Abstract
The α-, β-, and γ-protocadherins (Pcdhα, Pcdhβ, and Pcdhγ) comprise a large family of single-pass transmembrane proteins predominantly expressed in the nervous system. These proteins contain six cadherin-like extracellular domains, and proteolysis of Pcdhα and Pcdhγ by the γ-secretase complex releases their intracellular domains into the cytoplasm where they may function locally and/or enter the nucleus and affect gene expression. Thus, cleavage of Pcdhs may function to link intercellular contacts and intracellular signaling. Here we report that shedding of the Pcdhα extracellular domain and subsequent processing by γ-secretase require endocytosis and that Pcdhs interact with the regulator of vesicular sorting ESCRT-0 in undifferentiated cells. We also find that the accumulation of Pcdh cleavage products is regulated during development. Differentiation leads to an increase in the interactions between Pcdh proteins and a decrease in the accumulation of cleavage products. We conclude that Pcdh processing requires endocytosis and that the level of cleavage products is regulated during neuronal differentiation.
Keywords: γ-secretase, hepatocyte growth factor-regulated tyrosine kinase substrate, neurodevelopment, protein–protein interactions, proteolysis
Members of the cadherin superfamily of proteins play critical roles in intercellular adhesion and cell–cell communication. The clustered protocadherins (Pcdhs) are a subgroup of this superfamily that are predominantly expressed in the nervous system, and Pcdh genes are organized into three large clusters spanning nearly 1 Mb of genomic DNA (1, 2). On the basis of the unusual organization and combinatorial expression of Pcdh genes, and the cell surface localization of Pcdh proteins, the Pcdhs have been proposed to function in the specification of neuronal interactions in the brain (1, 2). There are >50 distinct Pcdh isoforms encoded by three closely linked gene clusters named Pcdhα, Pcdhβ, and Pcdhγ (2). Individual neurons stochastically express distinct combinations of Pcdh isoforms, thus generating enormous cell surface diversity that may contribute to the specificity of synaptic interactions (3, 4).
Individual Pcdh proteins consist of six cadherin-like ectodomains (ECs), a single transmembrane (TM) domain, and a C-terminal intracellular domain (ICD). For each isoform, a single large exon (termed a variable or V exon) encodes the six ECs, the TM domain, and a short stretch of the ICD. Each Pcdhβ protein is entirely encoded by a single V exon. In the case of Pcdhα and Pcdhγ, an individual V exon is spliced to three subtype-specific constant (Con) exons that encode the remainder of the Pcdhα or Pcdhγ ICD (5, 6). Therefore, the majority of the ICD is identical in all Pcdhα isoforms or in all Pcdhγ isoforms.
Despite their potential roles in synaptic interactions, the functions of the clustered Pcdhs are not well understood. Their extracellular domains have the capacity to interact with each other both homophilically and heterophilically (7–9). However, their interactions are much weaker than those of the classic cadherins such as N-cadherin (N-Cad) (10). Thus, it seems likely that Pcdhs facilitate communication between neighboring cells rather than function as adhesion molecules.
Mutations in the Pcdhγ gene cluster have been shown to negatively influence synapse formation and lead to increased death of interneurons in the spinal cord and retina (11–13). Depending on the cellular context, mutations in the Pcdhγ gene cluster display both cell autonomous and non-cell–autonomous effects (11, 14). Mutations in the Pcdhα gene cluster do not appear to affect cell viability or synapse number, but have been reported to disrupt the formation of proper glomeruli in the olfactory bulb and the innervation of target areas by serotonergic neurons (15, 16). In addition, both Pcdhα and -γ have been shown to bind to each other and the tyrosine kinases PYK2, FAK (17), and Ret (18) and to a variety of other signaling molecules. Thus, Pcdhs function, at least in part, by linking intercellular contacts to intracellular signaling pathways.
The potential role of clustered Pcdh proteins in intracellular signaling is further supported by the finding that Pcdhα, Pcdhβ, and Pcdhγ are proteolytically cleaved by the γ-secretase complex. This cleavage produces soluble intracellular fragments that have the potential to enter the nucleus (8, 19–21). These cleavage fragments may directly or indirectly affect gene expression, similar to other γ-secretase substrates such as Notch and N-cadherin (22, 23). Proteolytic processing of Pcdhs proceeds in two steps, a feature common to all γ-secretase substrates. First, the majority of the Pcdhα or -γ extracellular domains are removed to generate a transmembrane C-terminal fragment (CTF1). In at least some cases, Pcdhα and -γ CTF1s are produced by the metalloproteinase ADAM10 (a disintegrin and metalloprotease 10) (8, 19). CTF1 then undergoes a second round of endoproteolysis, this time by presenilin, the catalytic subunit of γ-secretase. Presenilin cleaves CTF1 within the transmembrane domain or at the inner membrane surface to produce a soluble fragment called CTF2. CTF2 is most abundant in the cytoplasm, but is also detected in the nucleus (19, 21). For almost all γ-secretase substrates, the shedding of the extracellular domain is a prerequisite for intramembrane cleavage (24). The mechanisms that control Pcdh cleavage are not known.
Here we show that the cleavage of Pcdhs is tightly linked to subcellular trafficking of the proteins. In undifferentiated cells, endocytosis is required for the shedding of the Pcdhα extracellular domain. Differentiation is accompanied by a redistribution of the protein and a decrease in binding to endosomal proteins. We also show that the steady-state levels of protocadherin cleavage products decrease during the development of the nervous system and during differentiation of neuron-like cells in culture. Differentiation leads to increased interactions between Pcdh isoforms, which correlates with a decrease in Pcdh cleavage products. In addition, we show that interactions between the extracellular domains of Pcdh isoforms contribute to this decrease. These data suggest that in early brain development or in undifferentiated cell lines, rapid endocytosis of Pcdh proteins facilitates the shedding of their extracellular domains and efficient γ-secretase–dependent cleavage. This process leads to the constitutive generation of a cytosolic cleavage product (CTF2). As with other γ-secretase substrates, this fragment may enter the nucleus and regulate gene expression. As development progresses or cells differentiate, a complex of Pcdh proteins forms and Pcdh-CTF2 is no longer produced. This complex may then be poised for proteolysis in response to signals yet to be identified.
Results
As noted in the previous section, full-length Pcdh proteins undergo two-step proteolysis. First, the extracellular domain is cleaved to generate a transmembrane stub called CTF1. CTF1 is then processed by the γ-secretase complex to generate a soluble intracellular fragment called CTF2. We often find that Pcdh CTF1s exist as species of multiple molecular weights (see, for example, Fig. 1 and Figs. S1–S3). There are several nonmutually exclusive explanations for this observation, including the presence of multiple cleavage sites, differential posttranslational modification, and, in the case of endogenous proteins, differing molecular weights between isoforms. To investigate the possibility that the levels of Pcdh cleavage products are regulated during development or neuronal differentiation, we probed Western blots of endogenous Pcdhα or Pcdhγ proteins with polyclonal antibodies against their constant regions. We first examined lysates of whole brain from different developmental time points. As shown in Fig. 1 A and B, the levels of Pcdhα-CTF1 and Pcdhγ-CTF1 significantly decrease during mouse development.
Fig. 1.
The levels of Pcdhα and -γ cleavage products decrease during both neuronal development and differentiation in culture. (A and B) Western blots of endogenous Pcdhα or Pcdhγ in whole brain extracts from mice at different developmental time points. Blots were probed with polyclonal antibody against the constant region of Pcdhα (αCon) or Pcdhγ (γCon). (C and D) Western blots of endogenous Pcdhα or Pcdhγ in cell lysates from primary neurospheres cultured for 4 d in vitro and treated with the γ-secretase inhibitor DAPT (+) or vehicle control (−). (E and F) Western blots of lysates from undifferentiated (UD) and differentiated (D) CAD cells stably expressing Pcdhα4-TAP or γb7-TAP. Cells were differentiated for 0 (UD) or 72 (D) h and the blots were probed with anti-FLAG and the loading controls anti-calnexin (CNX) or anti–β-actin. *Nonspecific, cross-reacting bands; CTF1, C-terminal fragment 1; FL, full-length Pcdh. An unidentified band in E that disappears during differentiation is indicated by an arrow.
We also find that in neurosphere cultures, Pcdhα and -γ CTF1s can be stabilized by DAPT, a γ-secretase inhibitor that blocks the conversion of CTF1 into CTF2 (Fig. 1 C and D). This observation indicates that cleavage of endogenous Pcdh proteins occurs in primary cells enriched for neuronal precursors and that a γ-secretase–dependent cleavage pathway is active. If protocadherin cleavage is an indication of signaling activity, then the cleavage product may be highly active during early development of the nervous system.
To study the generation of Pcdh cleavage products in more detail, we used the neuroblastoma cell line Cath.a-differentiated (CAD) (25). CAD cells express several neuron-specific proteins, and they can be induced to undergo morphological differentiation in response to serum withdrawal (25, 26). We also generated plasmid constructs in which a tandem affinity purification (TAP) tag containing both FLAG and HA epitopes is fused to the intracellular domain of Pcdhα4 or -γb7. Alternatively, EGFP was fused to the short intracellular domain of Pcdhβ17 (Fig. S1). When expressed in CAD cells, the fusion proteins are also cleaved to generate Pcdh CTF1 and CTF2 fragments, and this process requires γ-secretase (Figs. S1–S3). As with endogenous protein in the brain, in vitro differentiation of CAD cells results in a significant decrease in the levels of CTF1 derived from Pcdhα4-TAP or Pcdhγb7-TAP (Fig. 1 E and F). This decrease may be due to either a decrease in CTF1 production or an increase in CTF1 turnover.
Treatment of cells with the proteasome inhibitor lactacystin stabilized CTF2 in undifferentiated, but not in differentiated CAD cells, indicating that it is no longer produced at detectable levels following differentiation (Fig. S4). By contrast, proteasome inhibition did not affect CTF1 levels in either undifferentiated or differentiated CAD cells, suggesting that CTF1 may be subject to a proteasome-independent mechanism of turnover such as lysosomal degradation. These results show that presenilin-dependent processing of Pcdhα is a developmentally regulated event that decreases as cells differentiate.
We next tested whether cellular differentiation alters the interaction of Pcdh proteins with each other. We find that higher levels of endogenous Pcdhβ and -γ coimmunoprecipitate with Pcdhα4-TAP in differentiated CAD cells, compared with undifferentiated CAD cells (Fig. 2 and Fig. S5). Interestingly, a previous study reported that the overexpression of Pcdhα inhibits cleavage of Pcdhγ (19). Taken together, these findings suggest the possibility that a differentiation-dependent increase in Pcdh–Pcdh interaction may be linked to a decrease in the levels of Pcdh cleavage products.
Fig. 2.
Differentiation increases Pcdh–Pcdh binding. Coimmunoprecipitation of Pcdhα4-TAP and endogenous Pcdhβ and -γ is shown. Pcdhα4-TAP was immunoprecipitated from lysates of undifferentiated (UD) or differentiated (D) CAD cells and precipitates were probed with antibodies against FLAG, γCon, and the extracellular domain of Pcdhβ17.
We further examined the correlation between differentiation, Pcdh–Pcdh interaction, and Pcdh cleavage by using a TAP-tagged Pcdhα4 construct that lacks EC domains 1–5 (Pcdhα4ΔEC1-5-TAP; Fig. 3A). Immunoprecipitation of this TAP-tagged, truncated protein showed that the association of the truncated protein with endogenous Pcdhγ is less than that observed with full-length Pcdhα4-TAP (Fig. 3B). This result is in agreement with a report that the extracellular domains are required for Pcdh interactions (9). Strikingly, upon differentiation, CTF1 derived from Pcdhα4ΔEC1-5 is present at undifferentiated levels (Fig. 3C). Taken together, these results suggest that efficient Pcdh–Pcdh interaction is necessary for the decrease in cleavage products that accompanies differentiation and that the protease that carries out cleavage is still active in differentiated cells.
Fig. 3.
Pcdh–Pcdh interactions play roles in cleavage suppression in differentiated CAD cells. (A) Schematic of full-length Pcdhα4-TAP (α4FL-TAP) and a mutant lacking the first five ECs (α4ΔEC1-5-TAP). (B) Coimmunoprecipitation of Pcdhα4-TAP fusions and endogenous Pcdhγ. α4FL-TAP or α4ΔEC1-5-TAP was precipitated from lysates of CADs differentiated for 24 h, and precipitates were blotted for γCon and FLAG. *IgG band in IP. (C) Western blot of cell lysates from undifferentiated and differentiated CAD cells stably expressing α4FL-TAP or α4ΔEC1-5-TAP.
Our data support the idea that differentiation leads to increased interactions between distinct Pcdh subtypes and that Pcdh–Pcdh interactions are required to lower the levels of cleavage products in differentiated cells. We also find that the proteolytic activity responsible for the generation of Pcdhα4-CTF1 is still present in differentiated cells, and weak Pcdh–Pcdh binding allows for the constitutive presence of CTF1. The decrease in the levels of Pcdh cleavage products may be due to a decrease in the rate of their production, an increase in the rate of their turnover, or some combination of these two.
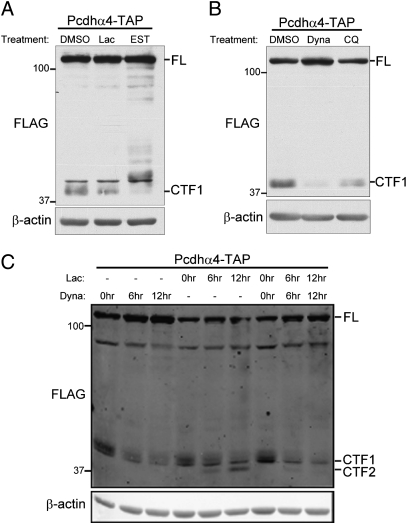
Next, we examined the cellular processes required for efficient Pcdh cleavage in undifferentiated CAD cells. In neurons, a significant portion of the Pcdh proteins are found outside of synapses in vesicles and tubulovesicular structures within the soma, axons, and dendrites (11, 27, 28). Additionally, a recent report suggests that the Pcdhγ proteins are subject to regulated intracellular trafficking to sites of cell–cell contact during neuronal development and synapse formation (29). To further examine the roles that dynamic subcellular trafficking may play in the regulation of the Pcdh proteins, we first asked whether Pcdh proteins are subject to endocytosis and trafficking to the lysosome for degradation. Undifferentiated CAD cells were briefly treated with EST, an inhibitor of cysteine proteases including the lysosomal cathepsins (30), or with the proteasome inhibitor lactacystin (Fig. 4A). EST caused an accumulation of fragments intermediate in size between full-length Pcdhα4 and Pcdhα4 CTF1. These fragments were not observed following inhibition of the proteasome with lactacystin. The fragments stabilized by EST treatment likely represent degradation products from full-length Pcdh protein that was endocytosed and trafficked to the lysosome. These fragments may be generated by the activity of an unknown protease on full-length Pcdh proteins.
Fig. 4.
Endocytosis is required for Pcdhα cleavage. (A) Western blot of cell lysates from CAD cells treated for 4 h with DMSO, lactacystin (Lac), or the cysteine protease inhibitor EST. (B) Western blot of cell lysates from CAD cells treated for 4 h with DMSO, the dynamin inhibitor dynasore (Dyna), or the weak base chloroquine (CQ). (C) Western blot of cell lysates from stably transfected CAD cells treated with lactacystin and/or dynasore for the indicated times.
Previous studies have shown that some substrates of the γ-secretase complex are endocytosed before cleavage (31, 32). We therefore carried out experiments to test whether endocytosis is required for the production of Pcdhα4 CTF1 or CTF2. Specifically, we treated cells with dynasore, a dynamin inhibitor that blocks the formation of endosomes (33), or chloroquine, a membrane-impermeable base that is taken up into endosomes and blocks their acidification (34). Both of these treatments inhibited the production of Pcdhα4 CTF1, indicating that endocytosis is indeed required for shedding of the Pcdhα4 EC domains (Fig. 4B). As EC shedding is a prerequisite for γ-secretase processing, the blockade of endosome formation and maturation should block the production of both membrane-bound CTF1 and soluble CTF2. We tested this possibility by inhibiting the proteasome with lactacystin and endocytosis with dynasore. As expected, treatment with lactacystin alone stabilizes CTF2, but both CTF1 and CTF2 production is abolished in the presence of dynasore (Fig. 4C). We conclude that endocytosis is required for proteolytic shedding of the Pcdh EC domain and the generation of CTF1 and is therefore required for the production of soluble CTF2.
Because our data show that endocytosis and vesicular trafficking are required for the cleavage of Pcdh proteins, we explored the mechanisms that control these processes. The Notch receptor requires endocytosis before γ-secretase cleavage (31), and four protein complexes known as endosomal sorting complex required for transport-0, -I, -II, and -III (ESCRT-0, -I, -II, and -III) direct Notch-positive endosomes to the multivesicular body pathway and the lysosome (35–38). To determine whether Pcdh proteins associate with ESCRT complexes in a manner similar to that of Notch, we transfected a component of ESCRT-0 [HGF-regulated tyrosine kinase substrate fused to GFP (Hrs-GFP)] into CAD cells stably expressing Pcdhα4-TAP or Pcdhγb7-TAP. In undifferentiated CAD cells, we observe a striking colocalization between intracellular Pcdh puncta and Hrs-GFP. By contrast, in differentiated cells, the Pcdh staining pattern is much more diffuse, there are fewer and smaller Pcdh-positive puncta, and colocalization with Hrs-GFP is significantly diminished (Fig. 5A and Figs. S6–S8). This change in colocalization occurs despite similar levels of total Pcdh and Hrs proteins (Fig. 5 B and C). These results suggest that there may be an interaction between the Pcdh proteins and ESCRT complexes that is regulated by differentiation.
Fig. 5.
Pcdhα is bound by ESCRT-0 in undifferentiated cells. (A) Confocal imaging of Pcdhα4-TAP and ESCRT-0. CAD cells stably expressing Pcdhα4-TAP were transfected with the ESCRT-0 subunit Hrs fused to GFP. After 0 or 72 h of differentiation, cells were fixed and stained with anti-HA (red or grayscale), anti-GFP (green or grayscale), and DAPI (blue). (B) Coimmunoprecipitation of Pcdhα4-TAP and endogenous Hrs. CAD cells stably transfected with an empty vector or Pcdhα4-TAP were differentiated for 0 h (UD) or 72 h (D). Pcdh was immunoprecipitated from cell lysates with anti-FLAG antibody, and Western blots were carried out for Hrs and FLAG. A nonspecific band comigrates with Pcdhα4-CTF1 following FLAG IP. (C) Coimmunoprecipitation of endogenous Hrs and Pcdhα4-TAP. Lysates of undifferentiated cells stably transfected with an empty vector or Pcdhα4-TAP were used for immunoprecipitation with a polyclonal antibody against Hrs or a control IgG. Western blots were then carried out against FLAG and Hrs. (D) Coimmunoprecipitation of α4ΔEC1-5-TAP and Hrs. α4ΔEC1-5-TAP was immunoprecipitated from lysates of undifferentiated or differentiated cells and a Western blot was performed against Hrs and FLAG.
In agreement with our colocalization data, immunoprecipitation of Pcdhα4-TAP followed by Western blot for endogenous Hrs reveals a physical interaction between the two proteins that is significantly diminished during differentiation (Fig. 5B). The converse experiment, Hrs immunoprecipitation followed by Pcdhα4-TAP Western blot, reveals that Pcdhα4 CTF1 is associated with ESCRT-0 (Fig. 5C). Remarkably, the Pcdhα4ΔEC1-5 mutant that is constitutively cleaved following differentiation is also constitutively bound by Hrs (Fig. 5D). Cleavage of the mutant protein is inhibited by dynasore, similar to that of the wild-type protein (Fig. S9). Taken together, these observations suggest that endocytosis, extracellular domain cleavage, γ-secretase cleavage, and ESCRT-dependent trafficking maybe coregulated during differentiation.
Discussion
We show that that the levels of Pcdhα and -γ cleavage products decrease during development and during differentiation of neuron-like cells in culture. This differentiation-dependent decrease occurs in parallel with an increase in the interactions between Pcdh isoforms. In addition, mutations in the extracellular domain that limit Pcdh–Pcdh binding lead to constitutive cleavage. We also show that the Pcdhs are subject to vesicular trafficking and that Pcdh cleavage requires endocytosis. Interfering with endosome formation or maturation blocks the formation of both Pcdhα4 CTF1 and CTF2. Full-length Pcdhα4 and Pcdhα4 CTF1 also associate with ESCRT-0 in undifferentiated CAD cells, and a significant portion of the Pcdh proteins are subject to degradation in the lysosome.
The peak in Pcdh protein cleavage products in early developmental stages may reflect broad, Pcdh-dependent signaling during periods of active neurogenesis, synaptogenesis, and neurite outgrowth. The decrease in Pcdh cleavage products over time may be a consequence of the progression of synapse maturity. In cell culture, we observe that Pcdh CTFs appear to be present constitutively in undifferentiated CAD cells. This result is similar to the cleavage in cell lines that has been observed for Pcdhα, Pcdhγ, and other γ-secretase substrates (19–21, 39). As CAD cells differentiate in vitro, the level of Pcdh CTFs decreases in a manner that mirrors the decrease in cleavage products in the brain during development.
The differentiation-dependent decrease in cleavage products is correlated with an increase in association between Pcdhs, and full-length isoforms in complex with each other become the predominant form of the proteins. Disrupting interactions between Pcdhs by mutating the extracellular domain leads to the constitutive presence Pcdh CTFs in differentiated cells. Taken together, these results suggest that Pcdh cleavage is in part regulated by signals mediated through the EC domains. At present, the signals upstream of active cleavage are not known. Our data suggest that Pcdh–Pcdh binding is involved in decreasing the levels of Pcdh cleavage products and is not responsible for cleavage induction. Pcdh cleavage may therefore be dependent on binding to a signaling molecule, as is the case in the Notch–Delta system, or on changes in other cellular conditions such as neuronal activity or calcium influx. Indeed, cleavage of both N-cadherin and Pcdhγc3 is induced by glutamate (8, 23) and we find that, in differentiated CAD cells, the protease responsible for Pcdh extracellular cleavage remains active. Neuronal activity may therefore allow an increase in CTF levels in mature cells. Additionally, we find that in mature neurons and differentiated CAD cells, the Pcdhs bind to the receptor tyrosine kinase Ret and participate in GDNF-inducible signaling that is independent of γ-secretase cleavage (18). Therefore, in differentiated neuronal cells, the Pcdhs may be poised for both cleavage-dependent and cleavage-independent signaling.
We find that Pcdhα cleavage requires endocytosis and that disrupting this process with dynasore or chloroquine inhibits CTF1 production. In addition, Pcdh proteins are bound by the ESCRT-0 complex in undifferentiated CAD cells, but not in differentiated CAD cells, and at least some full-length protein is trafficked to the lysosome. As is the case with cleavage, Pcdh endocytosis and trafficking may be in part regulated by the EC domains, as a mutant lacking EC domains 1–5 is constitutively bound by Hrs in differentiated cells. These observations share some parallels to the Notch signaling pathway. Endocytosis is required before γ-secretase releases the Notch intracellular domain into the cytoplasm (31). However, ESCRT complexes also direct some Notch proteins to the lysosome for degradation before γ-secretase cleavage can occur. This process limits Notch signaling and mutations in the ESCRT pathway lead to Notch hyperactivation (35–38). Endocytosis and trafficking in undifferentiated cells may therefore buffer Pcdh signaling by both activating cleavage and limiting the amount of full-length Pcdh that is available. It is not known how endocytosis leads to the sequential cleavage of Pcdh proteins, but several nonmutually exclusive modes of regulation are possible. For example, endocytic vesicles may deliver Pcdh cargo to a subcellular compartment where proteases are active. Additionally, the change in pH that accompanies endosome maturation may be necessary for Pcdh cleavage.
Endocytosis, trafficking, and cleavage of the Pcdhs may also be regulated by signal-dependent events that require the Pcdh intracellular domain or intracellular Pcdh binding factors. This proposal is consistent with a report that regulated vesicular trafficking controls the formation of Pcdh–Pcdh contacts between neurons in a manner that requires the Pcdh ICDs (29). For the Notch receptor, endocytosis and γ-secretase cleavage are dependent on monoubiquitination, and sorting of Notch to the lysosome is dependent on the Nedd4 family of E3 ligases (31, 40, 41–43). Processing of Pcdh may be regulated in a manner similar to that of Notch.
Here we present insights into the regulation of Pcdh cleavage. The levels of Pcdh cleavage products depend on cellular context and the highest CTF levels are found in the early stages of brain development. We also show that cleavage is regulated by vesicular trafficking and Pcdh–Pcdh interactions. The investigation of the mechanisms that govern Pcdh binding and cleavage and the identification of targets that are downstream of Pcdh processing will further our understanding of the roles Pcdh proteins play in the nervous system.
Materials and Methods
Plasmids.
A TAP-tag containing two HA sequences, one FLAG sequence, and a TEV protease cleavage site was fused to the C terminus of mouse Pcdhα4, Pcdhα4ΔEC1-5, or Pcdhγb7 in pcDNA3. EGFP was fused C terminally to mouse full-length Pcdhα4, the Pcdhα4ICD, or Pcdhβ17 in pEGFP-N2 (Clontech). EGFP was fused C terminally to human Hrs in pDEST47 (Invitrogen). Transfections of primary and immortalized cell lines were carried out with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Chemical Reagents.
The following chemical reagents were used: lactacystin in DMSO at a final concentration of 5 μg/mL (Calbiochem), DAPT in DMSO at 25 μM (Calbiochem), EST in EtOH at 50 μM (Calbiochem), chloroquine diphosphate in water at 125 μM (Sigma), and dynasore in DMSO at 100 μM (Sigma).
Cell Lines and Culture.
CAD cells were cultured in DMEM (Gibco) and 10% Fetalclone III Serum (HyClone). Stably transfected lines were produced by introducing the TAP-tagged plasmids and selecting with G418. Differentiation was carried out by withdrawing serum for 72 h.
Primary Neurosphere Culture.
Brains of E12–E14 CD1 mouse embryos (Charles River Laboratories) were dissected and dissociated by papain digestion (Worthington Biochemical Corporation). Cells were resuspended at a density of 25,000–50,000 cells/mL in DMEM:F12 (Gibco) supplemented with N2 (Gibco), B27 (Gibco), 10 ng/mL EGF (Invitrogen), and 5 ng/mL FGF (Invitrogen) and plated on bacterial dishes.
Western Blot and Immunoprecipitation.
Cells and tissues were lysed in 1% Nonidet P-40 buffer [50 mM Tris·HCl (pH 7.4), 1% Nonidet P-40, 150 mM NaCl, 30 mM NaF, 5 mM EDTA, 10% glycerol, 40 mM β-glycerophosphate, and protease inhibitors]. The following commercial antibodies were used for Western blot: anti-FLAG (clone M2; Sigma), anti-GFP (Clone 7.1+13.1; Roche), anti–β-actin (ab8226; Abcam), and anti-Hrs (clone 989; Bethyl Laboratories). Anti-αCon polyclonal sera were produced by fusing the mouse Pcdhα constant region to GST, purifying the protein from bacterial lysates, removing the GST tag, and immunizing rabbits. Antibodies were affinity purified with the Pcdhα constant region alone. Anti-β17 polyclonal sera were produced by immunizing rabbits with a synthetic peptide (RRARIISQENKEHLQLNLQS) coupled to Keyhole Limpet Hemocyanin. Antibodies were affinity purified with the peptide alone. Anti-γCon antibody was a gift from Greg Phillips (Mount Sinai School of Medicine, New York). Anti-calnexin antibody was a gift from David B. Williams (University of Toronto, Toronto). Western blots were carried out according to standard protocols and protein was visualized with secondary antibodies coupled to HRP (Jackson ImmunoResearch) and Immobilon ECL reagent (Millipore). Densitometry was performed in ImageJ (National Institutes of Health). Immunoprecipitations were carried out for 4 h at 4° with anti–FLAG-agarose (M2; Sigma) or with primary antibody in the presence of Protein G-Sepharose (GE Healthcare). Precipitates were washed in Nonidet P-40 buffer alone, or IP Wash Buffer A [10 mM Tris·HCl (pH 7.2), 1% Nonidet P-40, 0.5% sodium deoxycholate, 100 mM NaCl, 1 mM EDTA], IP Wash Buffer B [10 mM Tris·HCl (pH 7.2), 0.1% Nonidet P-40, 1 M NaCl] and Nonidet P-40 buffer.
Immunocytochemistry.
CAD cells were grown on coverslips coated with poly-d-lysine and laminin (BD Biosciences), fixed in 4% paraformaldehyde, and permeabilized with 0.2% Triton X-100. Where appropriate, anti-EGFP coupled to Alexa 488 (A21311; Invitrogen) or anti-HA (HA.11; Covance) was added overnight. Fluorescently coupled secondary antibodies were obtained from Jackson ImmunoResearch. Coverslips were mounted in Vectashield with DAPI (Vector Laboratories). Images were collected with a Zeiss LSM 510 Meta confocal microscope.
Supplementary Material
Acknowledgments
We thank Hon-Ren Huang and other members of the Maniatis laboratory for advice and discussion. This work was supported by National Institutes of Health Grant 5R01GM042231 (to T.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013105107/-/DCSupplemental.
References
- 1.Kohmura N, et al. Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 2.Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 3.Esumi S, et al. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet. 2005;37:171–176. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko R, et al. Allelic gene regulation of Pcdh-alpha and Pcdh-gamma clusters involving both monoallelic and biallelic expression in single Purkinje cells. J Biol Chem. 2006;281:30551–30560. doi: 10.1074/jbc.M605677200. [DOI] [PubMed] [Google Scholar]
- 5.Tasic B, et al. Promoter choice determines splice site selection in protocadherin alpha and gamma pre-mRNA splicing. Mol Cell. 2002;10:21–33. doi: 10.1016/s1097-2765(02)00578-6. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Su H, Bradley A. Molecular mechanisms governing Pcdh-gamma gene expression: Evidence for a multiple promoter and cis-alternative splicing model. Genes Dev. 2002;16:1890–1905. doi: 10.1101/gad.1004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obata S, et al. Protocadherin Pcdh2 shows properties similar to, but distinct from, those of classical cadherins. J Cell Sci. 1995;108:3765–3773. doi: 10.1242/jcs.108.12.3765. [DOI] [PubMed] [Google Scholar]
- 8.Reiss K, et al. Regulated ADAM10-dependent ectodomain shedding of gamma-protocadherin C3 modulates cell-cell adhesion. J Biol Chem. 2006;281:21735–21744. doi: 10.1074/jbc.M602663200. [DOI] [PubMed] [Google Scholar]
- 9.Murata Y, Hamada S, Morishita H, Mutoh T, Yagi T. Interaction with protocadherin-gamma regulates the cell surface expression of protocadherin-alpha. J Biol Chem. 2004;279:49508–49516. doi: 10.1074/jbc.M408771200. [DOI] [PubMed] [Google Scholar]
- 10.Morishita H, et al. Structure of the cadherin-related neuronal receptor/protocadherin-alpha first extracellular cadherin domain reveals diversity across cadherin families. J Biol Chem. 2006;281:33650–33663. doi: 10.1074/jbc.M603298200. [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR. Gamma-protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development. 2008;135:4141–4151. doi: 10.1242/dev.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, et al. Gamma protocadherins are required for survival of spinal interneurons. Neuron. 2002;36:843–854. doi: 10.1016/s0896-6273(02)01090-5. [DOI] [PubMed] [Google Scholar]
- 13.Weiner JA, Wang X, Tapia JC, Sanes JR. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad T, Wang X, Gray PA, Weiner JA. A differential developmental pattern of spinal interneuron apoptosis during synaptogenesis: Insights from genetic analyses of the protocadherin-gamma gene cluster. Development. 2008;135:4153–4164. doi: 10.1242/dev.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa S, et al. The protocadherin-alpha family is involved in axonal coalescence of olfactory sensory neurons into glomeruli of the olfactory bulb in mouse. Mol Cell Neurosci. 2008;38:66–79. doi: 10.1016/j.mcn.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Katori S, et al. Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas. J Neurosci. 2009;29:9137–9147. doi: 10.1523/JNEUROSCI.5478-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, et al. Alpha- and gamma-protocadherins negatively regulate PYK2. J Biol Chem. 2008;284:2880–2890. doi: 10.1074/jbc.M807417200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schalm SS, Ballif BA, Buchanan SM, Phillips GR, Maniatis TM. Phosphorylation of protocadherin proteins by the receptor tyrosine kinase Ret. Proc Natl Acad Sci USA. 2010;107:13894–13899. doi: 10.1073/pnas.1007182107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonn S, Seeburg PH, Schwarz MK. Combinatorial expression of alpha- and gamma-protocadherins alters their presenilin-dependent processing. Mol Cell Biol. 2007;27:4121–4132. doi: 10.1128/MCB.01708-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haas IG, Frank M, Véron N, Kemler R. Presenilin-dependent processing and nuclear function of gamma-protocadherins. J Biol Chem. 2005;280:9313–9319. doi: 10.1074/jbc.M412909200. [DOI] [PubMed] [Google Scholar]
- 21.Hambsch B, Grinevich V, Seeburg PH, Schwarz MK. Gamma-protocadherins, presenilin-mediated release of C-terminal fragment promotes locus expression. J Biol Chem. 2005;280:15888–15897. doi: 10.1074/jbc.M414359200. [DOI] [PubMed] [Google Scholar]
- 22.Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- 23.Marambaud P, et al. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Struhl G, Adachi A. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell. 2000;6:625–636. doi: 10.1016/s1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- 25.Qi Y, Wang JK, McMillian M, Chikaraishi DM. Characterization of a CNS cell line, CAD, in which morphological differentiation is initiated by serum deprivation. J Neurosci. 1997;17:1217–1225. doi: 10.1523/JNEUROSCI.17-04-01217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips GR, et al. Gamma-protocadherins are targeted to subsets of synapses and intracellular organelles in neurons. J Neurosci. 2003;23:5096–5104. doi: 10.1523/JNEUROSCI.23-12-05096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emond MR, Jontes JD. Inhibition of protocadherin-alpha function results in neuronal death in the developing zebrafish. Dev Biol. 2008;321:175–187. doi: 10.1016/j.ydbio.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Monreal M, Kang S, Phillips GR. Gamma-protocadherin homophilic interaction and intracellular trafficking is controlled by the cytoplasmic domain in neurons. Mol Cell Neurosci. 2009;40:344–353. doi: 10.1016/j.mcn.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamai M, et al. In vitro and in vivo inhibition of cysteine proteinases by EST, a new analog of E-64. J Pharmacobiodyn. 1986;9:672–677. doi: 10.1248/bpb1978.9.672. [DOI] [PubMed] [Google Scholar]
- 31.Gupta-Rossi N, et al. Monoubiquitination and endocytosis direct gamma-secretase cleavage of activated Notch receptor. J Cell Biol. 2004;166:73–83. doi: 10.1083/jcb.200310098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urra S, et al. TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal gamma-secretase-mediated release of the p75 intracellular domain. J Biol Chem. 2007;282:7606–7615. doi: 10.1074/jbc.M610458200. [DOI] [PubMed] [Google Scholar]
- 33.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 34.Maxfield FR. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982;95:676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moberg KH, Schelble S, Burdick SK, Hariharan IK. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev Cell. 2005;9:699–710. doi: 10.1016/j.devcel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Thompson BJ, et al. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev Cell. 2005;9:711–720. doi: 10.1016/j.devcel.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Vaccari T, Bilder D. The Drosophila tumor suppressor vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev Cell. 2005;9:687–698. doi: 10.1016/j.devcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Vaccari T, Lu H, Kanwar R, Fortini ME, Bilder D. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;180:755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haass C, et al. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 40.Sakata T, et al. Drosophila Nedd4 regulates endocytosis of notch and suppresses its ligand-independent activation. Curr Biol. 2004;14:2228–2236. doi: 10.1016/j.cub.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 41.Wilkin MB, et al. Regulation of notch endosomal sorting and signaling by Drosophila Nedd4 family proteins. Curr Biol. 2004;14:2237–2244. doi: 10.1016/j.cub.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 42.Litterst C, et al. Ligand binding and calcium influx induce distinct ectodomain/gamma-secretase-processing pathways of EphB2 receptor. J Biol Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunan J, Small DH. Regulation of APP cleavage by alpha-, beta- and gamma-secretases. FEBS Lett. 2000;483:6–10. doi: 10.1016/s0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.