Abstract
The phagocyte NAPDH–oxidase complex consists of several phagocyte oxidase (phox) proteins, generating reactive oxygen species (ROS) upon activation. ROS are involved in the defense against microorganisms and also in immune regulation. Defective ROS formation leads to chronic granulomatous disease (CGD) with increased incidence of autoimmunity and disturbed resolution of inflammation. Because regulatory T cells (Tregs) suppress autoimmune T-cell responses and are crucial in down-regulating immune responses, we hypothesized that ROS deficiency may lead to decreased Treg induction. Previously, we showed that in p47phox-mutated mice, reconstitution of macrophages (Mph) with ROS-producing capacity was sufficient to protect the mice from arthritis. Now, we present evidence that Mph-derived ROS induce Tregs. In vitro, we showed that Mph ROS-dependently induce Treg, using an NADPH-oxidase inhibitor. This finding was confirmed genetically: rat or human CGD Mph with mutated p47phox or gp91phox displayed hampered Treg induction and T-cell suppression. However, basal Treg numbers in these subjects were comparable to those in controls, indicating a role for ROS in induction of peripheral Tregs. Induction of allogeneic delayed-type hypersensitivity with p47phox-mutated Mph confirmed the importance of Mph-derived ROS in Treg induction in vivo. We conclude that NAPDH oxidase activity in Mph is important for the induction of Tregs to regulate T cell-mediated inflammation.
Keywords: chronic granulomatous disease, NADPH oxidase, neutrophil cytosolic oxidase 1, redox
Reactive oxygen species (ROS) not only are harmful and mediators of oxidative stress, but also have immune regulatory functions, especially when produced in lower amounts (1–3). The mechanisms by which ROS affect the immune system are just beginning to become clear. For example, mitochondrial ROS oxidize released high-mobility group protein B1 during apoptosis, thereby preventing immune activation and allowing induction of tolerance (4), and myeloid-derived suppressor cells suppress antitumor T-cell responses in a phagocytic NADPH–oxidase (Nox2) complex-dependent way (5). This Nox2 complex consists of multiple components (i.e., the membrane-expressed cytochrome b558 consisting of gp91phox and p22phox and the cytosolic components p47phox, p67phox, and p40phox). Chronic granulomatous disease (CGD) develops when any of these components is absent or functionally hampered, and ROS production is defective. CGD is characterized by recurrent bacterial and fungal infections and abnormal granuloma formation. These granulomas are mostly sterile and often respond to steroid therapy without antibiotics (6). In addition, CGD patients suffer from autoimmune diseases more frequently than does the healthy population (7, 8). These features point to a defect in immune regulation caused by the absence of ROS.
We observed previously that mice and rats with alleles of the neutrophil cytosolic factor 1 (Ncf1) gene (encoding p47phox) that encode a less functional Nox2 and thus have lower ROS production are more susceptible to induced autoimmune diseases than are their wild-type littermates (9, 10). This observation is in line with observations in CGD patients. Interestingly, the reduced ROS-producing capacity in our congenic rat model mediated higher susceptibility to pristane-induced arthritis in a T-cell–dependent fashion (9, 11). However, T cells express no or only very low levels of Nox2, suggesting that other cells determine the T-cell response by producing ROS (12). One study, however, does show low levels of Nox2 in T cells (13). Because antigen-presenting cells (APC) interact with T cells during antigen presentation, and APC express Nox2, they may affect T-cell responses via ROS production. Indeed, ROS produced during antigen presentation affect the immune response (14, 15) by interfering in signal transduction (16, 17). In addition, ROS generated in phagosomes/endosomes determines the ability to cross-present antigen, both in mice and humans (18, 19). We showed that among murine APCs, macrophages (Mph) were most efficient at producing ROS (20). Transgenic mice expressing functional p47phox only in Mph on a p47phox-mutated background were as equally protected against collagen-induced arthritis as their fully wild-type littermates. These observations indicate that Mph-derived ROS are sufficient to inhibit T-cell responses (20).
Regulatory T cells (Tregs) can suppress activation and proliferation of effector T cells and thereby diminish immune responses. Autoimmunity therefore can be the result of a defective Treg system (21), and successful treatment of autoimmune disease with Tregs has been reported in mouse models (22, 23). We hypothesized that if Mph-derived ROS prevent T-cell–mediated immune responses, it could do so by inducing Tregs. Previously we showed that antiinflammatory Mph can induce potent Tregs, in contrast to proinflammatory Mph (24). Here we investigated whether Mph-derived ROS influence the induction of Tregs in humans in vitro as well as in rats in vivo. We observed that Mph can induce Tregs in a ROS-dependent fashion in vitro and, more importantly, that Mph from ROS-deficient CGD patients are significantly less efficient in inducing Tregs. We conclude that Mph can modulate T-cell responses by producing ROS and can induce Tregs in a ROS-dependent fashion.
Results
Human Mph Produce ROS upon Stimulation.
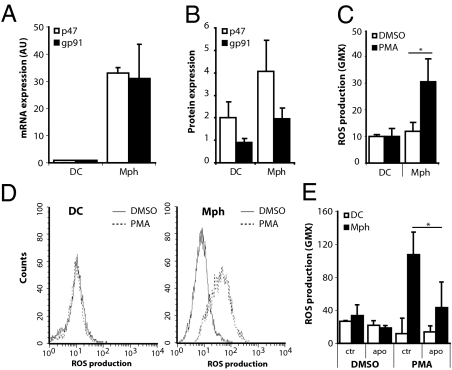
To investigate the role of ROS in antigen presentation by different APC, the expression of two Nox2 members was determined: p47phox (Ncf1) and gp91phox (Cybb) in human dendritic cells (DC) and in Mph colony-stimulating factor (M-CSF)–differentiated Mph. mRNA expression for both p47phox and gp91phox was about 30 times higher in Mph than in DC (Fig. 1A). By intracellular FACS analysis, we observed that the expression of gp91phox protein was very low in DC, whereas p47phox was clearly present. In contrast, Mph showed significant expression of both gp91phox and p47phox (Fig. 1B). Functionally, human Mph efficiently generated ROS upon phorbol 12-myristate 13-acetate (PMA) stimulation (Fig. 1 C and D). In contrast, DC hardly produced ROS, in line with the low gp91phox expression. Mph differentiated in GM-CSF (proinflammatory Mph) produced only marginal amounts of ROS (Fig S1A). The ROS production by Mph could be blocked to background levels with apocynin, an inhibitor of Nox2 that binds p47phox and prevents translocation to the membrane (Fig. 1E).
Fig. 1.
Mph produce ROS. (A) mRNA and (B) protein expression levels of the two most important components of the Nox2 complex, p47phox and gp91phox, determined in DC and Mph by RT-PCR and flow cytometry, respectively. In A mRNA expression levels were corrected for GAPDH expression, and expression levels of DC were set to 1. B shows the ratio between specific staining and isotype control. Results shown are average and SD of the relative expression levels of four experiments with cells from four different donors. (C) The capacity of DC and Mph to produce ROS was measured after PMA stimulation. Results shown are average and SD of 6–10 independent experiments; the conditions after simulation with PMA or with vehicle (DMSO) are shown. (D) Representative FACS histograms of ROS production, measured by DHR123 fluorescence, by DC (Left) and Mph (Right) after PMA (dotted line) or DMSO (control; solid line) stimulation. (E) Production of ROS by DC and Mph was measured after PMA or vehicle (DMSO) activation and in the absence or presence of the specific p47phox inhibitor apocynin (1 mM). Results shown are the average and SD of three or four independent experiments. *P < 0.05.
Mph Suppress T-Cell Responses by Producing ROS.
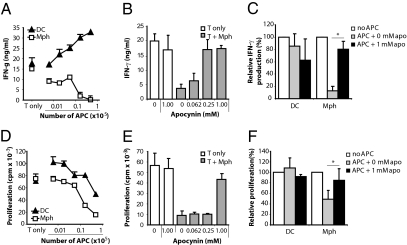
To investigate the suppressive capacity of ROS produced by APC on T-cell responses, purified T cells were activated with anti-CD3/CD28 mAbs in the presence of increasing numbers of Mph or DC. IFN-γ production and the proliferative response were determined at day 5. IFN-γ production (Fig. 2 A and C and Fig. S1B) and, to a lesser extent, T-cell proliferation (Fig. 2 D and F) were suppressed by Mph but not by DC. The Nox2 inhibitor apocynin abrogated the Mph-mediated suppressive effect in a dose-dependent manner (Fig. 2 B, C, E, and F). GM-CSF–differentiated proinflammatory Mph slightly suppressed T-cell activation, but this suppression was not reversible by apocynin and thus was not ROS-dependent (Fig. S1C).
Fig. 2.
Mph suppress T-cell activation in a ROS-dependent fashion. T cells (150,000) were activated with anti-CD3/28 Ab, and Mph or DC were added in increasing numbers (x axes). After 5 d of coculture, IFN-γ production (A) and proliferation (D) were determined by ELISA and 3H thymidine incorporation, respectively. Results shown are the average and SD of a representative experiment performed in triplicate. (B and E) We cocultured 150,000 T cells and 25,000 Mph in the absence or presence of different concentrations of apocynin (x axis) to study the effect of ROS on Mph-mediated T-cell suppression. After 5 d of coculture, IFN-γ production (B) and proliferation (E) were determined by ELISA and 3H thymidine incorporation, respectively. Representative experiments are shown. (C and F) Experiments were similar to those in A, B, D, and E, but the average and SD of three or four independent experiments are shown for an APC:T cell ratio of 1:6. Values shown are relative to the conditions without APC (100%, white bars). *P < 0.05
Mph Induce Tregs in a ROS-Dependent Fashion.
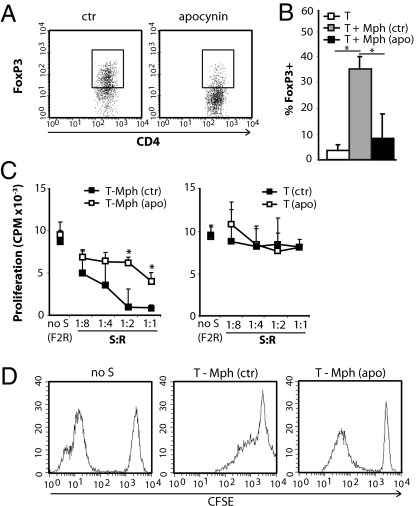
Mph differentiated with M-CSF induce CD4+CD25+FoxP3+ Tregs (24). The observed ROS-dependent suppression of T-cell activation by Mph could be mediated, at least in part, via induction of Tregs. To check this possibility, CD4+CD25− T cells were cocultured with Mph and anti-CD3/28 for 5 d, and the percentage of FoxP3+ cells among the CD3+CD4+CD25+ cells was assessed (Fig. 3 A, Left, and B). The percentage of FoxP3+ cells among the CD3+CD4+CD25+ cells was increased upon coculture of T cells with Mph, confirming their Treg-inducing capacity. Addition of apocynin to these cultures significantly reduced the number of Tregs (Fig. 3A, Right, and B), demonstrating the ROS dependency of Treg induction. To determine the functional capacity of these Tregs, suppression assays were performed. CD4+CD25− T cells were primed by Mph for 5 d in the presence of anti-CD3/28 and in the absence or presence of apocynin. These primed T cells were used as suppressor cells and combined with CD4+CD25− allogeneic carboxyfluorescein succinimidyl ester (CFSE)-labeled responder T cells and irradiated feeder cells. After 5 d, T-cell proliferation was assessed by 3H thymidine incorporation and CFSE dilution. T cells primed with Mph suppressed the proliferation of responder T cells in a dose-dependent fashion. In line with the observed ROS dependence of FoxP3 induction, priming in presence of apocynin prevented this suppressive activity (Fig. 3C, D). The ROS dependency of this effect was confirmed further by the observation that GM-CSF–differentiated Mph that hardly produced ROS did not induce Treg (Fig S2 A and B).
Fig. 3.
Mph induce Tregs via ROS. (A) Expression of FoxP3 within the CD3+CD4+CD25+ population of T cells primed with Mph in the absence (Left, ctr) or presence (Right) of apocynin. (B) CD4+CD25− T cells were primed with Mph in the presence [T + Mph (apo)] or absence [T + Mph (ctr)] of apocynin for 7 d, and then the percentage of FoxP3+ cells among CD3+CD4+CD25+ cells was analyzed. The white bar (T) represents CD4+CD25− T cells (cultured similarly but without Mph. Results shown are the average and SD of three experiments. (C) T cells were primed with (Left) or without (Right) Mph in presence or absence of apocynin (1 mM). After 5 d, these T cells were used as suppressor cells (S) and were combined with responder T cells (R) and irradiated feeder cells (F). Proliferation of responder cells was assessed by 3H thymidine incorporation (C) or by determining CFSE dilution (D). C shows average and SD of four experiments. In D, the 1:1 ratio from a representative experiment of three is shown, as well as the control condition without S but with twice the number of responders (F2R) to correct for crowding effects. *P < 0.05.
Mph from CGD Patients Show Disturbed Treg Induction.
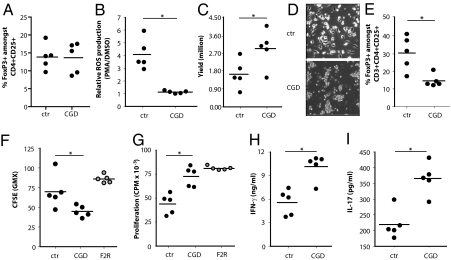
To obtain genetic proof of the ROS dependency of Treg induction, similar experiments were performed with Mph from CGD patients. The percentages of FoxP3+ cells among CD4+CD25+ cells were determined in peripheral blood from CGD patients and from healthy controls. No differences were observed (Fig. 4A). To study the role of ROS in the induction of Treg by Mph, monocytes from CGD patients and controls were isolated and cultured in M-CSF to obtain Mph. Mph from CGD patients and healthy controls showed similar morphology and expression levels of CD14 and CD163 (25), whereas the capacity to produce ROS was completely absent in Mph from CGD patients (Fig. 4B). Upon priming of CD4+CD25− T cells from one donor with either CGD or control Mph in presence of anti-CD3/28, we observed that CGD Mph allowed significantly more T-cell activation and expansion (Fig. 4 C and D). Moreover, CGD Mph induced significantly lower numbers of FoxP3+ T cells (Fig. 4E) than did control Mph. In a suppression assay, we observed that T cells primed by CGD Mph showed reduced inhibition of responder T-cell proliferation than did cells primed by control Mph (Fig. 4 F and G). In line with this observation, the levels of IFN-γ and IL-17 produced in these assays were significantly higher when suppressor cells were primed with CGD Mph than with control Mph (Fig. 4 H and I). These results provide genetic confirmation that production of ROS by Mph is involved in the induction of peripheral Tregs in human cells.
Fig. 4.
CGD Mph induce fewer Tregs. (A) The percentage of FoxP3+ cells among CD4+CD25+ cells in peripheral blood of CGD patients and healthy controls (ctr). (B) ROS production by Mph differentiated from healthy controls or CGD monocytes, as determined by flow cytometry after DHR123 staining. The ratio of ROS production after stimulation with PMA or DMSO is depicted. (C) CD4+CD25− T cells (106 per condition) from a single allogeneic donor were primed with Mph from either CGD patients or controls in presence of anti-CD3/28 Ab. After 5 d the number of viable T cells was determined. (D) Representative T-cell clustering observed after activation with anti-CD3/CD28 in presence of control Mph (Upper) or Mph derived from CGD patients (Lower). (Scale bars: 25 μm.) (E) T cells primed with Mph derived from CGD patients or controls were analyzed by flow cytometry for the percentage of FoxP3+ cells among CD4+CD25+ cells. (F) CFSE-labeled responder cells were cocultured with the T cells that were primed with ctr or CGD Mph and irradiated feeder cells (F2R) in the presence of anti-CD3/28, and dilution of CFSE was measured by flow cytometry after 4 d. (G) In parallel experiments, 3H thymidine incorporation was determined. Gray dots are control conditions in the absence of Mph-primed suppressor T cells but with double amounts of responder cells (F2R) to correct for crowding effects. In the supernatants of this suppression assay, IFN-γ (H) and IL-17 (I) levels were determined. *P < 0.05.
Mph Induce Tregs in Vivo in a ROS-Dependent Fashion.
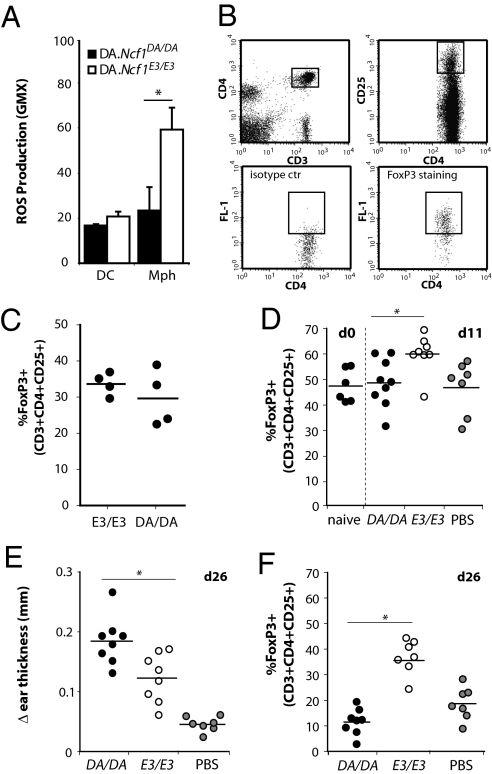
To investigate whether Mph induce Tregs in a ROS-dependent fashion in vivo, we used the congenic rat model (9). Dark Agouti (DA).Ncf1DA/DA rats have a reduced ROS-producing capacity because of SNPs (M106V and M153T) in Ncf1. DA.Ncf1E3/E3 congenic rats express the allelic variant that leads to higher ROS production. First, ROS production by Mph and DC cultured from bone marrow of both strains was determined. Similar to the human and murine (20) situation, Mph of the DA.Ncf1E3/E3 strain were able to produce significant levels of ROS after stimulation, whereas DC were far less efficient (Fig. 5A). To compare circulating Treg numbers, the percentage of FoxP3+ cells among CD3+CD4+CD25+ cells was measured, but no differences between the two strains were observed, similar to findings in CGD patients and controls (Fig. 5 B and C). To study the effect of Mph ROS on Treg induction and T-cell responses in vivo, allogeneic Lewis rats were primed with Mph from either DA.Ncf1E3/E3 or DA.Ncf1DA/DA rats. Eleven days later, all rats were immunized with irradiated splenocytes from DA.Ncf1DA/DA rats to boost the anti-DA response. At this time point Lewis rats primed with DA.Ncf1DA/DA Mph had lower numbers of CD3+CD4+CD25+FoxP3+ cells than those primed with ROS-sufficient DA.Ncf1E3/E3 Mph (Fig. 5D), whereas the number of activated T cells (CD4+CD25+) cells was comparable (Fig S3A). Two weeks later, all rats were challenged with irradiated DA.Ncf1DA/DA splenocytes in the ear to evoke a delayed-type hypersensitivity (DTH) response. After 24 h, rats initially primed with ROS-producing DA.Ncf1E3/E3 Mph showed significantly less ear swelling than rats primed with Mph from DA.Ncf1DA/DA rats (Fig. 5F). Moreover, these rats still showed higher levels of FoxP3+ cells in their peripheral blood (Fig. 5G), whereas the number of activated T cells did not differ between groups (Fig S3B). These results indicate that Mph induce Tregs in a ROS-dependent fashion in vivo, thereby leading to lower T-cell responses.
Fig. 5.
Mph suppress DTH responses in vivo in a ROS-dependent fashion. (A) Rat bone marrow cells were cultured with rat GM-CSF and IL-4 or human M-CSF to obtain DC and Mph, respectively. ROS production by PMA-stimulated DC and Mph generated from DA.Ncf1DA/DA or congenic Ncf1-wild-type DA.Ncf1E3/E3 rats was measured by DHR123 staining. Results shown are average and SD of four experiments. (B) Treg gating strategy on peripheral blood. (C) The percentage of FoxP3+ cells among CD3+CD4+CD25+ cells in peripheral blood of naïve DA.Ncf1E3/E3 (E3/E3) rats or DA.Ncf1DA/DA (DA/DA) rats. (D) Lewis rats were primed with Mph from DA.Ncf1DA/DA rats (DA/DA) or DA.Ncf1E3/E3 rats (E3/E3) or with PBS at day 0. At day 11, the percentage of FoxP3+ cells among CD3+CD4+CD25+ T cells was determined and compared with the levels in naïve Lewis rats. (E) After rats were immunized at day 11 and challenged in the ear at day 25 with irradiated DA/DA splenocytes, the difference in ear thickness, as a measure for the DTH reaction, was determined at day 26. (F) The percentage of FoxP3+ cells among CD3+CD4+CD25+ T cells at day 26 after priming. *P < 0.05.
Discussion
Here we show that, in both humans and rats, Mph-derived ROS suppress T-cell responses by induction of Tregs. This finding was confirmed by using Mph from CGD patients. Importantly, we show that Mph induces Tregs in a ROS-dependent fashion both in vitro and in vivo.
ROS can inhibit T-cell activation (14, 15, 26). For example, in cancer patients, granulocyte-derived H2O2 mediates impairment of T-cell function (27). The mechanism by which ROS affect T-cell responses still is unclear. Rats with defective ROS production because of an SNP in Ncf1 have more reduced proteins at their T-cell surfaces. The functional implications of these high cell-surface thiol levels were shown by arthritis transfer experiments: Decreasing the number of thiols on CD4+ T-cell surfaces abrogated their ability to transfer disease from sick DA.Ncf1DA/DA rats to naïve DA.Ncf1E3/E3 rats (12). Alternatively, ROS may pass the cell membrane and affect signal transduction proteins such as ζ-chain–associated protein kinase 70 (ZAP70) and linker of activation of T cell (LAT) (28). Another role for ROS has been suggested in the kynurenine pathway of tryptophan catabolism. In ROS-deficient mice with aspergillosis an O2-dependent step in the kynurenine pathway was blocked, contributing to the observed acute lung inflammation and unrestrained γδ T-cell activity (29). On the other hand, CGD Mph previously have been shown to have a normal tryptophan metabolism (30), and recently the microsomal cytochrome b5 rather than O2− was shown to activate indoleamine 2,3 dioxygenase (31). (However, this activity may vary among species.) Finally, it has been reported that ROS can induce apoptosis in T cells (32, 33), thereby decreasing the number of activated T cells. In our studies we did not see increased levels of apoptosis upon coincubation of Mph and T cells, so it is unlikely that increased apoptosis is the reason for the observed inhibition of activation. APC may affect T cells during antigen presentation through the production of ROS. This activity may take place in the immunological synapse, hence creating a micromilieu that allows oxidation of specific proteins; however, this notion has yet to be investigated.
DC generally are considered to be the most powerful APC (34). We show here, as was shown previously (20, 35), that DC are not very efficient at producing ROS. In contrast, the Mph we investigated were very efficient in producing ROS. These Mph are differentiated from monocytes with M-CSF and have an antiinflammatory phenotype (36–38). Our Mph2 and Mph1 may not represent exactly all Mph occurring in vivo, but they provided good polar models to answer our research question (39). We already have shown that these Mph, unlike proinflammatory-type Mph, are able to induce potent Tregs (24). Tregs play a critical role in the prevention of autoimmunity and resolution of inflammation (40). Because Mph prevented T cell-mediated autoimmunity in the mouse by producing ROS, we here studied the effect of ROS produced by Mph on T-cell activation and Treg induction, both in CGD patients and in an Ncf1-mutated rat model. Although ROS may have direct effects on T cells by oxidation of certain intracellular or membrane-bound proteins important for T-cell signaling, ROS also may induce Tregs through Mph. We chose to use T-cell activation by Mph in an allogeneic setting to exclude effects of ROS on antigen processing and presentation, which have been described previously (19). We showed that Mph induce Tregs from a CD4+CD25− population only when they are able to produce ROS. Both pharmacologic and genetic inhibition of ROS production abrogated the ability of Mph to induce Tregs. Interestingly, the percentages of Tregs were comparable in the peripheral blood of CGD patients vs. control subjects or in DA.Ncf1DA/DA vs. DA.Ncf1E3/E3 rats. This observation suggests that, in contrast to peripheral Treg induction, the number of natural Tregs induced in the thymus, is not affected by Mph ROS, although expansion of natural Tregs in the Lewis rats in the DTH experiments could not be excluded. Previously we have shown that Tregs induced by antiinflammatory Mph use membrane-bound TGF-β for suppression (24). Although we did not address the role of TGF-β in this study, it has been reported previously that ROS can activate TGF-β (41, 42). However, these ROS were not cell-derived, so it is unknown whether ROS produced by Mph upon interaction with a T cell has similar effects on membrane-bound TGF-β, and if such activation would affect Treg induction. It has also been shown that T cells themselves can produce ROS after anti-CD3/28 Ab activation and that these ROS activate TGF-β, leading to Treg induction (43). However, this ROS is produced intracellularly, and this mechanism probably is not comparable to our system. The exact mechanisms, however, still need to be investigated.
It is an attractive idea that, as long as activation signals remain below a certain threshold level, Mph prevent unwanted inflammation and autoimmunity by regulating T-cell responses via the production of ROS (3). Upon potent immune activation (e.g., efficient antigen presentation by DC), the immune-suppressive effect of Mph may be overwhelmed. This hypothesis is in line with the observation that both patients with CGD and mice with a nonfunctional Nox2 are more prone to develop autoimmunity (8, 10, 44, 29, 45). This observation suggests that Mph-derived ROS may protect against (auto-)immune activation. Indeed, altered monocyte function caused by aberrant inflammatory gene expression has been observed in CGD patients (46, 47). It would be interesting to investigate whether the increased autoimmunity and defective granuloma resolution in CGD patients result from a defect in Treg induction. Recently, a role for Treg in granuloma clearing was described in Wegener’s granulomatosis. Treg number and function were reduced in patients with this disease, and the reduction was most pronounced in subjects with most active disease (48).
To investigate if ROS production by Mph also could inhibit allogeneic T-cell responses in vivo, DTH experiments were performed in a rat model. These experiments showed that Mph can prevent T-cell responses and induce Tregs in a ROS-dependent fashion in vivo, thereby decreasing the allogeneic response. We thus demonstrated that the ability to produce ROS by APC plays a critical role in determining whether these APC will activate or suppress T cells. In conclusion, we show that Mph, by producing ROS, suppress T-cell activation and induce Tregs both in vitro and in vivo.
Methods
Animals.
Rats (DA or Lewis) were from Harlan or our own breeding (DA.Ncf1E3/E3; founders originating from Medical Inflammation Research, Karolinska Institute, Stockholm, Sweden) (9). For DTH experiments littermates were used (DA.Ncf1DA/DA and DA.Ncf1E3/E3). Rats were used at 8–12 wk of age, and groups were matched by sex and age. Rats were kept in polystyrene cages and fed standard rodent chow. Animal experiments were approved by the committee of medical ethics (CEM) of the Leiden University Medical Center.
Patients.
Peripheral blood was obtained from five CGD patients with mutations identified in the genes encoding p47phox (AR-CGD, homozygous Δdeletion in NCF1) or gp91phox (X-linked CGD, mutations in CYBB). Patients signed informed consents. PBMC were isolated as described below. The number of viable monocytes was not different from that in healthy controls.
Myeloid Cell Culture and T-Cell Isolation.
Human.
Monocytes were isolated from buffy coats by positive selection of CD14+ cells from the Ficoll interphase by MACS (Miltenyi) (38). Monocytes were cultured for 7 d in 10 ng/mL IL-4 plus 5 ng/mL GM-CSF (both from Biosource) to obtain DC or in 5 ng/mL Mph colony-stimulating factor (M-CSF) or GM-CSF (R&D Systems) to obtain Mph (38). Cells were cultured at 1.5 × 106cells per well in six-well plates, and medium containing cytokines was refreshed twice. For DC, the nonadherent cells were used. Mph were harvested by gentle scraping after short trypsin incubation (3 min, 37 °C). T cells were isolated from buffy coats by sheep RBC rosetting. For Treg experiments, CD4+ cells were isolated by MACS (negative selection kit; Miltenyi). CD25+ depletion was performed by panning on petri dishes coated with goat anti-mouse Ab and capturing T cells positively labeled with anti-CD25 Ab. These cells were >90% CD4+ and >99% CD25−.
Rat.
For rat, bone marrow cells were cultured in rat GM-CSF+ rat IL-4 (Biosource) to obtain DC or in human M-CSF (5 ng/mL) to obtain Mph. Medium was refreshed every other day. The culture medium contained additional l-glutamine (2 mM) and Fungizone (Gibco) but no β-mercaptoethanol. Cells were used after 7 d. T cells were isolated from spleens by magnetic sorting for CD3+ cells (Dynabeads; Invitrogen).
Mixed Leukocyte Reaction.
We cocultured 150,000 allogeneic T cells with irradiated (40 Gy) myeloid cells in a mixed leukocyte reaction in different ratios, with soluble anti-CD3 (IxE; 1 μg/mL) and anti-CD28 (CLB-CD28/1; 0.25 μg/mL) both kindly provided by L. A. Aarden (Sanquin, Amsterdam). After 5 d, supernatant was assayed for IFN-γ production (eBioscience), and cells were cultured for 16 h in presence of 3H thymidine (0.5 μCi). Thymidine incorporation was determined as a measure for proliferation. Anti-CD28 alone had no effect, and the Ab had no effect on myeloid cell proliferation in absence of T cells. Apocynin (Sigma) was used in concentration ranges up to 1 mM.
Flow Cytometry.
Expression levels of surface proteins were measured by flow cytometry (FACScalibur; BD Biosciences) after staining with specific conjugated Ab or unconjugated Ab detected by conjugated secondary Ab. ROS production was determined by incubating the cells with dihydrorhodamine123 (DHR123), 5 μM in RPMI++ at 37 °C for 10 min. Subsequently, oxidative burst was induced by adding PMA (200 ng/mL for 20 min at 37 °C). Human and rat Tregs were detected by staining with anti-CD25- phycoerythrin (PE), anti-CD3- peridinin-chlorophyll protein complex, and anti-CD4-allophycocyanin (BD Biosciences). Subsequently, cells were permeabilized, fixed (BD Biosciences), and stained with FITC-labeled anti-FoxP3 or FITC-labeled isotype control, according to the manufacturer's recommendations (eBioscience). The lymphocyte fraction was selected, and the percentage of FoxP3+ among CD3+CD4+CD25bright cells was determined. Apoptotic cells were determined by Annexin-V FITC/propidium iodide (BD Bioscences) double staining. Single- and double-positive cells were considered apoptotic and dead cells, respectively. The expression levels of p47phox and gp91phox also were determined by intracellular staining as described above for FoxP3 staining using mouse anti-human p47phox and gp91phox Ab (Santa Cruz Biotechnology) detected with goat anti-mouse PE (DAKO).
T-Cell Suppression Assay.
CD4+CD25− T cells from one donor were cultured for 5 d with Mph from another donor in a ratio of 6:1, in the presence of anti-CD3/28. After 5 d, T cells were harvested and depleted for HLA ClassII+ cells and used as suppressor (S) cells (<1% Mph contamination). Freshly isolated CD4+CD25− T cells were used as responder (R) cells. CD4− cells from the same donor were irradiated (40 Gy) and used as feeder (F) cells. The R:F:S ratio ranged from 8:16:32 to 8:16:1. A condition to control for equal cell numbers in all conditions with feeders and double the number of responder T cells (F2R) instead of FR was taken along to correct for possible crowding effects in the absence of suppressor cells as present in experimental conditions. Cells were stimulated with a low dose (1 μg/mL) of PHA or anti-CD3/28 (49). After 5 d cells were labeled for 8–16 h with 3H thymidine, and incorporated radioactivity was determined. Supernatant was subjected to cytokine analysis by ELISA or Luminex bead-based assay. In some experiments, responder cells were labeled with 5 μM CFSE, and proliferation was determined by FACS analysis, by gating on the CFSE-labeled population and quantifying the CFSE dilution.
mRNA Isolation and Quantitative PCR.
mRNA was isolated using a Qiagen kit. After making cDNA, semiquantitative PCRs were performed on a Bio-Rad Icycler machine using the following primers: p47phox (Forward: CCTGACGAGACGGAAGAC; Reverse: GGGAAGTAGCCTGTGACG), gp91phox (Forward: TAGTGGGAGCAGGGATTG; Reverse: TCAAAGGCATGTGTGTCC). The following GAPDH primers were used for normalization: Forward TTCCAGGAGCGAGATCCCT and reverse CACCCATGACGAACATGGG.
DTH Experiments.
Priming of Lewis rats at day 0 was done by i.v. injection of 5 × 106 Mph generated from either DA.Ncf1E3/E3 or DA.Ncf1DA/DA. Rats were immunized i.p. 11 d later with irradiated DA.Ncf1DA/DA splenocytes. On this day blood was drawn to determine the percentage of Tregs. After another 14 d, at day 25, rats were challenged in the left ear with irradiated splenocytes in PBS. The right ears were injected with PBS only. Ear swelling was measured before challenge and after 24 h and was expressed as the difference in thickness between the challenged and the control ear.
Statistics.
Results of different independent, although similarly performed, experiments were pooled, and the averages of the values were subjected to statistical analysis. Significant differences were determined using the Mann–Whitney U test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. M. Hultqvist for genotyping and E. van Beelen for Luminex assays. K.A.G. was supported by Grant KSPB07.0003 from the Dutch Kidney Foundation and VENI 916.86.049 from The Netherlands Organization for Scientific Research. M.D.K. was supported by Grant KSPB07.0001 from the Dutch Kidney Foundation. N.D.L.S. and T.H.M.O. are supported by the Netherlands Leprosy Relief Foundation, the Turing Foundation, and the European Commission. We are grateful for support from the Swedish Foundation for Strategic Research and the Masterswitch Grant HEALTH-F2-2008-223404 from the European Union.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012016107/-/DCSupplemental.
References
- 1.Hultqvist M, Olsson LM, Gelderman KA, Holmdahl R. The protective role of ROS in autoimmune disease. Trends Immunol. 2009;30:201–208. doi: 10.1016/j.it.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ghezzi P, Bonetto V, Fratelli M. Thiol-disulfide balance: From the concept of oxidative stress to that of redox regulation. Antioxid Redox Signal. 2005;7:964–972. doi: 10.1089/ars.2005.7.964. [DOI] [PubMed] [Google Scholar]
- 3.Gelderman KA, et al. Rheumatoid arthritis: The role of reactive oxygen species in disease development and therapeutic strategies. Antioxid Redox Signal. 2007;9:1541–1567. doi: 10.1089/ars.2007.1569. [DOI] [PubMed] [Google Scholar]
- 4.Kazama H, et al. Induction of immunological tolerance by apoptotic cells requires caspase-dependent oxidation of high-mobility group box-1 protein. Immunity. 2008;29:21–32. doi: 10.1016/j.immuni.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corzo CA, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chin TW, Stiehm ER, Falloon J, Gallin JI. Corticosteroids in treatment of obstructive lesions of chronic granulomatous disease. J Pediatr. 1987;111:349–352. doi: 10.1016/s0022-3476(87)80452-3. [DOI] [PubMed] [Google Scholar]
- 7.Rosenzweig SD. Inflammatory manifestations in chronic granulomatous disease (CGD) J Clin Immunol. 2008;28(Suppl 1):S67–S72. doi: 10.1007/s10875-007-9160-5. [DOI] [PubMed] [Google Scholar]
- 8.Schäppi MG, Jaquet V, Belli DC, Krause KH. Hyperinflammation in chronic granulomatous disease and anti-inflammatory role of the phagocyte NADPH oxidase. Semin Immunopathol. 2008;30:255–271. doi: 10.1007/s00281-008-0119-2. [DOI] [PubMed] [Google Scholar]
- 9.Olofsson P, et al. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet. 2003;33:25–32. doi: 10.1038/ng1058. [DOI] [PubMed] [Google Scholar]
- 10.Hultqvist M, et al. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci USA. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmberg J, et al. Pristane, a non-antigenic adjuvant, induces MHC class II-restricted, arthritogenic T cells in the rat. J Immunol. 2006;176:1172–1179. doi: 10.4049/jimmunol.176.2.1172. [DOI] [PubMed] [Google Scholar]
- 12.Gelderman KA, Hultqvist M, Holmberg J, Olofsson P, Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci USA. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 14.Matsue H, et al. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J Immunol. 2003;171:3010–3018. doi: 10.4049/jimmunol.171.6.3010. [DOI] [PubMed] [Google Scholar]
- 15.Tse HM, et al. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- 16.Forman HJ, Torres M. Signaling by the respiratory burst in macrophages. IUBMB Life. 2001;51:365–371. doi: 10.1080/152165401753366122. [DOI] [PubMed] [Google Scholar]
- 17.DeYulia GJ, Jr, Cárcamo JM, Bórquez-Ojeda O, Shelton CC, Golde DW. Hydrogen peroxide generated extracellularly by receptor-ligand interaction facilitates cell signaling. Proc Natl Acad Sci USA. 2005;102:5044–5049. doi: 10.1073/pnas.0501154102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantegazza AR, et al. NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood. 2008;112:4712–4722. doi: 10.1182/blood-2008-01-134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savina A, et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell. 2006;126:205–218. doi: 10.1016/j.cell.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 20.Gelderman KA, et al. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J Clin Invest. 2007;117:3020–3028. doi: 10.1172/JCI31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 22.Bluestone JA, Tang Q. Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells. Proc Natl Acad Sci USA. 2004;101(Suppl 2):14622–14626. doi: 10.1073/pnas.0405234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Q, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savage ND, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181:2220–2226. doi: 10.4049/jimmunol.181.3.2220. [DOI] [PubMed] [Google Scholar]
- 25.Xu W, et al. Human peritoneal macrophages show functional characteristics of M-CSF-driven anti-inflammatory type 2 macrophages. Eur J Immunol. 2007;37:1594–1599. doi: 10.1002/eji.200737042. [DOI] [PubMed] [Google Scholar]
- 26.Khan N, et al. Hydrogen peroxide inhibits IL-12 p40 induction in macrophages by inhibiting c-rel translocation to the nucleus through activation of calmodulin protein. Blood. 2006;107:1513–1520. doi: 10.1182/blood-2005-04-1707. [DOI] [PubMed] [Google Scholar]
- 27.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 28.Gringhuis SI, et al. Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J Immunol. 2000;164:2170–2179. doi: 10.4049/jimmunol.164.4.2170. [DOI] [PubMed] [Google Scholar]
- 29.Romani L, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 30.Murray HW, et al. Role of tryptophan degradation in respiratory burst-independent antimicrobial activity of gamma interferon-stimulated human macrophages. Infect Immun. 1989;57:845–849. doi: 10.1128/iai.57.3.845-849.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maghzal GJ, Thomas SR, Hunt NH, Stocker R. Cytochrome b5, not superoxide anion radical, is a major reductant of indoleamine 2,3-dioxygenase in human cells. J Biol Chem. 2008;283:12014–12025. doi: 10.1074/jbc.M710266200. [DOI] [PubMed] [Google Scholar]
- 32.Kuang DM, et al. Tumor-educated tolerogenic dendritic cells induce CD3epsilon down-regulation and apoptosis of T cells through oxygen-dependent pathways. J Immunol. 2008;181:3089–3098. doi: 10.4049/jimmunol.181.5.3089. [DOI] [PubMed] [Google Scholar]
- 33.Tripathi P, Hildeman D. Sensitization of T cells to apoptosis—a role for ROS? Apoptosis. 2004;9:515–523. doi: 10.1023/B:APPT.0000038033.14925.02. [DOI] [PubMed] [Google Scholar]
- 34.Ueno H, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 35.Elsen S, et al. Cryptic O2- -generating NADPH oxidase in dendritic cells. J Cell Sci. 2004;117:2215–2226. doi: 10.1242/jcs.01085. [DOI] [PubMed] [Google Scholar]
- 36.Verreck FA, et al. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porta C, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci USA. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu W, et al. IL-10-producing macrophages preferentially clear early apoptotic cells. Blood. 2006;107:4930–4937. doi: 10.1182/blood-2005-10-4144. [DOI] [PubMed] [Google Scholar]
- 39.Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol. 2010;10:453–460. doi: 10.1038/nri2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esensten JH, Wofsy D, Bluestone JA. Regulatory T cells as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:560–565. doi: 10.1038/nrrheum.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 42.Pociask DA, Sime PJ, Brody AR. Asbestos-derived reactive oxygen species activate TGF-beta1. Lab Invest. 2004;84:1013–1023. doi: 10.1038/labinvest.3700109. [DOI] [PubMed] [Google Scholar]
- 43.Amarnath S, Dong L, Li J, Wu Y, Chen W. Endogenous TGF-beta activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25- T cells. Retrovirology. 2007;4:57. doi: 10.1186/1742-4690-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Q, et al. p47phox deficiency induces macrophage dysfunction resulting in progressive crystalline macrophage pneumonia. Am J Pathol. 2009;174:153–163. doi: 10.2353/ajpath.2009.080555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.George-Chandy A, et al. Th17 development and autoimmune arthritis in the absence of reactive oxygen species. Eur J Immunol. 2008;38:1118–1126. doi: 10.1002/eji.200737348. [DOI] [PubMed] [Google Scholar]
- 46.Brown KL, et al. ROS-deficient monocytes have aberrant gene expression that correlates with inflammatory disorders of chronic granulomatous disease. Clin Immunol. 2008;129:90–102. doi: 10.1016/j.clim.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Selmeczy Z, Szelényi J, Német K, Vizi ES. The inducibility of TNF-alpha production is different in the granulocytic and monocytic differentiated forms of wild type and CGD-mutant PLB-985 cells. Immunol Cell Biol. 2003;81:472–479. doi: 10.1046/j.1440-1711.2003.01190.x. [DOI] [PubMed] [Google Scholar]
- 48.Morgan MD, et al. Patients with Wegener’s granulomatosis demonstrate a relative deficiency and functional impairment of T-regulatory cells. Immunology. 2010;130:64–73. doi: 10.1111/j.1365-2567.2009.03213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, van Dongen H, Scherer HU, Huizinga TW, Toes RE. Suppressor activity among CD4+,CD25++ T cells is discriminated by membrane-bound tumor necrosis factor alpha. Arthritis Rheum. 2008;58:1609–1618. doi: 10.1002/art.23460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.