Abstract
Plant RHO GTPases (RAC/ROPs) mediate multiple extracellular signals ranging from hormone to stress and regulate diverse cellular processes important for polarized cell growth, differentiation, development, reproduction, and responses to the environment. They shuttle between the GDP-bound inactive state and the GTP-bound activated state and their activation is predominantly mediated by a family of guanine nucleotide exchange factors (GEFs) referred to as ROPGEFs. Using the Arabidopsis ROPGEF1 as bait, we identified members of a receptor-like kinase (RLK) family as potential upstream regulators for RAC/ROP signaling. NADPH oxidase-derived reactive oxygen species (ROS) are emerging as important regulators for growth and development and play a crucial role in mediating RAC/ROP-regulated root hair development, a polarized cell growth process. We therefore screened T-DNA insertion mutants in these RLKs for root hair defects and found that mutations in one of them, At3g51550 encoding the FERONIA (FER) receptor-like kinase, induced severe root hair defects. We show that the fer phenotypes correlated with reduced levels of active RAC/ROPs and NADPH oxidase-dependent, auxin-regulated ROS accumulation in roots and root hairs and that up-regulating RAC/ROP signaling in fer countered the mutant phenotypes. Taken together, these observations strongly support FER as an upstream regulator for the RAC/ROP-signaled pathway that controls ROS-mediated root hair development. Moreover, FER was pulled down by ROP2 GTPase in a guanine nucleotide-regulated manner implying a dynamic signaling complex involving FER, a ROPGEF, and a RAC/ROP.
Keywords: RAC/ROP, reactive oxygen species, ROPGEF, signal transduction, surface regulator
RHO GTPases are a large family of related monomeric GTP-binding proteins that serve diverse signaling functions (1). Plant RAC/ROPs form a unique clade of RHO-like GTPases and play important roles in regulating cell growth and polarity establishment, hormone- and stress-induced responses that underlie growth, development, reproduction, and interactions with the environment (2–4). Several RAC/ROP effectors have been identified (see Fig. S1A). These include a family of CRIB-domain proteins (RICs) and a coiled-coil domain protein (ICR1). RICs are known to mediate intracellular [Ca2+] and actin dynamics in polarized pollen tubes and coordinate actin and microtubule organization in leaf epidermal cells (2), whereas ICR1 regulates secretion and auxin distribution during early embryo development in Arabidopsis (5, 6). Interaction between RAC/ROPs and NADPH oxidase underlies activation of pathogen-elicited reactive oxygen species (ROS)-mediated defense response in rice (7). Upstream of RAC/ROPs, the hormones auxin and abscisic acid can activate and inactivate, respectively, these small GTPases, which in turn signal hormone-regulated responses (8–10). Given their involvement in diverse biological processes, RAC/ROPs may act as integration points for crosstalk between multiple signaling pathways (2).
Besides being mediators of stress-induced responses, increasing evidence shows that ROS also serve important regulatory functions in growth and development (11–15). In plants, multiple transmembrane spanning NADPH oxidases, homologous to the catalytic gp91phox subunit of the mammalian enzyme, have been found to underlie several RAC/ROP-mediated, ROS-regulated growth and stress-induced responses. In studies involving the Arabidopsis ROOT HAIR DEFECTIVE2 (RHD2/RBOHC) gene encoding a NADPH oxidase and SUPERCENTIPEDE, which encodes a RAC/ROP negative regulator, guanine nucleotide dissociation inhibitor (GDI) (see Fig. S1A), Dolan and colleagues provided compelling evidence for a RAC/ROP-mediated NADPH oxidase-dependent pathway for ROS-regulated root hair development (16, 17). Specifically, they showed that NADPH oxidase-derived ROS is required for polarized root hair growth and regulated RAC/ROP activity controls production and spatially regulated accumulation of ROS at the tip of emerging and elongating root hairs. Similarly, up-regulating RAC/ROP activity by overexpressing a constitutively active RAC/ROP in transgenic Arabidopsis seedlings also induced ectopic accumulation of NADPH oxidase-derived ROS and defective root hairs (18).
Although relying on guanine nucleotide exchange for activation is conserved for RAC/ROPs (Fig. S1A), their activation is largely dependent on a unique guanine exchange factor (GEF) family referred to as ROPGEFs (19–21). ROPGEFs have variable amino- and carboxyl-terminal domains and a conserved centrally located nucleotide exchange activity domain, which exclusively activates RAC/ROPs but not RHO GTPases from other organisms. Little is known about signaling components upstream of ROPGEFs. To date, two pollen-specific leucine-rich repeat receptor-like kinases (LRR RLKs) (22), the tomato LePRK2, and Arabidopsis AtPRK2a are the only surface receptors known to interact with ROPGEFs. These PRKs regulate RAC/ROP-mediated pollen tube growth, another polarized cell type in plant (23–25).
Of the hundreds of RLKs in Arabidopsis (26), several of the 17-membered Catharanthus roseus receptor-like kinase (RLK)-related family have been identified as regulators for various growth-related processes (27). Loss of function in one of these, THESEUS1 (THE1), suppressed the short hypocotyl phenotype in a cellulose synthase mutant, leading to the suggestion that it acts as a sensor for cell wall defects to signal growth inhibition (28). Although single mutations in THE1 and the related HERCULES1 (HERK1) did not induce noticeable phenotype, the1 herk1 double mutant showed inhibited growth (29). Reducing the level of another related RLK, FERONIA (FER), also resulted in growth inhibition and induced altered hormone responses (29, 30). FER was identified as a regulator for female fertility, mediating pollen tube rupture in the female gametophyte to discharge sperm for fertilization and preventing multiple tube entrance into an already fertilized ovule (31–33). The pollen-specific ANXUR1 and ANXUR2 are needed for pollen tube growth as double anx1 anx2 mutant pollen tubes rupture precociously and fail to reach the ovules for fertilization (34, 35). Together, these findings suggest that these RLKs play important roles in controlling cell growth and integrity, mediating consequences that could be as diagonally opposed as growth and nongrowth. Here, we show that FER is a ROPGEF-interacting RLK and regulates RAC/ROP-signaled and ROS-mediated root hair development, revealing a surface regulator for a signaling pathway that regulates ROS-mediated root hair growth and also broadly impacts growth and development.
Results
FER RLK Is a Broadly Expressed ROPGEF-Interacting RLK.
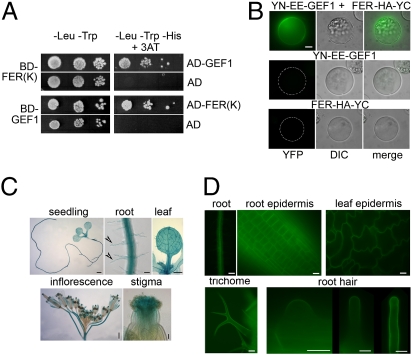
Using ROPGEF1 as bait in a yeast two-hybrid screen, we identified members of the Arabidopsis CrRLK family as potential interacting proteins for ROPGEFs (Fig. 1A and Fig. S1 B and C). Focusing on one of these, At3g51550, we used bimolecular fluorescence complementation (BiFC) assays (36) in protoplasts to confirm that this RLK also interacted with ROPGEF1 in plant cells (Fig. 1B). Given the importance of RAC/ROP signaling to polarized cell growth (15–18) and that root hairs are more accessible than pollen tubes for observation, we initially screened T-DNA insertion mutants in these RLKs for root hair defects (see SI Materials and Methods) and found that mutations in At3g51550, which turned out to be identical to FER when its sequence was elucidated (33), uniquely induced severe root hair defect (see below). Hence we adopt the FER nomenclature and the family as FER related. We also included in our studies siréne (srn), whose mutation is allelic with fer, the other originally described mutations, and inducing similar female gametophytic defects as fer (31–33).
Fig. 1.
FER is a broadly expressed ROPGEF-interacting RLK. (A) The kinase domain of FER [FER(K)] and ROPGEF1 (GEF1) interact in yeast two-hybrid assays, complementing histidine deficiency (First and Third rows). FER(K) interacts with several other GEFs and ROPGEF1 interacts with RAC/ROP (Fig. S1 B and C) (19, 20). (B) FER and ROPGEF1 interact in BiFC assays in protoplasts. YFP image for the interacting YN–EE–GEF1 and FER–HA–YC was acquired by autoexposure (Top); the same exposure condition was used for control protoplasts expressing one of the partners (Middle and Bottom). (Scale bar, 10 μm.) (C) pFER::GUS expression pattern in 10-d-old seedlings and roots and leaf from 10-d-old plants. Arrowheads, root hairs. [Scale bars, 1 mm (seedling and inflorescence), 50 μm (root and stigma), and 500 μm (leaf).] (D) FER–GFP localization in pFER::FER-GFP transformed Arabidopsis. [Scale bars, 100 μm (root), 50 μm (trichome), and 10 μm (root, leaf epidermis, and root hairs).]
FER promoter (pFER)::GUS analysis in transgenic Arabidopsis indicates that in addition to expression in the ovule (33), FER is broadly expressed (Fig. 1C) as previously shown by transcript analyses (33, 37). pFER expressed FER–GFP was localized to the plasma membrane of diverse cell types, including emerging and growing root hairs (Fig. 1D).
FER Is Essential for Root Hair Development.
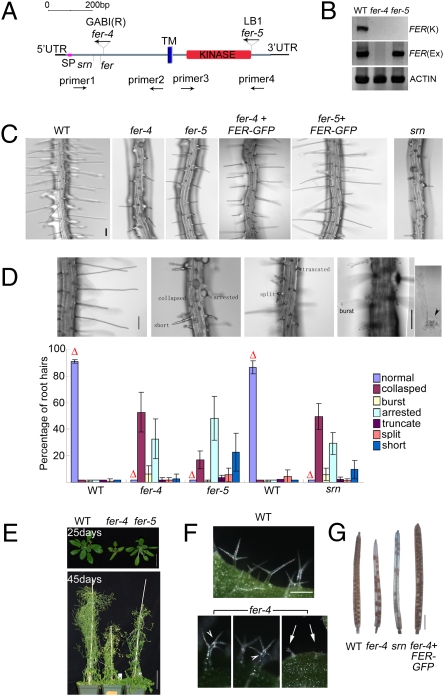
We identified two T-DNA–induced alleles, fer-4 and fer-5 (Fig. 2A and Fig. S2A). The originally described fer and srn are both in the vicinity of fer-4 (33) (Fig. 2A). RT-PCR analysis showed that fer-4, with its T-DNA insertion in the extracellular domain-coding region, is a null mutant, whereas fer-5 harbors truncated transcripts (Fig. 2B), consistent with its T-DNA insert being closed to the end of its kinase domain-coding region of the gene. fer-4 and srn showed indistinguishable and multiple phenotypes, whereas fer-5’s defects were milder and more restricted (see below).
Fig. 2.
Mutations in FER induce root hair and other vegetative phenotypes. (A) Domain map for FER. Locations of the T-DNA insertion alleles fer-4 (GABI_GK106A06) and fer-5 (Salk_ 029056c), fer, and srn (31–33) and primers used in PCR analyses are indicated. SP, signal peptide; TM, transmembrane domain; UTR, untranslated region. Mature FER is ∼807 aa long. Genomic DNA analysis for fer-4 and fer-5 is shown in Fig. S2A. (B) RT-PCR analysis for FER expression. FER(K), PCR used primers 3 and 4; FER(Ex), PCR used primers 1 and 2. (C) Root hairs from 4-d-old wild-type (WT), fer-4, fer-5 and srn, and pFER::FER-GFP complemented fer-4, -5 seedlings. (Scale bar, 100 μm.) See Fig. S2B for FER–GFP expression in the complemented fer seedlings. (D) fer mutations induce a broad spectrum of root hair defects. Top shows normal root hairs (Left) and fer mutation-induced collapsed (the most severe), burst [seen with discharged cytoplasm (arrowhead)], arrested, truncated, split, and short (the least severe) root hairs. (Scale bar, 100 μm.) The histogram shows distribution of the different classes of root hairs in WT and mutant seedlings. Each data bar represents the mean ± SD where n = 1,200 root hairs from 15–24 four-day-old seedlings. Δs highlight normal root hairs from each sample. (E) fer-induced growth suppression. (Scale bars, 10 mm (25 d) and 50 mm (45 d.) (F) fer-4 induces trichome defects. Arrows, collapsed; arrowheads, more than three branches. Approximately 30% of trichomes in fer-4 seedlings were normal compared with >90% in WT (Fig. S2C). (G) fer-4 and srn show similar reproductive phenotype. (Scale bar, 2 mm.)
Consistent with the high expression level of FER in root hairs (37) (Fig. 1C), fer-4, fer-5, and srn showed readily observable root hair defects that ranged from collapsed, burst, to short (Fig. 2 C and D). Although the level of normal root hairs was similarly low, the majority of fer-4 and srn root hairs collapsed shortly after emergence, whereas many of the fer-5 root hairs were shorter than wild type (WT) or burst after some growth was achieved (Fig. 2 C and D). fer-5 was negligibly affected in growth and development, whereas fer-4 showed significantly reduced stature, developed severe trichome defects (Fig. 2 E and F and Fig. S2C), and reproductively mimicked fer and srn (Fig.2G). The root hair defects in fer-4, -5 were complemented by pFER::FER-GFP (Fig. 2C and Fig. S2B), as were other growth and reproduction phenotypes in fer-4 (e.g., Fig. 2G). Together these observations imply that FER’s role in growth and development is considerably broader than regulating female gametophytic functions (31–33). Although kinase-deleted and extracellular domain-located mutations in some RLKs act in a dominant negative manner (38, 39), the fact that the T-DNA insert is at the end of the kinase domain and that heterozygous fer-5 plants were nondistinguishable from wild-type plants suggest fer-5 is more likely to have lost some aspects of FER’s multiple functions that rely on an intact C-terminal region, whereas the null mutants fer-4, srn, and fer have lost all of FER’s functions.
FER Functions in a RAC/ROP Signaling Pathway and Mediates Auxin-Regulated Root Hair Development.
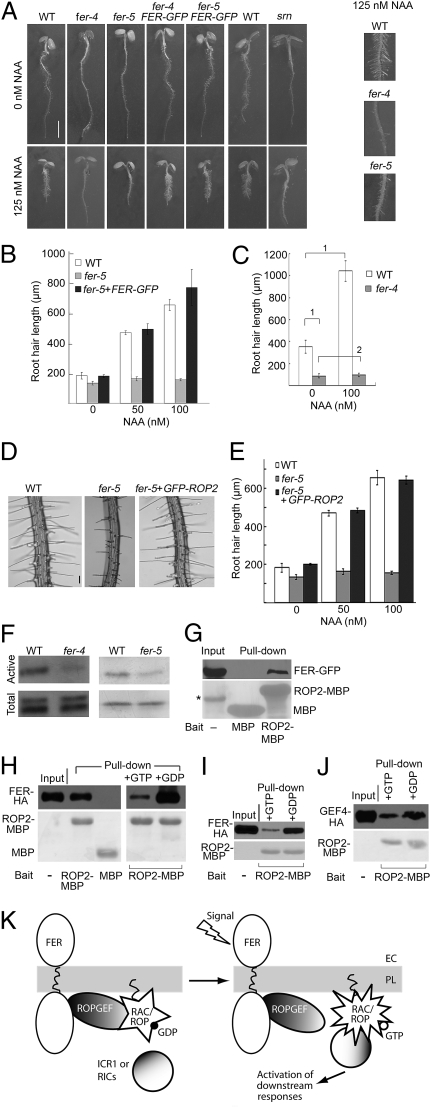
It is well established that auxin plays an important role in regulating root hair development (e.g., refs. 40, 41) and we showed earlier that RAC/ROPs mediate auxin-signaled gene derepression via 26S proteasome-mediated repressor proteolysis (9, 10). We therefore asked whether FER functions in a RAC/ROP signaling pathway and also acts as a regulator for auxin-regulated root hair development. We observed that when grown in the presence of auxin, differences between WT and fer root hairs were further amplified because root hair elongation in WT seedlings was stimulated by auxin, whereas fer-4, fer-5, and srn root hairs failed to respond and remained short (Fig. 3 A–C and Fig. S2D). pFER::FER-GFP complemented this phenotype, restoring auxin-stimulated root hair growth in fer seedlings (Fig. 3 A and B).
Fig. 3.
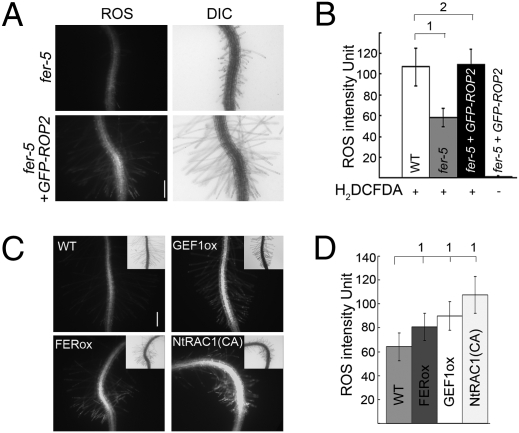
fer mutants show reduced auxin and RAC/ROP signaling capacity. (A) Four-day-old WT, mutant, and pFER::FER-GFP complemented mutant seedlings grown under standard (0 nM NAA) or auxin-supplemented conditions. Auxin-treated WT and fer-4, -5 mutants are shown magnified on the Right. (Scale bar, 2 mm.) (B) Root hair length comparison between WT, fer-5, and complemented fer-5 under standard and auxin-supplement growth conditions. Data bars represent average of the mean root hair lengths from triplicate samples ± SE. For each replicate, 300 root hairs from at least nine plants were measured. (C) Root hair length comparison between WT and fer-4 (Fig. S2D shows data for srn). (D and E) Overexpression of GFP–ROP2 rescued fer-5–induced root hair morphological defects (D), restored root hair elongation (E, compare white and black bars), and auxin-stimulated root hair elongation (E, compare 0, 50, and 100 nM NAA data bars). See Fig. S3 for genotyping of these rescued plants. (Scale bar, 100 μm.) (F) Pulldown of active RAC/ROPs. ICR1–MBP was used for fer-4; PBD–GST (8, 9) was used for fer-5. Anti-NtRac1 antibody (9) was used for detection. The fer-4 blot was visualized by chemiluminescence, the fer-5 blot by alkaline phosphatase reaction. Quantification of data is shown in Fig. S2E. (G–J) ROP2–MBP pulldown of pFER-expressed FER–GFP from microsomal proteins from transformed seedlings (G), of FER–HA expressed in transiently transfected mesophyll (H) and root (I) protoplasts, and of GEF4–HA expressed in mesophyll protoplasts (J). Lower panels show input bait proteins on Ponçeau-stained blots. Anti-GFP (G) and anti-HA (H–J) were used for detection. *band in G was unrelated to the experiment. (K) Sketch showing guanine nucleotide-regulated FER–RAC/ROP interaction as suggested by results shown in G–J. Presumably, in vivo, signal activation-induced changes in the interacting ROPGEF and RAC/ROP would weaken the GEF–RAC/ROP interaction, recycling the ROPGEF, and releasing activated RAC/ROP to interact with effectors for downstream signaling. Data for WT, fer-5, fer5 + FER–GFP and fer5 + GFP–ROP2 shown in B and E were collected in the same experiments thus the data for WT and fer-5 are used in both panels. Mutant root hair data are overestimations because only hairs with measurable lengths were included in the data set. Brackets and the numbers 1 and 2 in C denote statistical comparisons (1, significant; 2, insignificant) as described in Materials and Methods; these differences are representative for data shown in B and E.
To establish that FER indeed acted upstream of RAC/ROPs, we examined whether increasing RAC/ROP signaling capacity by overexpressing GFP–ROP2 may counteract the root hair defects in fer-4 and fer-5. Indeed fer-5 root hairs were restored to normal, including ability to respond to auxin stimulation (Fig. 3 D and E and see also Fig. S3), consistent with reduced RAC/ROP signaling as underlying the fer-induced root hair defects under normal growth conditions and decreased sensitivity to auxin stimulation of root hair growth. However, a rescued fer-4 was not recovered. Possibly, the higher level of RAC/ROP overexpression required to counteract fer-4’s deficiency could have prevented transgenic plant regeneration. Alternatively, and more pertinent to FER’s multiple functionality as suggested by the multiple phenotypes induced by fer-4 and other null or knockdown mutations (29–33), it is possible that fer-5 is defective only in FER’s RAC/ROP-interacting capacity, whereas fer-4 lacks all of FER’s functions, including those potentially requiring an intact extracellular domain.
To substantiate the FER and RAC/ROP signaling linkage on a biochemical level, we used a functional pulldown assay that specifically targets activated RAC/ROPs (5, 8, 9). Indeed, the level of activated RAC/ROPs was reduced in fer-4, -5 seedlings relative to WT (Fig. 3F and Fig. S2E) consistent with FER being necessary for RAC/ROP activation thus working as an upstream regulator for these small GTPases. Moreover, ROP2 maltose-binding protein (MBP) efficiently pulled down pFER-expressed FER–GFP from seedling microsomal proteins or when FER–HA was overexpressed in mesophyll or root protoplasts (Fig. 3 G–I), suggesting complex formation via their mutual interaction with ROPGEFs, several of which are known to interact with FER (Fig. S1B).
It is known that most ROPGEFs preferentially bind to GDP-bound RAC/ROPs versus the GTP-bound activated form (19, 20), presumably upon nucleotide exchange stimulated by upstream signaling, the activated GTPases would be released to interact with downstream effectors. Therefore the presence of a signaling complex involving an upstream regulator, a ROPGEF and a RAC/ROP should exist but would likely be transient in vivo (see Fig. 3K). To substantiate that the observed pulldown of FER by RAC/ROPs (Fig. 3 G and H Left) was functionally relevant, we included either GDP or GTP in the pulldown reaction to determine whether these guanine nucleotides differentially affect the pulldown efficiency. GDP and GTP would shift RAC/ROPs to the inactive or activated state, respectively, mimicking the action of these guanine nucleotides under physiological conditions. Indeed, pulldown of FER–HA was substantially enhanced by GDP but reduced by GTP (Fig. 3 H Right and I), consistent with the observation that most ROPGEFs (e.g., ROPGEF4, Fig. 3J) are preferentially pulled down by GDP-bound RAC/ROPs but not as efficiently by the GTP-bound form (19, 20). Together with observations that ROPGEFs interact with FER (Fig. 1 A and B and Fig. S1B) and with RAC/ROPs (19, 20; Fig. S1C), results from these pulldown assays are consistent with a dynamic signaling complex involving interactions between FER, a ROPGEF, and a RAC/ROP, mediating nucleotide exchange and activating the small GTPases (Fig. 3J).
FER Regulates RAC/ROP-Mediated NADPH Oxidase-Dependent ROS Accumulation in Root and Root Hairs.
The root hair defects in fer mutants closely resemble those seen in rhd2 (Fig. S4). Together with the FER, RAC/ROP signaling linkage (Fig. 3) and the RAC/ROP linkage to NADPH oxidase-dependent ROS-mediated root hair development (15–18), it seemed highly plausible that FER acted upstream of the RAC/ROP-regulated pathway. We therefore examined whether fer mutants were compromised in RAC/ROP-regulated ROS accumulation.
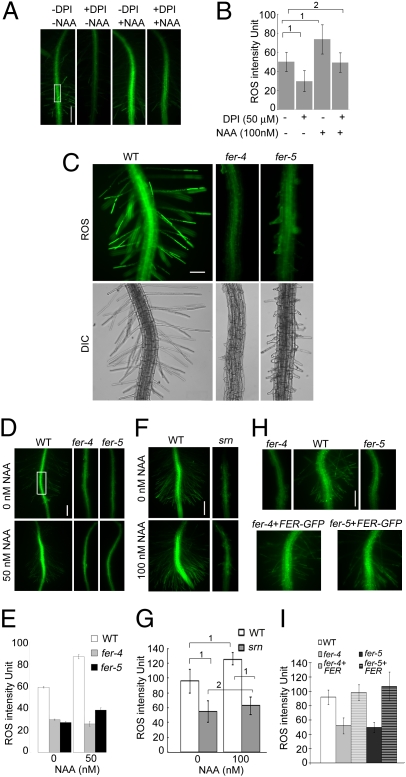
Roots and root hairs are known to show auxin-regulated diphenylene iodonium (DPI)-sensitive NADPH oxidase-dependent ROS accumulation and growth (e.g., refs. 13, 16, 17, 41, 42; Fig. 4 A and B; Fig. S5A). When treated with H2DCF–DA, a commonly used ROS-detection stain, fer root and root hairs showed that they accumulated significantly lower levels of ROS relative to WT (Fig. 4 C and D–G, 0 nM NAA) and they were also nonresponsive to auxin-stimulated ROS accumulation (Fig. 4 D–G, NAA-treated samples). Like the root hair morphological defects, the reduced ROS phenotype in fer was complemented by pFER::FER-GFP (Fig. 4 H and I) and countered by overexpressing ROP2–GFP (Fig. 5 A and B and see also Fig. S5B and Fig. S6 A and B). The corollary experiment of overexpressing RAC/ROPs, ROPGEF1, or FER also resulted in increased ROS accumulation in transgenic seedling roots (Fig. 5 C and D and Fig. S6 C and D), further supporting that FER, ROPGEFs, and RAC/ROPs act in a common pathway that controls NADPH oxidase-dependent ROS production.
Fig. 4.
FER regulates NADPH dependent- and auxin-regulated ROS production in root and root hairs. (A and B) ROS accumulation and auxin-stimulated ROS production in Arabidopsis primary root. DPI inhibition reflects NADPH oxidase-dependent ROS production (see e.g., refs. 16, 17, 41). Seedlings were treated with H2DCF–DA to monitor ROS levels (see SI Materials and Methods). The rectangle in A indicates a representative region of interest (ROI) where average root ROS intensity was quantified for this and other samples; comparative data are shown in B. All images were acquired using the control (−DPI, −NAA) image acquisition condition. Each data bar in the histogram represents the mean ± SD of ROS intensity measured from nine roots in one representative experiment. All other experiments involving root ROS intensity comparison (D–I; Fig. 5) follow the same sampling and analysis methods. (C) ROS in WT, fer-4 and fer-5 root and root hairs. The WT ROS image was acquired by autoexposure; all other images were acquired using the WT exposure conditions. (Scale bar, 100 μm.) (D–I) ROS in the primary roots of WT, fer-4, -5 (D and E), srn (F and G), and pFER::FER-GFP complemented fer-4, -5 seedlings [H and I; under the ROS imaging conditions, FER–GFP signal was negligible (see Fig. S5B)] in normal and auxin-treated growth conditions. (E, G, and I) ROS level quantification at representative ROIs. (Scale bars, 100 μm.) 1, significant; 2, insignificant differences. Statistical differences shown in G are representative for data shown in E and I, except for fer-5’s weak but significant response to NAA (E, P = 0.002376), reflecting its weaker phenotype.
Fig. 5.
FER and RAC/ROPs function in a common pathway regulating ROS production. (A and B) GFP–ROP2 restores ROS accumulation in fer-5 roots and root hairs. Image acquisition and quantitative analyses were as described in Fig. 4. ROS images (A) were acquired using exposure conditions for a H2DCF–DA-treated WT control under which GFP–ROP2 signal was negligible (fourth data bar in B; see also Fig. S5B). (Scale bar, 200 μm.) Note also the root hairs in the GFP–ROP2-expressing fer-4 seedlings. (B) Quantified comparative ROS levels. Root hair ROS was also quantified using dihydroethidium for detection (54) (Fig. S6 A and B). (C and D) Overexpression of CaMV35S-driven FER–HA (Fig.S6C), ROPGEF1 or NtRAC1(CA) (9) augments ROS production in roots and root hairs. Insets in C show DIC images. Quantified comparative ROS levels are shown in D. These transgenic plants did not show readily noticeable phenotypes other than mild root hair depolarization (e.g., as seen in GEF1ox seedling shown here), reflecting increased Rac/Rop signaling; their root hair lengths were within the range seen in control seedlings. (Scale bar in C, 200 μm.) 1, significant; 2, insignificant differences.
Discussion
In showing that FER regulates RAC/ROP-signaled NADPH oxidase-dependent ROS-mediated root hair growth, our results reveal a surface regulator for a well-established signaling pathway that is not only important for the growth of these polarized cells but also broadly implicated in growth, development, and stress management (11–15). That FER is broadly expressed (Fig. 1) and loss-of-function fer mutants are pleiotropic and severely growth suppressed (Figs. 2–4) suggest FER plays an important and general role in growth promotion throughout development. The presence of ROS in the growing regions of vegetative tissues and that inhibiting NADPH oxidase-dependent ROS production correlates with impaired growth (e.g., refs. 14, 16, 42) are consistent with the suggestion that FER targets the NADPH oxidase-regulated pathway to promote growth in a broad range of cell types. The fact that ROS both promote and repress growth suggests the functional involvement of ROS in cell growth is complex. Using the diversely functioning RAC/ROPs as regulators for NADPH oxidase would be a versatile strategy that could maximize input regulation of ROS production and output modulation of ROS-mediated responses. Discovery of FER as an upstream regulator for ROS-regulated growth, potentially in a large number of cell types, opens the way to dissect how ROS-mediated signaling may be regulated to meet their roles under diverse conditions. On the other hand, given the multiple downstream pathways that RAC/ROPs are known to regulate (2–6), it is also possible that FER activates other RAC/ROP effectors to control additional target systems, in different or even the same cell types. The fact that FER is required for pollen tube rupture inside the female gametophyte (31–33) suggests cell death, albeit noncell autonomously, as the ultimate target. Therefore, a complete dissection of FER functions throughout growth and development will be needed to fully establish the potentially multiple signal-response pathways that the broadly expressed FER is likely to mediate.
Establishing the FER to RAC/ROP signaling linkage suggests members of the broader FER-like RLK family (27) may also act as surface regulators for signal relay to regulate these molecular switches. Thus far, four other Arabidopsis FER-like RLKs have been implicated in regulating growth, reproduction, and stress-related processes (28, 29, 34, 35). These seemingly diverse pathways may nonetheless share certain functional themes. For instance, with the well-established role of apoplastic ROS in controlling wall stiffness and extensibility (14, 43–45), it is conceivable that FER would mediate a ROS environment that weakens the pollen tube wall, whereas ANX1 and ANX2 would do so to maintain its integrity. Given the role of NADPH oxidase-derived ROS in secondary cell wall synthesis and that RAC/ROPs have been implicated as regulating this process (46), it is conceivable for ROS to be involved in the THE1-mediated cell wall integrity-dependent growth regulatory process. Taken together, available information on these FER-related RLKs suggests that the functional roles for at least a subset of these receptors may be induced by or impact cell wall integrity and that ROS, with their ability to support cell expansion and induce wall stiffness, could be central to the processes that these RLKs mediate.
Defined signal-receptor linkages for RAC/ROP-regulated pathways remain to be established. The observation of an α-subunit of a heterotrimeric G protein acting upstream of RAC/ROP-mediated pathogen-induced responses in rice (47) implies that members of the seven transmembrane-spanning receptor family could serve as RAC/ROP surface regulators and pathogen-derived elicitor ligands for these receptors. Two cysteine-rich proteins, one pollen and the other stigma produced, interact with LePRK2 (48, 49) and a 3-kDa stylar-secreted peptide specifically dephosphorylates LePRK2 and stimulates pollen tube growth (25, 50). These candidate ligands for LePRK2 could modulate pollen functions differentially before and upon landing on the stigmatic surface and during its growth in the pistil. Elucidating the ligands for FER in root hair development will fill a critical connection to signals that stimulate RAC/ROP regulation of ROS-mediated growth. Multiple hormonal and nutrient factors are known to affect root hair development (e.g., refs. 40, 41, 51, 52). The observations that fer mutants were altered in brassinosteroid and ethylene responses (30) and deficient in auxin-stimulated ROS production and root hair elongation (Figs. 3 and 4) are consistent with the notion that FER-regulated RAC/ROP signaling and auxin signaling may somehow intersect. The identification of FER as a surface regulator for ROS-mediated root hair elongation in this study not only provides a critical discovery, filling in the gap to upstream signal mediation for a well-established RAC/ROP signaling pathway, it also provides a portal to discover surface regulatory mechanisms that link a broad range of signals to multiple cellular responses.
Materials and Methods
Plant growth and transformation, protoplast transfection, molecular and biochemical methods followed standard procedures (see SI Materials and Methods). Arabidopsis thaliana Col-0 was used as control for fer-4, -5 and C24 as control for srn, respectively. Chimeric genes are described in SI Materials and Methods.
Protein–Protein Interaction Analysis.
ROPGEF1 was used as bait in yeast two-hybrid to screen a 3-d-old etiolated Arabidopsis seedling cDNA library (CD4-22 from the Arabidopsis Biological Resource Center). BiFC (36) was carried out in transfected root protoplasts using 5–10 μg of one (controls) or both of the split YFP fused with ROPGEF1or FER.
Root Hair Analysis.
Root hairs located between 1.5 and 3.5 mm from the primary root tip of 4-d-old seedlings were observed microscopically (see SI Materials and Methods). Image J was used to measure the length of root hairs observed in the same focal plane. ROS were visualized in the primary root and root hairs by H2DCF–DA (2′,7′-dichlorodihydro-fluorescein diacetate, Sigma) (16, 17, 42, 53) and the superoxide-detecting dihydroethidium (DHE) as an alternate dye (see SI Materials and Methods) (54). DPI, a commonly used inhibitor of membrane-associated NADPH oxidase and other flavin-containing enzymes was used (50 μM) to differentiate NADPH oxidase produced ROS from those generated from other sources (e.g., refs. 16, 17, 42, 53). ROS were imaged using a FITC filter (Ex460-500, DM505, Em510-560) for H2DCF–DA signal or a rhodamine (Ex546/10, DM565LP, Em 590LP filter for dihydroethidium signal). ROS fluorescence intensity within a fixed region of interest (ROI) was quantified using Adobe Photoshop v.7.0. Experiments were repeated at least three times. Student’s t-tests were used for statistical analyses. Pairwise data comparisons in Figs. 3–5 are indicated by brackets above the data bars; 1 denotes significant (P < 0.05), 2 denotes insignificant (P > 0.05) difference. Statistics details are shown in SI Materials and Methods.
Pulldown Assays.
Pulldown assays for activated RAC/ROPs in 7-d-old WT and fer seedling roots used ICR1 (5)–MBP or PBD–GST (refs. 8, 9 and SI Materials and Methods) as bait, based on the rationale that direct effectors of RHO GTPases specifically bind to their activated form. ROP2–MBP was used to pull down pFER-expressed FER–GFP from seedlings, 35S-expressed FER–HA and ROPGEF4–HA from mesophyll and root protoplasts. When guanine nucleotides were included in these assays, ROP2–MBP resin was pretreated with 10 mM GTP or 10 mM GDP for 2 h and the pulldown assays were carried out with 1 mM GTP or 10 mM GDP in the buffer. Details are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. F. Berger (National University of Singapore) for srn seeds and Dr. J.-S. Lee (Ewha Womans University, Korea) for ROS detection protocol. We thank Dr. L. Tao for generating the Arabidopsis 35S-NtRac1(CA) transgenic plants. We thank a reviewer for suggesting DHE as an alternate dye for ROS detection. This work was supported by National Science Foundation Grant IOB0544222 and US Department of Agriculture Grant CSREES 2004-35304-14837.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 17461.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005366107/-/DCSupplemental.
References
- 1.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 2.Nibau C, Wu H-M, Cheung AY. RAC/ROP GTPases: ‘Hubs’ for signal integration and diversification in plants. Trends Plant Sci. 2006;11:309–315. doi: 10.1016/j.tplants.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Fu Y. ROP/RAC GTPase signaling. Curr Opin Plant Biol. 2007;10:490–494. doi: 10.1016/j.pbi.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kost B. Spatial control of Rho (Rac-Rop) signaling in tip-growing plant cells. Trends Cell Biol. 2008;18:119–127. doi: 10.1016/j.tcb.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Lavy M, et al. A Novel ROP/RAC effector links cell polarity, root-meristem maintenance, and vesicle trafficking. Curr Biol. 2007;17:947–952. doi: 10.1016/j.cub.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 6.Hazek O, et al. A rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol. 2010;8:e1000281. doi: 10.1371/journal.pbio.1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong HL, et al. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell. 2007;19:4022–4034. doi: 10.1105/tpc.107.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemichez E, et al. Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 2001;15:1808–1816. doi: 10.1101/gad.900401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tao LZ, Cheung AY, Wu HM. Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell. 2002;14:2745–2760. doi: 10.1105/tpc.006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tao LZ, Cheung AY, Nibau C, Wu HM. RAC GTPases in tobacco and Arabidopsis mediate auxin-induced formation of proteolytically active nuclear protein bodies that contain AUX/IAA proteins. Plant Cell. 2005;17:2369–2383. doi: 10.1105/tpc.105.032987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner E. GTPases and reactive oxygen species: Switches for killing and signaling. J Cell Sci. 2004;117:143–153. doi: 10.1242/jcs.00937. [DOI] [PubMed] [Google Scholar]
- 12.Torres MA. ROS in biotic interactions. Physiol Plant. 2010;138:414–429. doi: 10.1111/j.1399-3054.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 13.Jaspers P, Kangasjärvi J. Reactive oxygen species in abiotic stress signaling. Physiol Plant. 2010;138:405–413. doi: 10.1111/j.1399-3054.2009.01321.x. [DOI] [PubMed] [Google Scholar]
- 14.Swanson S, Gilroy S. ROS in plant development. Physiol Plant. 2010;138:384–392. doi: 10.1111/j.1399-3054.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- 15.Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: Lessons from root hairs. J Exp Bot. 2006;57:1829–1834. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- 16.Foreman J, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 17.Carol RJ, et al. A RhoGDP dissociation inhibitor spatially regulates growth in root hair cells. Nature. 2005;438:1013–1016. doi: 10.1038/nature04198. [DOI] [PubMed] [Google Scholar]
- 18.Jones MA, Raymond MJ, Yang Z, Smirnoff N. NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot. 2007;58:1261–1270. doi: 10.1093/jxb/erl279. [DOI] [PubMed] [Google Scholar]
- 19.Berken A, Thomas C, Wittinghofer A. A new family of RhoGEFs activates the Rop molecular switch in plants. Nature. 2005;436:1176–1180. doi: 10.1038/nature03883. [DOI] [PubMed] [Google Scholar]
- 20.Gu Y, Li S, Lord EM, Yang Z. Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control Rho GTPase-dependent polar growth. Plant Cell. 2006;18:366–381. doi: 10.1105/tpc.105.036434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu D, Le J, Zakharova T, Mallery EL, Szymanski DB. A SPIKE1 signaling complex controls actin-dependent cell morphogenesis through the heteromeric WAVE and ARP2/3 complexes. Proc Natl Acad Sci USA. 2008;105:4044–4049. doi: 10.1073/pnas.0710294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torii KU. Leucine-rich repeat receptor kinases in plants: Structure, function, and signal transduction pathways. Int Rev Cytol. 2004;234:1–46. doi: 10.1016/S0074-7696(04)34001-5. [DOI] [PubMed] [Google Scholar]
- 23.Kaothien P, et al. Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J. 2005;42:492–503. doi: 10.1111/j.1365-313X.2005.02388.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, McCormick S. A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2007;104:18830–18835. doi: 10.1073/pnas.0705874104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang D, et al. The pollen receptor kinase LePRK2 mediates growth-promoting signals and positively regulates pollen germination and tube growth. Plant Physiol. 2008;148:1368–1379. doi: 10.1104/pp.108.124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiu S-H, Bleecker AB. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003;132:530–543. doi: 10.1104/pp.103.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hématy K, Höfte H. Novel receptor kinases involved in growth regulation. Curr Opin Plant Biol. 2008;11:321–328. doi: 10.1016/j.pbi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Hématy K, et al. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol. 2007;17:922–931. doi: 10.1016/j.cub.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Guo H, et al. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc Natl Acad Sci USA. 2009;106:7648–7653. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant. 2010;3:626–640. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]
- 31.Rotman N, et al. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr Biol. 2003;13:432–436. doi: 10.1016/s0960-9822(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 32.Huck N, Moore JM, Federer M, Grossniklaus U. The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development. 2003;130:2149–2159. doi: 10.1242/dev.00458. [DOI] [PubMed] [Google Scholar]
- 33.Escobar-Restrepo JM, et al. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki S, et al. ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr Biol. 2009;19:1327–1331. doi: 10.1016/j.cub.2009.06.064. [DOI] [PubMed] [Google Scholar]
- 35.Boisson-Dernier A, et al. Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development. 2009;136:3279–3288. doi: 10.1242/dev.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerppola TK. Bimolecular fluorescence complementation: Visualization of molecular interactions in living cells. Methods Cell Biol. 2008;85:431–470. doi: 10.1016/S0091-679X(08)85019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 2004;136:2621–2632. doi: 10.1104/pp.104.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diévart A, et al. CLAVATA1 dominant-negative alleles reveal functional overlap between multiple receptor kinases that regulate meristem and organ development. Plant Cell. 2003;15:1198–1211. doi: 10.1105/tpc.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shpak ED, Lakeman MB, Torii KU. Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. Plant Cell. 2003;15:1095–1110. doi: 10.1105/tpc.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones AR, et al. Auxin transport through non-hair cells sustains root-hair development. Nat Cell Biol. 2009;11:78–84. doi: 10.1038/ncb1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pitts RJ, Cernac A, Estelle M. Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J. 1998;16:553–560. doi: 10.1046/j.1365-313x.1998.00321.x. [DOI] [PubMed] [Google Scholar]
- 42.Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001;126:1055–1060. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knight MR. New ideas on root hair growth appear from the flanks. Proc Natl Acad Sci USA. 2007;104:20649–20650. doi: 10.1073/pnas.0710632105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macpherson N, et al. NADPH oxidase involvement in cellular integrity. Planta. 2008;227:1415–1418. doi: 10.1007/s00425-008-0716-2. [DOI] [PubMed] [Google Scholar]
- 46.Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A. The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol. 1999;119:849–858. doi: 10.1104/pp.119.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suharsono U, et al. The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA. 2002;99:13307–13312. doi: 10.1073/pnas.192244099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang W, Kelley D, Ezcurra I, Cotter R, McCormick S. LeSTIG1, an extracellular binding partner for the pollen receptor kinases LePRK1 and LePRK2, promotes pollen tube growth in vitro. Plant J. 2004;39:343–353. doi: 10.1111/j.1365-313X.2004.02139.x. [DOI] [PubMed] [Google Scholar]
- 49.Tang W, Ezcurra I, Muschietti J, McCormick S. A cysteine-rich extracellular protein, LAT52, interacts with the extracellular domain of the pollen receptor kinase LePRK2. Plant Cell. 2002;14:2277–2287. doi: 10.1105/tpc.003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wengier DL, Mazzella MA, Salem TM, McCormick S, Muschietti JP. STIL, a peculiar molecule from styles, specifically dephosphorylates the pollen receptor kinase LePRK2 and stimulates pollen tube growth in vitro. BMC Plant Biol. 2010;10:33. doi: 10.1186/1471-2229-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang YJ, Lynch JP, Brown KM. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. J Exp Bot. 2003;54:2351–2361. doi: 10.1093/jxb/erg250. [DOI] [PubMed] [Google Scholar]
- 52.Perry P, Linke B, Schmidt W. Reprogramming of root epidermal cells in response to nutrient deficiency. Biochem Soc Trans. 2007;35:161–163. doi: 10.1042/BST0350161. [DOI] [PubMed] [Google Scholar]
- 53.Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bucana C, Saiki I, Nayar R. Uptake and accumulation of the vital dye hydroethidine in neoplastic cells. J Histochem Cytochem. 1986;34:1109–1115. doi: 10.1177/34.9.2426339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.