that lead to sIL-1Ra production in monocytes. The results show that the induction of sIL-1Ra production by GA occurs through pathways comprising PI3Kδ, Akt, MEK1/2, ERK1/2, and GSK3.
Results
GA Triggers PI3K/Akt and MEK/ERK Pathways in Monocytes.
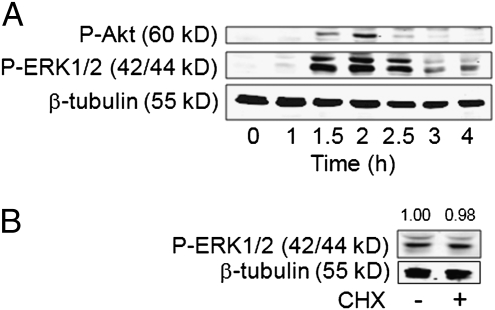
PI3Ks and extracellular signal-regulated kinase-1/2 (ERK1/2) have been shown to contribute to the induction of sIL-1Ra in monocytes. To assess whether GA was able to turn on PI3K/Akt and MAPK pathways, monocytes were activated by an optimal concentration of GA (25 μg/mL) that was previously determined (29). GA induced the phosphorylation of Akt and ERK1/2, i.e., downstream elements of PI3Ks and MEK1/2, both kinases being coordinately phosphorylated, reaching a maximum at 2 h (Fig. 1A). Transduction signals were directly induced by GA, as shown by the phosphorylation of ERK1/2 after 2 h activation in the presence or absence of the protein synthesis inhibitor cycloheximide (Fig. 1B). These results suggest that GA, per se, induced signal transduction in monocytes and did not operate via an autocrine or paracrine loop.
Fig. 1.
GA triggers both PI3K and MAPK pathways in human monocytes. (A) Monocytes were stimulated by GA. After the indicated time, cell lysates were subjected to Western blot analysis with the indicated antibody. (B) Monocytes were preincubated for 30 min in the presence or absence of 10 μM cycloheximide (CHX) before activation by GA for 2 h. Cell lysates were subjected to Western blot analysis.
PI3Kδ Is the PI3K Isoform Involved in the Induction of sIL-1Ra Production.
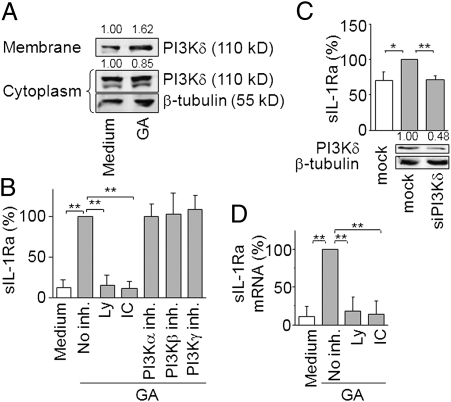
Because PI3Kδ controls sIL-1Ra induction in monocytes activated by several stimuli, we first assessed its activation in response to GA treatment. The catalytic subunit of PI3Kδ was localized in membrane fraction of monocytes exposed to GA (Fig. 2A), demonstrating the activation of PI3Kδ by GA. To ascertain the involvement of PI3Kδ in sIL-1Ra production and rule out involvement of other class I PI3K isoforms, we assessed the effects of both specific PI3K knockdown by siRNA and blockade by pharmacological inhibitors. The PI3K pan-inhibitor Ly294002 suppressed GA-induced production of sIL-1Ra (Fig. 2B), suggesting that PI3K pathway activation controlled sIL-1Ra production induced by GA. Specific blockade of PI3Kα, PI3Kβ, and PI3Kγ activity with optimal doses of inhibitors did not affect sIL-1Ra production. In contrast, IC87114, a specific inhibitor of PI3Kδ, reduced GA-induced sIL-1Ra production to basal level (Fig. 2B), suggesting that PI3Kδ controlled sIL-1Ra production in response to GA. The results obtained with kinase inhibitors were confirmed by the silencing of the different class I PI3K catalytic subunits in monocytes. As previously shown (36), transfection of monocytes with siRNA markedly increased the basal production of sIL-1Ra, i.e., from 465 ± 239 pg/mL to 1,173 ± 696 pg/mL. However, GA clearly enhanced sIL-1Ra production in mock-transfected monocytes reaching 1,806 ± 634 pg/mL, i.e., a significant 1.54-fold enhancement (P < 0.01). In contrast to PI3Kα, PI3Kβ, and PI3Kγ silencing (Fig. S1A), the specific knockdown of PI3Kδ abolished GA-induced production of sIL-1Ra (Fig. 2C), indicating a major role of this PI3K isoform in GA-induced sIL-1Ra production. The involvement of PI3Kδ was further confirmed by the exquisite sensitivity of GA-induced sIL-1Ra production to Ly294002 and IC87114; the production of sIL-1Ra reverting to basal levels at 2.5 μM of either inhibitor (Fig. S1 B and C). To ascertain that PI3Kδ controlled the steady state levels of sIL-1Ra mRNA, we measured the effect of specific inhibitors on sIL-1Ra transcript expression in response to GA-stimulation. Both PI3K pan-inhibitor and PI3Kδ specific inhibitor decreased sIL-1Ra mRNA expression to basal level in GA-treated monocytes (Fig. 2D). Together, these results identify PI3Kδ as the PI3K isoform controlling sIL-1Ra expression in GA-activated monocytes.
Fig. 2.
PI3Kδ controls sIL-1Ra induction by GA in human monocytes. (A) Monocytes were stimulated by GA. Cell membrane and cytoplasm fractions were analyzed by Western blot. (B) Monocytes were preincubated or not preincubated with 10 μM Ly294002 (Ly), 5 μM IC87114 (IC), 50 nM PI3Kα inhibitor, 50 nM PI3Kβ inhibitor, or 500 nM PI3Kγ inhibitor as indicated. Cells were activated with GA (gray columns) or left unactivated (white column). Production of sIL-1Ra was measured in supernatants and presented as percentage of production observed in the absence of inhibitor (no inh. = 100% = 1989 ± 867 pg/mL sIL-1Ra). (C) Monocytes were nucleofected with stealth siRNA for PI3Kδ or negative control (mock). PI3Kδ silencing was assessed by Western blot (Lower). PI3Kδ knockdown or mock transfected monocytes were activated (gray columns; 100% = 1806 ± 634 pg/mL sIL-1Ra) or not activated (white column; sIL-1Ra concentration = 1173 ± 696 pg/mL) with GA and sIL-1Ra measured in culture supernatants. (D) Monocytes were treated with 10 μM Ly294002 (Ly) or 5 μM IC87114 (IC) before activation (gray columns) by GA or left unactivated (white column). sIL-1Ra mRNA was analyzed by qPCR and presented as percentage of transcript level induced by GA in the absence of inhibitor. Results are either representative of three experiments (Western blots) or mean ± SD of three independent experiments carried out with monocytes isolated from three different individuals. **P < 0.01, *P < 0.05 as determined by Student t test.
GA Triggers Production of sIL-1Ra through a PI3Kδ/Akt Pathway.
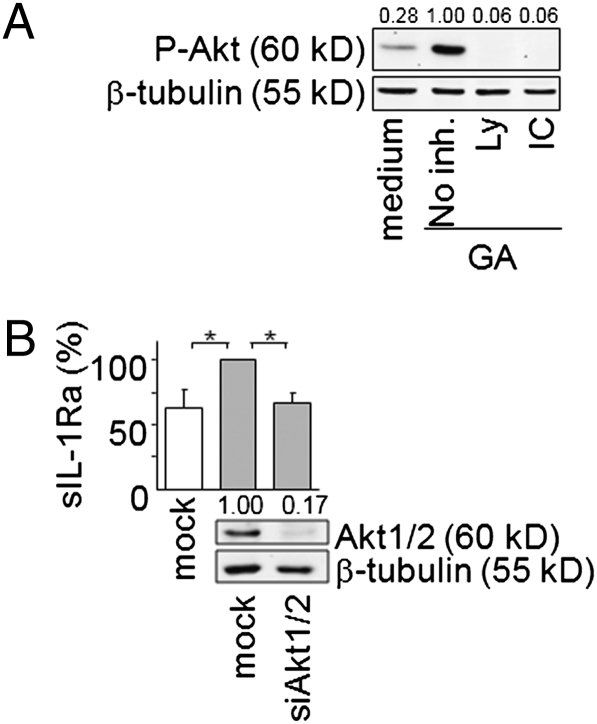
To assess the contribution of Akt downstream PI3Kδ in the control of sIL-1Ra production in GA-activated monocytes, the phosphorylation of Akt was first ascertained in GA-activated monocytes in the presence of PI3K and PI3Kδ inhibitors. The GA-induced phosphorylation of Akt was inhibited to similar extent when monocytes were pretreated with the PI3K pan-inhibitor Ly294002 or the PI3Kδ specific inhibitor IC87114 (Fig. 3A). These results indicated that PI3Kδ controlled Akt phosphorylation in monocytes activated by GA. To confirm the implication of Akt in the control of sIL-1Ra production, we evaluated the effect of siRNA specific for Akt1/2 on sIL-1Ra production induced by GA in monocytes. The silencing of Akt knocked down its expression by 83% (Fig. 3B). Concomitantly, GA-induced production of sIL-1Ra was reduced to basal level (Fig. 3B). Collectively, these observations indicate that PI3Kδ/Akt pathway is mandatory to the induction of sIL-1Ra production by GA-activated monocytes.
Fig. 3.
GA triggers the production of sIL-1Ra through a PI3Kδ/Akt pathway. (A) Monocytes were preincubated with or without 10 μM Ly294002 (Ly) or 5 μM IC 87114 (IC) before stimulation by GA. Cell lysates were subjected to Western blot analysis. (B) Monocytes were nucleofected with stealth siRNA for Akt1/2 or negative control (mock). The efficiency of Akt1/2 silencing was assessed by Western blot (Lower); a representative experiment out of 3 is presented. Akt1/2 knockdown or mock transfected monocytes were GA activated (gray columns; 100% = 2174 ± 467 pg/mL sIL-1Ra) or left unactivated (white column; sIL-1Ra concentration = 1,346 ± 467 pg/mL). sIL-1Ra was measured in culture supernatants and presented as described in legend of Fig. 2B. Data are mean ± SD of three independent experiments. *P < 0.05 as determined by Student t test.
MEK1, MEK2, and ERK1/2 Control GA-Induced sIL-1Ra Production.
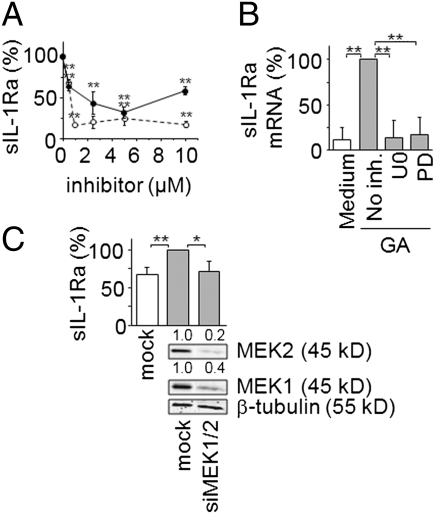
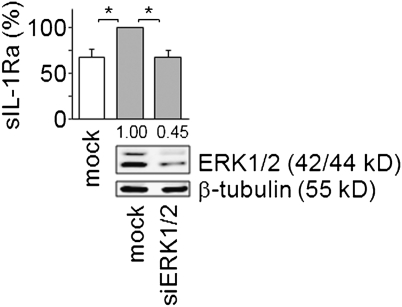
We next investigated the role of the MEK/ERK pathway in GA-induced sIL-1Ra production, as GA induced the phosphorylation of ERK1/2 in monocytes (Fig. 1). The MEK1 specific inhibitor, PD980159, and the MEK1 and MEK2 dual inhibitor, U0126, diminished sIL-1Ra production induced by GA in a dose–response manner (Fig. 4A). The inhibition of sIL-1Ra production by U0126 was more pronounced than that observed in the presence of PD98059 at all doses. More particularly, sIL-1Ra production was abolished in the presence of 1 μM U0126, whereas basal levels of sIL-1Ra were hardly reached in the presence of PD98059. These data suggest that both MEK1 and MEK2 take part in the control of sIL-1Ra production in response to GA. To optimize the inhibition of sIL-1Ra production after GA induction, a concentration of 5 μM of either inhibitor was used in the experiments described below (unless otherwise stated). Both MEK1 and MEK1/2 inhibitors diminished sIL-1Ra mRNA levels (Fig. 4B), suggesting that these kinases participate in the control of sIL-1Ra transcript steady state levels in response to GA activation. The results obtained with the kinase inhibitors were confirmed by silencing MEK1 and MEK2 in monocytes. Transfection of monocytes with MEK1 and MEK2 siRNA significantly diminished the expression of both kinases, as demonstrated by Western blot analysis (Fig. 4C). As observed above (Figs. 2C and 3B), the production of sIL-1Ra was significantly enhanced following nucleofection of monocytes with siRNA. However, the silencing of MEK1/2 resulted in the inhibition of sIL-1Ra production induced by GA that reverted to basal levels (Fig. 4C). To address the involvement of ERK1/2, the canonical substrates of MEK1/2, in the control of sIL-1Ra production, the kinases were silenced by specific stealth siRNA. The transfection of monocytes with ERK1/2 siRNA strongly reduced protein expression of each kinase (Fig. 5). The silencing of ERK1/2 abolished GA-induced production of sIL-1Ra (Fig. 5). Together the results of Figs. 4 and 5 demonstrate the implication of the MEK/ERK pathway in sIL-1Ra production downstream GA-activation of monocytes.
Fig. 4.
MEK1/2 controls GA-induced sIL-1Ra production. (A) Monocytes were preincubated in the absence or presence of increasing concentration of U0126 (○) or PD98059 (●) before the addition of GA. sIL-1Ra production was measured in supernatants. Results are presented as described in Fig. 2B (no inh. = 100% = 2177 ± 837 pg/mL sIL-1Ra). (B) Monocytes were treated with 5 μM U0126 (U0) or 5 μM PD98059 (PD) before addition (gray columns) or no addition (white column) of GA. mRNA was isolated and analyzed by real-time qPCR. Results are presented as in Fig. 2D. (C) Monocytes were nucleofected with stealth siRNA for MEK1 and MEK2 or negative control (mock). Efficiency of MEK1/2 silencing was assessed by Western blot (Lower), and sIL-1Ra production by MEK1/2 knocked-down or mock transfected monocytes activated (gray columns; 100% = 1933 ± 656 pg/mL sIL-1Ra) or not activated (white column; sIL-1Ra concentration = 1211 ± 547 pg/mL) by GA was measured in culture supernatants. Results are presented as mean ± SD of three independent experiments. **P < 0.01, *P < 0.05 as determined by Student t test.
Fig. 5.
ERK1/2 controls GA-induced sIL-1Ra production. Monocytes were nucleofected with stealth siRNA for ERK1/2 or negative control (mock). Efficiency of ERK1/2 silencing was assessed by Western blot (Lower); one representative experiment among three is presented. ERK1/2 knocked-down or mock-transfected monocytes were activated (gray columns; 100% = 2,334 ± 556 pg/mL sIL-1Ra) or not activated (white column; sIL-1Ra concentration = 1,555 ± 767 pg/mL) with GA and sIL-1Ra measured in culture supernatants. Results are presented as mean ± SD of three independent experiments; *P < 0.05 as determined by Student t test.
PI3Kδ/Akt and MEK/ERK Are Part of Two Parallel Pathways Controlling sIL-1Ra Production.
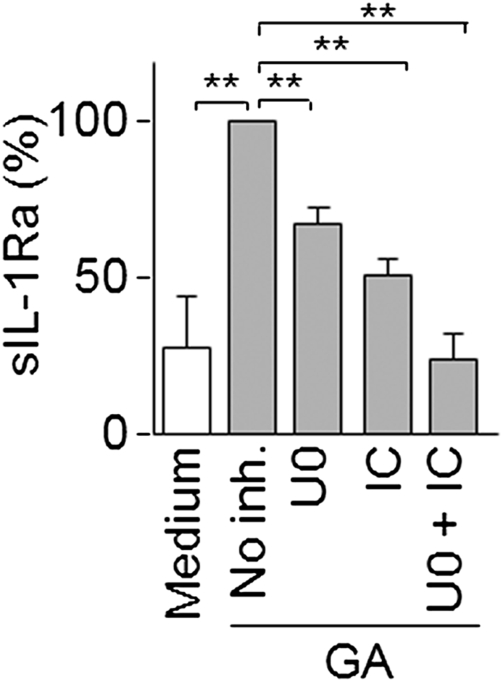
To determine whether PI3Kδ and MEK1/2 integrated a common pathway to regulate sIL-1Ra production in response to GA in monocytes, we assessed the effect of inhibitors in combination or alone. Because signals induced by GA were exquisitely sensitive to kinase inhibitors, we used suboptimal concentrations of inhibitors as determined by dose–response curves (Fig. S1C and Fig. 4A), i.e., 0.5 μM IC87114 and 0.5 μM U0126. At such inhibitor concentrations, we observed a decrease of sIL-1Ra production by 45 ± 5% and 69 ± 6% with PI3Kδ and MEK1/2 inhibitor, respectively (Fig. 6). When both IC87114 and U0126 were combined, the GA-induced production of sIL-1Ra was abolished, reaching basal level. These results suggest that the PI3Kδ/Akt and the MEK/ERK pathways act in parallel to regulate sIL-1Ra production in monocytes activated by GA.
Fig. 6.
PI3Kδ and MEK1/2 are part of two different pathways controlling sIL-1Ra production. (A) Monocytes were preincubated in the absence or presence of 0.5 μM U0126 (U0, MEK1/2 inhibitor), 0.5 μM IC87114 (IC, PI3Kδ inhibitor) or a mixture of both inhibitors (U0 + IC). Cells were then activated (gray columns) or not activated (white column) by GA. Production of sIL-1Ra was measured in harvested supernatants. Results are presented as described in Fig. 2B (no inh. = 100% = 2720 ± 1726 pg/mL sIL-1Ra). Data are mean ± SD of three experiments carried out with monocytes prepared from blood of three different donors. **P < 0.01 as determined by Student t test.
PI3Kδ/Akt and MEK/ERK Pathways Converge on GSK3α/β to Control GA-Induced sIL-1Ra Production.
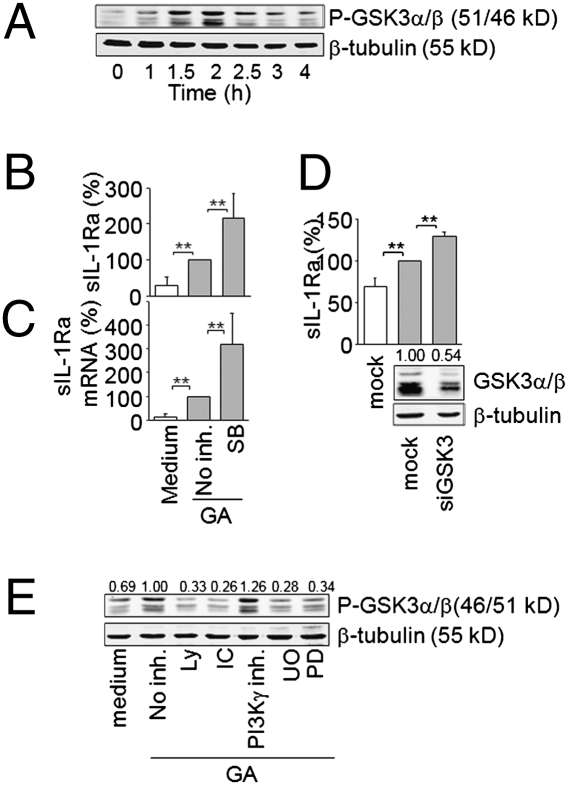
GSK3 is a constitutively active Ser-Thr kinase, whose phosphorylation downstream PI3K/Akt or MAPK pathway results in its inhibition (37, 38). To assess the contribution of GSK3 to GA-induced sIL-1Ra production by monocytes, the phosphorylation of GSK3 at Ser21 for GSK3α and Ser9 for GSK3β was first analyzed. Likewise Akt and ERK1/2 phosphorylation (Fig. 1A), the phosphorylation of GSK3α/β was induced by GA, reaching a maximum at 2 h (Fig. 7A). To evaluate the involvement of GSK3 in the control of sIL-1Ra production, monocytes were pretreated with the GSK3 inhibitor SB216763 before activation by GA. When monocytes were pretreated with SB216763, the production of sIL-1Ra induced by GA increased 2.15- ± 0.69-fold (Fig. 7B). Concomitantly, pretreatment of monocytes with SB216763 enhanced sIL-1Ra transcript expression in response to GA (Fig. 7C), indicating that active GSK3α/β repressed sIL-1Ra production in GA-activated monocytes. To confirm results obtained with the inhibitor, we examined the effect of siRNA-mediated GSK3α/β silencing on GA-induced sIL-1Ra production. The silencing of GSK3α/β reduced its expression by 46% (Fig. 7D) and further increased the induction of sIL-1Ra production by 1.95- ± 0.05-fold after monocyte stimulation by GA (Fig. 7D). To assess whether GSK3 inactivation was controlled by the PI3Kδ/Akt or the MEK/ERK pathway, we tested the effects of PI3K and MEK inhibitors on GSK3α/β phosphorylation. Inhibitors of both pathways decreased GSK3α/β phosphorylation, whereas the PI3Kγ inhibitor was ineffective (Fig. 7E). These data demonstrate that activation of the PI3Kδ/Akt and MEK/ERK pathways by GA converge on GSK3 and repress its activity to control sIL-1Ra expression in monocytes.
Fig. 7.
GSK3α/β controls GA-induced sIL-1Ra production. (A) Monocytes were stimulated by GA for indicated time, and cell lysates were subjected to Western blot analysis. (B and C) Monocytes were preincubated for 45 min in the presence or absence of 10 μM SB216763 (SB) and then cultured in the absence (white columns) or presence (gray columns) of GA. (B) Production of sIL-1Ra was measured in harvested supernatants (no inh. = 100% = 2020 ± 636 pg/mL sIL-1Ra) and (C) mRNA analyzed by real-time quantitative PCR; results are presented as described in Fig. 2 B and D, respectively. (D) Monocytes were nucleofected with stealth siRNA for GSK3α and GSK3β (siGSK3) or negative control (mock). Efficiency of GSK3α/β silencing was assessed by Western blot (Lower). sIL-1Ra was measured in culture supernatants of GSK3α/β knocked-down or mock-transfected monocytes activated (gray columns; 100% = 2174 ± 467 pg/mL sIL-1Ra) or not activated (white column; sIL-1Ra concentration = 1,344 ± 676 pg/mL) by GA. (E) Monocytes were preincubated in the absence or presence of 10 μM Ly294002 (Ly); 5 μM IC87114 (IC); 500 nM PI3Kγ inhibitor, 5 μM U0126 (U0), or 5 μM PD98059 (PD) before activation by GA. Cell lysates were subjected to Western blot analysis. **P < 0.01 as determined by Student's t test.
Discussion
This study demonstrates that GA triggers at least two different parallel pathways (PI3Kδ/Akt and MEK/ERK) that converge on GSK3 to promote sIL-1Ra production in human monocytes (Fig. S2). PI3Kδ is activated in monocytes exposed to GA, as suggested by the recruitment of p110δ catalytic subunit at monocyte membranes. Our data further indicate that PI3Kδ is the only PI3K isoform involved in the control of sIL-1Ra production in monocytes upon GA-activation. PI3Kδ controls sIL-1Ra production through the activation of its downstream element, Akt, which phosphorylates GSK3 and turns off its repressive activity. In addition, both MEK1 and MEK2 control sIL-1Ra production through the activation of their canonical substrates ERK1/2 and GSK3. The PI3Kδ/Akt and MEK/ERK pathways are likely to act in parallel upstream GSK3, although the present results do not rule out other cross-talks. The demonstration that GA, which is a mixture of peptides with different sequences of Glu, Lys, Ala, and Tyr, induces signal transduction leading to the production of sIL-1Ra, strongly suggests that monocytes express a specific receptor/sensor that binds/recognizes GA or a part of its peptides.
The existence of a specific GA sensor/receptor in monocytes is sturdily suggested by our observations demonstrating the induction of signal transduction pathways. In vitro, GA was shown to interact with empty, recombinant MHC class II molecules, competing with the binding of myelin basic protein into the peptide-binding groove (39). GA also interacts promiscuously with MHC class II molecules at the surface of living APCs (40). The latter study demonstrates that binding of GA to MHC class II molecules occurred very rapidly after GA application, i.e., GA binding at time = 0 (80%) was not different from that at time = 20 h. This contrasts with the present results showing delayed signal transduction with maximum Akt and ERK1/2 phosphorylation at 2 h. Because signal transduction induced by ligation of MHC class II molecules by antibodies, their natural ligand (LAG-3), or superantigens occurs within minutes (41–43), it is likely that GA signaling is mediated by other receptors/sensors. Moreover, contrasting with GA effects, IL-1β production is induced in monocytes upon ligation of MHC class II by antibodies, F(ab) from the latter, and superantigens (42, 44–46). As GA induces sIL-1Ra but not IL-1β production in human monocytes (29), it is unlikely that MHC class II molecules may act as GA receptors to induce sIL-1Ra production. Furthermore, the demonstration that in MHC class II-deficient mice, GA induces type II monocyte differentiation favoring the production of anti-inflammatory cytokines, strongly indicates that GA may affect monocyte function in the absence of MHC class II molecules (47). In addition to MHC class II, GA has been shown to interact with Mac-1 (CD11b/CD18 integrin) (48). Ligation of β2-integrins by either CD23 or antibodies induces the production of IL-1β in human monocytes that depends on the early (2–5 min) phosphorylation of ERK1/2 (49). Therefore, alike MHC class II molecules, Mac-1 is unlikely to act as the GA receptor on monocytes involved in sIL-1Ra induction.
Our results further support the premise that PI3Kδ activation is a key requirement to optimal secretion of sIL-1Ra in monocytes upon various stimulatory conditions. Indeed, we recently reported that PI3Kδ accounts for the PI3K-dependent signaling ruling the production of sIL-1Ra in monocytes activated by lipopolysaccharides (LPS), i.e., a prototypical stimulus of acute inflammation, and by contact with stimulated T cells, i.e., a stimulus likely to reflect chronic inflammatory conditions (33). Interestingly, although PI3Kδ activity is required to sIL-1Ra induction independently of the stimulus, it dampens the production of proinflammatory cytokines in LPS-activated monocytes, but induces such production in T-cell contact-activated monocytes (33). Thus, PI3Kδ is likely to be a key element in the regulation of inflammatory effector functions of monocytes.
The activation of both MEK1 and MEK2 is required to optimal production of sIL-1Ra, which is controlled by their downstream substrates, ERK1/2. Of note, GA requires the activation of PI3Kδ/Akt and MEK/ERK pathways, which act in parallel to regulate sIL-1Ra production. Indeed, when suboptimal doses of inhibitors of either MEK1/2 or PI3Kδ were used, only 45–69% of inhibition of GA-induced sIL-1Ra production was observed. However, when inhibitors were combined, the induction of sIL-1Ra production was abolished, indicating that both pathways independently controlled sIL-1Ra following GA-activation. Because Akt, ERK1/2, and GSK3α/β were phosphorylated within the same time frame, i.e., 2 h after GA-activation, the two PI3Kδ/Akt and MEK/ERK pathways are likely to be concomitantly activated, both leading to GSK3 phosphorylation. Although PI3Kδ is used by other sIL-1Ra inducers, there are different factors ruling sIL-1Ra production downstream the different stimuli. In LPS-activated monocytes the production of sIL-1Ra is regulated via the PI3K pathway by GSK3, which acts upstream the MEK1/2/ERK1/2 pathway (34). Our current data are in agreement with these findings, demonstrating that GSK3 phosphorylation dampens sIL-1Ra production in GA-activated monocytes, although we identify GSK3 as a downstream effector of the MEK1/2/ERK1/2 pathway. GSK3 is a constitutively active Ser-Thr kinase whose phosphorylation downstream PI3K/Akt or MAPK pathway results in its inhibition (37, 38). GSK3 phosphorylates/inactivates multiple substrates, including transcription factors that are critical regulators of pro- and antiinflammatory cytokine production (50). In accordance with the present results, previous studies have demonstrated that active GSK3β negatively regulates the production of antiinflammatory cytokines while concurrently up-regulating production of proinflammatory cytokines (51).
We previously demonstrated that GA increases the circulating levels of sIL-1Ra in GA-treated MS patients and EAE mice (29). This observation corroborates the triggering of sIL-1Ra production by GA in isolated blood monocytes. Furthermore, in T-cell contact-activated monocytes, i.e., a mechanism relevant to chronic inflammation, GA strongly diminishes the expression of IL-1β and enhances expression of sIL-1Ra, contrasting with the effect of GA in monocytes activated upon acute inflammatory conditions (29). Similar effects were observed on elements of the IL-1 system in IFNβ-activated monocytes (52). Taken together, these findings suggest that both immunomodulators used in MS treatment, i.e., GA and IFNβ, affect the IL-1 system in comparable ways.
To conclude, our results demonstrate that GA signals through two parallel signaling pathways, i.e., the PI3Kδ/Akt and MEK/ERK cascades, which activities converge to phosphorylate GSK3α/β and result in the induction of sIL-1Ra production in monocytes. In addition to the direct activation of signal transduction by GA, this study strongly suggests the existence of a specific receptor/sensor of GA in human monocytes.
Materials and Methods
Details on materials and methods are given in SI Materials and Methods.
Monocytes.
Peripheral blood monocytes were prepared as described elsewhere (53).
sIL-1Ra Production, mRNA Quantification, and mRNA Silencing.
sIL-1Ra production, mRNA quantification, and mRNA silencing were carried out as previously described (29).
Western Blot Analysis.
Western blot analyses were carried out as described previously (54).
Translocation of PI3Kδ at Monocyte Membranes.
Monocytes (10 × 106 cells/mL; 1 mL) were activated by GA (25 μg/mL) for 2 h. Their membranes were isolated by ultracentrifugation and were subjected to Western blot analysis.
Statistical Analysis.
When required, significance of differences between groups was evaluated using a Student paired t test.
Supplementary Material
Acknowledgments
IC87114 was a kind gift from Calistoga Pharmaceuticals (Seattle, WA). This work was supported by Grants 320000-116259 (to D.B.) and #310030-113653 (to P.H.L.) from the Swiss National Science Foundation, grants from the Swiss Society for Multiple Sclerosis (to D.B. and P.H.L.), a grant from the Hans Wilsdorf Foundation (to D.B.), and a grant from the De Reuter Foundation (to D.B.). R.C. is a recipient of a postdoctoral fellowship from the Basque Country Government. N.M. is a recipient of an advanced researcher fellowship from the Swiss National Science Foundation (PA00A-119532).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009443107/-/DCSupplemental.
References
- 1.Burger D, Dayer JM. In: Cytokine Reference. Oppenheim JJ, Feldmann M, editors. London: Academic Press; 2000. pp. 319–336. [Google Scholar]
- 2.Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343:732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- 3.Burger D, Dayer JM, Palmer G, Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract Res Clin Rheumatol. 2006;20:879–896. doi: 10.1016/j.berh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 5.Aksentijevich I, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA. Mutations in cryopyrin: Bypassing roadblocks in the caspase 1 inflammasome for interleukin-1beta secretion and disease activity. Arthritis Rheum. 2007;56:2817–2822. doi: 10.1002/art.22841. [DOI] [PubMed] [Google Scholar]
- 7.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 8.Badovinac V, Mostarica-Stojković M, Dinarello CA, Stosić-Grujicić S. Interleukin-1 receptor antagonist suppresses experimental autoimmune encephalomyelitis (EAE) in rats by influencing the activation and proliferation of encephalitogenic cells. J Neuroimmunol. 1998;85:87–95. doi: 10.1016/s0165-5728(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 9.Furlan R, et al. HSV-1-mediated IL-1 receptor antagonist gene therapy ameliorates MOG(35-55)-induced experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene Ther. 2007;14:93–98. doi: 10.1038/sj.gt.3302805. [DOI] [PubMed] [Google Scholar]
- 10.Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 11.Martin D, Near SL. Protective effect of the interleukin-1 receptor antagonist (IL-1ra) on experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1995;61:241–245. doi: 10.1016/0165-5728(95)00108-e. [DOI] [PubMed] [Google Scholar]
- 12.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 13.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sela M, Teitelbaum D. Glatiramer acetate in the treatment of multiple sclerosis. Expert Opin Pharmacother. 2001;2:1149–1165. doi: 10.1517/14656566.2.7.1149. [DOI] [PubMed] [Google Scholar]
- 15.Balabanov R, Lisak D, Beaumont T, Lisak RP, Dore-Duffy P. Expression of urokinase plasminogen activator receptor on monocytes from patients with relapsing-remitting multiple sclerosis: Effect of glatiramer acetate (copolymer 1) Clin Diagn Lab Immunol. 2001;8:1196–1203. doi: 10.1128/CDLI.8.6.1196-1203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber MS, et al. Multiple sclerosis: Glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain. 2004;127:1370–1378. doi: 10.1093/brain/awh163. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, et al. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J Immunol. 2004;172:7144–7153. doi: 10.4049/jimmunol.172.11.7144. [DOI] [PubMed] [Google Scholar]
- 18.Vieira PL, Heystek HC, Wormmeester J, Wierenga EA, Kapsenberg ML. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J Immunol. 2003;170:4483–4488. doi: 10.4049/jimmunol.170.9.4483. [DOI] [PubMed] [Google Scholar]
- 19.Jung S, et al. Induction of IL-10 in rat peritoneal macrophages and dendritic cells by glatiramer acetate. J Neuroimmunol. 2004;148:63–73. doi: 10.1016/j.jneuroim.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Stasiolek M, et al. Impaired maturation and altered regulatory function of plasmacytoid dendritic cells in multiple sclerosis. Brain. 2006;129:1293–1305. doi: 10.1093/brain/awl043. [DOI] [PubMed] [Google Scholar]
- 21.Hussien Y, Sanna A, Söderström M, Link H, Huang YM. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J Neuroimmunol. 2001;121:102–110. doi: 10.1016/s0165-5728(01)00432-5. [DOI] [PubMed] [Google Scholar]
- 22.Liblau R. Glatiramer acetate for the treatment of multiple sclerosis: Evidence for a dual anti-inflammatory and neuroprotective role. J Neurol Sci. 2009;287(Suppl 1):S17–S23. doi: 10.1016/S0022-510X(09)71296-1. [DOI] [PubMed] [Google Scholar]
- 23.Kantengwa S, et al. Inhibition of naive Th1 CD4+ T cells by glatiramer acetate in multiple sclerosis. J Neuroimmunol. 2007;185:123–129. doi: 10.1016/j.jneuroim.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, et al. Copolymer 1 inhibits experimental autoimmune uveoretinitis. J Neuroimmunol. 2000;103:189–194. doi: 10.1016/s0165-5728(99)00239-8. [DOI] [PubMed] [Google Scholar]
- 25.Aharoni R, Sonego H, Brenner O, Eilam R, Arnon R. The therapeutic effect of glatiramer acetate in a murine model of inflammatory bowel disease is mediated by anti-inflammatory T-cells. Immunol Lett. 2007;112:110–119. doi: 10.1016/j.imlet.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Arnon R, Aharoni R. Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14593–14598. doi: 10.1073/pnas.0404887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sciacca FL, Canal N, Grimaldi LME. Induction of IL-1 receptor antagonist by interferon beta: Implication for the treatment of multiple sclerosis. J Neurovirol. 2000;6(Suppl 2):S33–S37. [PubMed] [Google Scholar]
- 28.Jungo F, Dayer JM, Modoux C, Hyka N, Burger D. IFN-beta inhibits the ability of T lymphocytes to induce TNF-alpha and IL-1beta production in monocytes upon direct cell-cell contact. Cytokine. 2001;14:272–282. doi: 10.1006/cyto.2001.0884. [DOI] [PubMed] [Google Scholar]
- 29.Burger D, et al. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1beta in human monocytes and multiple sclerosis. Proc Natl Acad Sci USA. 2009;106:4355–4359. doi: 10.1073/pnas.0812183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mikol DD, et al. REGARD study group Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): A multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7:903–914. doi: 10.1016/S1474-4422(08)70200-X. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez EG, Banks WA, Kastin AJ. Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J Neuroimmunol. 1994;55:153–160. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 32.Neuhaus O, Kieseier BC, Hartung HP. Pharmacokinetics and pharmacodynamics of the interferon-betas, glatiramer acetate, and mitoxantrone in multiple sclerosis. J Neurol Sci. 2007;259:27–37. doi: 10.1016/j.jns.2006.05.071. [DOI] [PubMed] [Google Scholar]
- 33.Molnarfi N, Brandt KJ, Gruaz L, Dayer JM, Burger D. Differential regulation of cytokine production by PI3Kdelta in human monocytes upon acute and chronic inflammatory conditions. Mol Immunol. 2008;45:3419–3427. doi: 10.1016/j.molimm.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Rehani K, Wang H, Garcia CA, Kinane DF, Martin M. Toll-like receptor-mediated production of IL-1Ra is negatively regulated by GSK3 via the MAPK ERK1/2. J Immunol. 2009;182:547–553. doi: 10.4049/jimmunol.182.1.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darragh J, Ananieva O, Courtney A, Elcombe S, Arthur JS. MSK1 regulates the transcription of IL-1ra in response to TLR activation in macrophages. Biochem J. 2010;425:595–602. doi: 10.1042/BJ20091062. [DOI] [PubMed] [Google Scholar]
- 36.Molnarfi N, Hyka-Nouspikel N, Gruaz L, Dayer JM, Burger D. The production of IL-1 receptor antagonist in IFN-beta-stimulated human monocytes depends on the activation of phosphatidylinositol 3-kinase but not of STAT1. J Immunol. 2005;174:2974–2980. doi: 10.4049/jimmunol.174.5.2974. [DOI] [PubMed] [Google Scholar]
- 37.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 38.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fridkis-Hareli M, et al. Binding motifs of copolymer 1 to multiple sclerosis- and rheumatoid arthritis-associated HLA-DR molecules. J Immunol. 1999;162:4697–4704. [PubMed] [Google Scholar]
- 40.Fridkis-Hareli M, et al. Direct binding of myelin basic protein and synthetic copolymer 1 to class II major histocompatibility complex molecules on living antigen-presenting cells—specificity and promiscuity. Proc Natl Acad Sci USA. 1994;91:4872–4876. doi: 10.1073/pnas.91.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andreae S, Buisson S, Triebel F. MHC class II signal transduction in human dendritic cells induced by a natural ligand, the LAG-3 protein (CD223) Blood. 2003;102:2130–2137. doi: 10.1182/blood-2003-01-0273. [DOI] [PubMed] [Google Scholar]
- 42.Palkama T, Hurme M. Signal transduction mechanisms of HLA-DR-mediated interleukin-1 beta production in human monocytes. Role of protein kinase C and tyrosine kinase activation. Hum Immunol. 1993;36:259–267. doi: 10.1016/0198-8859(93)90133-l. [DOI] [PubMed] [Google Scholar]
- 43.Kriegel MA, et al. Anti-HLA-DR-triggered monocytes mediate in vitro T cell anergy. Int Immunol. 2008;20:601–613. doi: 10.1093/intimm/dxn019. [DOI] [PubMed] [Google Scholar]
- 44.Palacios R. Monoclonal antibodies against human Ia antigens stimulate monocytes to secrete interleukin 1. Proc Natl Acad Sci USA. 1985;82:6652–6656. doi: 10.1073/pnas.82.19.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palkama T, Sihvola M, Hurme M. Induction of interleukin 1 alpha (IL-1 alpha) and IL-1 beta mRNA expression and cellular IL-1 production by anti-HLA-DR antibodies in human monocytes. Scand J Immunol. 1989;29:609–615. doi: 10.1111/j.1365-3083.1989.tb01164.x. [DOI] [PubMed] [Google Scholar]
- 46.Trede NS, Geha RS, Chatila T. Transcriptional activation of IL-1 beta and tumor necrosis factor-alpha genes by MHC class II ligands. J Immunol. 1991;146:2310–2315. [PubMed] [Google Scholar]
- 47.Weber MS, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 48.Stapulionis R, et al. Structural insight into the function of myelin basic protein as a ligand for integrin alpha M beta 2. J Immunol. 2008;180:3946–3956. doi: 10.4049/jimmunol.180.6.3946. [DOI] [PubMed] [Google Scholar]
- 49.Rezzonico R, Chicheportiche R, Imbert V, Dayer JM. Engagement of CD11b and CD11c beta2 integrin by antibodies or soluble CD23 induces IL-1beta production on primary human monocytes through mitogen-activated protein kinase-dependent pathways. Blood. 2000;95:3868–3877. [PubMed] [Google Scholar]
- 50.Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): Inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite effects of IFN beta on cytokine homeostasis in LPS- and T cell contact-activated human monocytes. J Neuroimmunol. 2004;146:76–83. doi: 10.1016/j.jneuroim.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 53.Hyka N, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–2389. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- 54.Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite regulation of IL-1beta and secreted IL-1 receptor antagonist production by phosphatidylinositide-3 kinases in human monocytes activated by lipopolysaccharides or contact with T cells. J Immunol. 2007;178:446–454. doi: 10.4049/jimmunol.178.1.446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.