Abstract
The immune response to tumor can be enhanced by targeting costimulatory molecules on T cells. As the CD70-CD27 costimulatory axis plays an important role in the activation, survival and differentiation of lymphocytes, we have examined the efficacy of agonistic anti-CD27 antibodies as monotherapies for established melanoma in a murine model. We demonstrate that this approach leads to a substantial reduction in the outgrowth of both experimental lung metastases and sub-cutaneous tumors. Anti-CD27 treatment supports the maintenance of tumor-specific CD8+ T cells within the tumor, reduces the frequency of FoxP3-expressing CD4+ T cells within tumors, and potentiates the ability of NK1.1+ and CD8+ tumor infiltrating cells to secrete IFNγ upon coculture with tumor cells. The enhanced effector function correlated with lower levels of PD-1 expression on CD8+ T cells from anti-CD27 treated mice. Despite the modulating effect of anti-CD27 on multiple cell types, only CD8+ T cells were absolutely required for tumor control. CD4+ T cells were dispensable, while NK1.1+ cells were needed during early stages of tumor growth but not for the effectiveness of anti-CD27. Thus, CD27-mediated costimulation provides a potent boost to multiple aspects of the endogenous responses to tumor, and may be exploited to enhance tumor immunity.
Keywords: immunotherapy, CD8+ T cells, CD27, FoxP3, PD-1
Introduction
The immune system’s ability to mount responses against tumor is now well-established (36). Tumor control generally requires the concerted activity of cytotoxic CD8+ T cells and NK cells, and in many instances can be enhanced by the expansion of tumor reactive helper CD4+ T cells. However, the capacity for lymphocytes to perform effector functions in established tumors can be limited either by suboptimal priming conditions (20), or by a variety of intrinsic and extrinsic mechanisms that suppress lymphocyte activation and/or function (13;15;17;22), culminating in diminished effector responses to the tumor.
The induction of T cell responses to tissue associated antigens usually requires cross-presentation of tumor-derived antigen by CD8α+ dendritic cells (DC) (3;16;27). The activation state and immunogenicity of DC is highly dependent upon engagement of CD40 by CD40L-expressing CD4+ T cells (6;33;35), but targeting CD40 for cancer therapy has shown limited results (43). The efficacy of anti-CD40 treatment in experimental models is significantly enhanced by the inclusion of innate receptor agonists (2;5;44). The mechanistic basis for enhanced immunogenicity of DC after CD40-stimulation/TLR stimulation has not been completely elucidated; however, we and others have demonstrated that the induction of CD70 (TNFSF7) on CD40-stimulated DC substantially contributes to their ability to elicit CD8+ T cell responses (10;18;34;41), and blocking CD70 reduces the efficacy of anti-CD40 treatment of experimental leukemias (18). Thus, the induced expression of CD70 likely plays an important role in the efficacy of CD40-targeting therapies.
While not absolutely required for most lymphocyte responses, CD70 engagement of its receptor, CD27 (TNFRSF7), enhances the activation and survival of CD8+ T cells and their subsequent differentiation into memory cells (24;25); the differentiation of CD4+ T cells into IFNγ-secreting cells (37;42;46); and the proliferation and effector activity of NK cells (31;40). Importantly, induced expression of CD70 in transgenic mice can lead to the reactivation of anergic or tolerant CD8+ T cells (29). Thus, targeting CD70 induction, or directly stimulating CD27, might be expected to augment immune responses against tumor. Supporting this notion, transgenic expression of CD70 on B cells has been shown to enhance protection against outgrowth of transplanted tumor (4). Further, recent studies have demonstrated that selective induction of CD70 expression on DC enhances their ability to support tumor control by co-transferred naïve transgenic CD8+ T cells (30). However, it is not certain whether stimulation of CD27 can independently control established, poorly immunogenic tumors, and the mechanistic basis by which targeting CD27 signaling enhances the control of tumor remains to be determined.
Here we show that a substantial proportion of the effector lymphocytes present in established tumors maintain expression of CD27, and that agonistic antibodies specific for CD27 support the control of both established lung metastases and subcutaneous tumor. The control of tumor was critically dependent upon CD8+ T cells and IFNγ, and correlated with both enhanced effector activity and reduced expression of the inhibitory molecule PD-1.
Materials and Methods
Animals
C57Bl/6 mice were obtained from Taconic (Germantown, NY). CD27−/− mice were generated by Dr Jannie Borst (Netherlands Cancer Institute) and provided by Dr Stephen Schoenberger (La Jolla Institute of Allergy and Immunology, CA). Mice were maintained in specific pathogen-free facilities and were treated in accordance with the guidelines established by the Animal Care and Use Committee at the University of Virginia.
Antibodies
To develop AT124-1, mouse CD27-huFc was produced in-house by transfected CHO cells and purified on Protein-A. LOU rats were immunized with 20-50ug CD27-huFc in CFA, IFA and PBS, the spleen cells were fused with NSI and resulting clones screened by ELISA on fusion protein followed by staining of Con A activated spleen cells. AT124-1 had similar binding to commercial Hamster anti-CD27 LG3 A10 (Becton Dickinson, San Jose, CA), was partially blocked by CD70-Fc fusion protein and clearly stained CD27 transfected cells (18). The development of RM27-3E5 has been described previously (38). Anti-PD-L1 (clone MIH5) was purchased from eBioscience (San Diego, CA). Depleting antibodies for CD8 (2.43), CD4 (GK1.5) and NK1.1 (PK136) were isolated from hybridoma cultures generated in the University of Virginia Lymphocyte Culture Core Laboratory. Control immunoglobulins were obtained from Sigma (St Louis, MO). All antibodies contained less that 100 pg/ml endotoxin. Fluorescently-conjugated antibodies specific for CD8, CD4, NK1.1, CD27, FoxP3, IFNγ, PD-1 and CTLA-4 were obtained from eBioscience and were titrated for optimal staining prior to experimental use.
Cell lines
B16F1 and LB15.13 were obtained from ATCC (Rockville, MD), were maintained in RPMI (Sigma) media containing 5% FBS (Hyclone, Logan, UT). B16cOVA were generated previously (28), and maintained in RPMI with 5% FBS and selected with blastocidin. Cell lines were verified for identity by microscopy and flow cytometric staining for the tyrosinase and ovalbumin proteins (B16F1/B16cOVA) and MHC class II expression and CD19 expression (LB15.13). Mycoplasma tests were periodically performed.
Peptides
Synthetic peptides were made, purified and validated as previously described (10).
Tumor treatment
For lung metastases studies, 4×105 B16cOVA or 1×105 B16F1 melanoma cells were injected intravenously. For s.c. tumors, 4×105 B16cOVA were injected into the sub-scapular space. 7d after injection, cohorts of mice (n=5) were treated with injections of 100 μg AT124-1, RM27-2E5 or cIg delivered i.p. every 3-4d. Mice were only used if the tumor was palpable. Lung metastases were evaluated on d21 by counting total visible lesions using a dissecting scope (x40 magnification). Tumor growth was measured at 24-48h intervals using a vernier caliper. Depletions of CD8, CD4 and NK1.1-expressing cells were performed either upfront by injecting 100μg of antibody i.p. 7d, 4d and 1d prior to establishing tumors, or on d6 and d7 after tumor had been established and each 7d thereafter. CD25+ regulatory T cells were depleted by treatment with 500 μg PC61.5 delivered i.p. 7d and 4d prior to tumor implantation. Depletion effectiveness was confirmed by flow cytometric analysis of tail vein bleeds.
Tumor infiltrating lymphocyte phenotyping and tetramer staining
H2-Kb-tetramers that had been folded around OVA257 were provided by Dr. Vic Engelhard, University of Virginia. Lymphocytes from lung tumors were isolated from perfused lungs on nycodenz gradients after collagenase, hyaluronidase and DNAse digestion as previously described (28). Lymphocytes were isolated from tumors and lymphoid organs by mechanical homogenization of excised tissue followed by Lympholyte-Mammal (Cederlane, Burlington, Canada) based density centrifugation. Enriched TIL were co-incubated for 30 min at 4°C with tetramer-APC, and fluorescently conjugated antibodies specific for CD8, CD4, CD3, NK1.1, CD27, CTLA-4 or PD-1. Detection of intracellular FoxP3 was performed after surface staining, according to the manufacturer’s directions. Staining was assessed by flow cytometry on a FACS Canto or Canto II using CellQuest software (BD Biosciences) and analyzed with FloJo software (Treestar).
Intracellular cytokine staining
Cytokine expression was examined ex vivo from TIL. isolated as described above, then incubated in vitro for 5h at a 1:1 ratio with B16cOVA, LB15.13 stimulator cells that had been incubated overnight in the presence of 100 μg/ml OVA257 or OVA323, or PMA/Ionomycin, in medium supplemented with 50 IU/ml IL-2 (Chiron) and 10 μg/ml brefeldin A (Calbiochem). Stimulated cultures were counterstained with anti-CD8, CD4 or NK1.1 (eBioscience), washed, then fixed and permeabilized in PermWash/Fix (BD Pharmingen), followed by staining with anti-IFN-γ. Values obtained from unpulsed stimulators were subtracted. Staining was assessed by flow cytometry as described above.
TIL cultures
Lymphocytes were isolated from tumors as above, then incubated in vitro in the presence or absence of LB15.13 cells pulsed with 10 μg/ml OVA257, with or without 10U/ml of IL-2 in the cultures. OVA257-specific CD8+ T cell numbers and function were assessed 7d later by MHC-tetramer staining and intracellular cytokine staining as above.
Statistics
Statistical significance of differences between comparison groups was determined by performing either unpaired two-tailed Mann-Whitney tests,with Bonferroni correction, for 95% confidence limits, or log-rank tests (for Kaplan-Meier survival curves) using GraphPad Prism software (San Diego, CA).
Results
Anti-CD27 is therapeutically effective in the treatment of established lung metastases
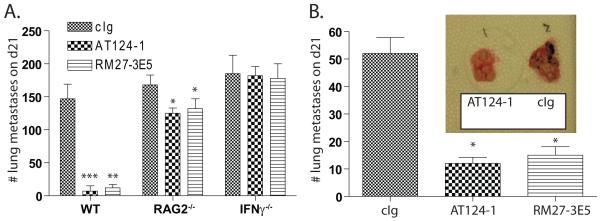
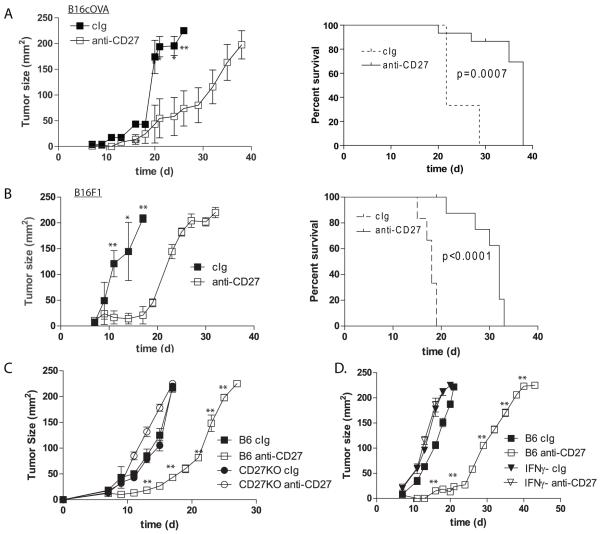
The ability for CD27-mediated costimulation to control the outgrowth of established tumors was directly assessed using two independently derived agonistic antibodies. Mice bearing 7d established B16cOVA experimental metastases were treated with 100 μg AT124-1 (18), RM27-3E5 (38) or control immunoglobulin (cIg) every 3-4d. 21d after initial tumor injection, mice were euthanized and the number of lung metastases was counted. Control treated mice bore high numbers of lung metastases (147 +/− 22 metastases). Those treated with either AT124-1 (7 +/− 8 metastases; P<0.001) or RM27-3E5 (12 +/− 5 metastases; P<0.01) had limited tumor burden at this time-point (Figure 1A). The majority of tumor control was lost when similar experiments were performed in RAG2- or IFNγ-deficient mice (Figure 1A), and no protection was achieved in CD27-knockout mice (Figure 2C). For a more stringent test, we next asked whether anti-CD27 treatment could control the less-immunogenic parental B16F1 tumor. Lung metastases or subcutaneous tumors were again established for 7d prior to the initiation of treatment. Lung were examined 21d after establishment, while s.c. tumors were measured every 24-48h. In confirmation of the results obtained with B16cOVA, mice treated with AT124-1 (12+/− 5 metastases; P<0.05) or RM27-3E5 (15 +/− 7; P<0.05) had significantly fewer tumor nodules compared to those treated with control antibody (52+/− 13 metastases) (Figure 1B), and delayed s.c. tumor growth (Figure 2B). These data indicate that targeting CD27 is a therapeutically effective maneuver that enhances tumor control by a mechanism that is substantially dependent upon IFNγ and adaptive immunity.
Figure 1. Control of established lung metastases by anti-CD27 treatment.
(A). WT, RAG2-knockout or IFNγ-knockout mice received 4×105 B16cOVA i.v. 7d after implantation, treatment with cIg, AT124-1 or RM27-3E5 (n=5 per cohort) was initiated. 21d after implantation, lung metastases were counted. *p<0.05; **p<0.01; ***p<0.001 compared to cIg. Data are representative of 2 similar experiments. (B). 1×105 B16F1 were i.v. transplanted into WT mice (n=5 per cohort). Treatment with AT124-1 or RM27-3E5 began on d7. *p<0.05. Data are representative of 3 independent experiments. Inset: photograph of representative lungs from AT124-1 or cIg treated mice.
Figure 2. Control of s.c. established tumor by CD27 stimulation and expression of CD27 on TIL.
(A and B) C57Bl/6 mice, (C) CD27 KO or (D) IFNγ KO mice were implanted s.c. with (A, C and D) 4×105 B16cOVA, or (B) 1×105 B16F1. On d7 treatment with AT124-1 proceeded as described in Figure 1. Tumor size was measured every 1-2d, and mice were euthanized if tumors reached protocol limits (survival plots). *p<0.05; **p<0.01, comparing anti-CD27 treated and cIg at the indicated timepoints. Survival plots were generated by the Kaplan-Meier method and significance established with the log-rank test. Experiment was repeated twice with similar results.
Expression of CD27 on tumor infiltrating lymphocytes
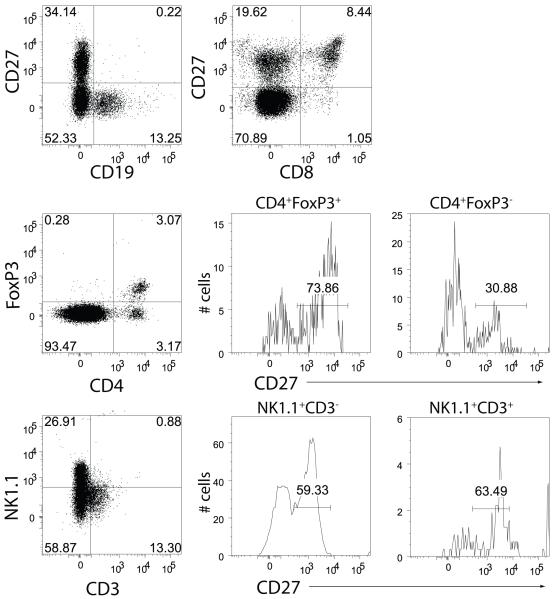
An assessment of the impact of CD27-mediated costimulation on the phenotypical and functional characteristics of tumor infiltrating lymphocytes (TIL) in small lung metastases is technically limited due to the close association of the metastases and the lung parenchyma. Therefore, we examined the impact of anti-CD27 on TIL associated with B16cOVA outgrowth in the subcutaneous (s.c.) setting. Treatment with AT124-1 significantly delayed the outgrowth of the established tumors, and prolonged the survival time of the tumor-bearing mice (Figure 2A). Similar results were obtained with RM27-3E5 (not shown), and control was again completely absent in CD27-deficient or IFNγ−/− mice (Figures 2C and 2D). To identify potential effector cells, we examined which lymphocytes present in d7 tumors express CD27 and thus might be influenced by CD27-mediated stimulation (Figure 3). Tumors were infiltrated with CD19+ B cells, though none expressed CD27. The majority of both the CD8+ T cells (80 +/− 5%) and the CD4+ T cells (60 +/− 8%) in tumor expressed CD27. Notably, a greater proportion of the FoxP3-expressing CD4+ T cells expressed CD27 (66 +/−11%) compared to CD4+ T cells in which FoxP3 was absent (31 +/− 4%). A considerable proportion of the TIL consisted of NK1.1+ cells. The majority (60 +/− 3%) of the CD3−NK1.1+ NK cells expressed CD27, as did the few CD3+NK1.1+ T cells (63 +/− 7%) that were evident in the tumor. Thus, CD27 is expressed on multiple types of tumor infiltrating lymphocytes that have the potential to modulate tumor outgrowth.
Figure 3. Expression of CD27 on TIL subpopulations.
D7 established tumors were excised and infiltrating lymphocytes were stained for the expression of CD19, CD8, CD4, NK1.1, CD3, FoxP3 and CD27. Numbers in dot plot quadrants and histograms describe percent of gated cells. Data are representative of 3 tumors.
CD27-mediated costimulation modulates presence of OVA257-specific CD8+ T cells in tumor
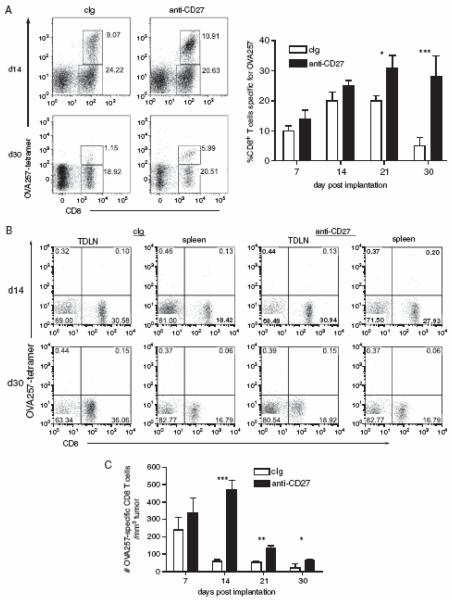
To define how anti-CD27 treatment might be enhancing tumor control, we compared the frequency and effector activities of TIL in cIg treated or anti-CD27 treated mice. Tumors were excised 7d, 14d and 23d after the initiation of anti-CD27 treatment, and stained for the presence of CD4+, NK1.1+ and CD8+ cells. The relative frequency of these populations of lymphocytes did not significantly differ between anti-CD27 and cIg treated mice over the duration of the assay (not shown). However, the proportion of CD8+ T cells that were specific for the immunodominant OVA257 epitope steadily increased over time in the tumors of anti-CD27 treated animals, while those in cIg treated mice dissipated (Figure 4A). In terms of absolute numbers of OVA257-specific CD8+ T cells/tumor, cIg treated tumors contained more on d14 and d21 due to the larger tumor size; however, anti-CD27 treated mice had significantly greater number of OVA257-specific CD8+ T cells per mm3 of tumor at all time points aside from d7 (Figure 4C). Notably, very few OVA257-specific CD8+ T cells were found in either TDLN or spleen after d7 (Figure 4B). Thus, anti-CD27 treatment supports the expansion and/or survival of tumor-specific CD8+ T cells and this correlates with reduced outgrowth of the tumor.
Figure 4. CD27 stimulation maintains the frequency of OVA257-specific CD8+ T cells in tumor.
Tumors were established by s.c. implantation of 4×105 B16cOVA. Mice were treated with anti-CD27 as described in Methods. Tumors were excised on d7, d14, d21 and d30 and TIL were isolated and costained for CD8, CD44 and OVA257-MHC tetramers. (A and B). Frequency of OVA257-specific CD8+ T cells within tumors and TDLN. Representative dot plots, gated on CD44hi lymphocytes (determined by FSC vs SSC characteristics), showing CD8 and OVA257-tetramer staining from either cIg (left plots) or anti-CD27 treated mice (right plots) on d14 and d30 of (A) tumors and (B) TDLN. Chart shows summary data of the frequency of OVA257-specific CD8+ T cells in TIL overtime. (C). Number of OVA257-specific CD8+ T cells with respect to the size of the tumor. For the charts, 5 tumors were assessed at each time point. *p<0.05; **p<0.01; ***p<0.001 compared to cIg treated. The experiment was repeated twice with similar results.
CD27-mediated costimulation enhances effector activity of CD8+ T cells and NK cells in TIL
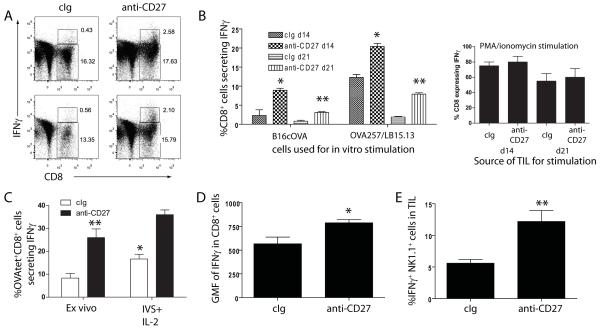
We next asked whether anti-CD27 treatment augmented effector activity of TIL, with a particular focus on IFNγ production as its absence abrogated the effectiveness of anti-CD27 therapy (Figures 1 and 2). Isolated lymphocytes from d14 tumors were co-cultured in vitro with B16cOVA cells, LB15.13 cells pulsed with OVA257, OVA323 or control peptides, or PMA/Ionomycin. Cultures were stained for the surface markers CD8, CD4 and NK1.1, and intracellularly for the expression of IFNγ. Approximately 10% of the CD8+ T cells from anti-CD27 treated tumors secreted IFNγ in response to B16cOVA (Figures 5A and 5B) and ~25% responded to OVA257 pulsed target cells (Figure 5B), while only ~2.5% and 12% of cIg treated CD8+ TIL responded to the respective stimulator cells (Figure 5B). In contrast, the proportion that secreted IFNγ after coculture with PMA/ionomycin was equivalent between the two groups (Figure 5B). Of the CD8+ T cells from d14 tumors that expressed IFNγ, those treated with anti-CD27 secreted ~50% higher levels of IFNγ on a per cell basis than those treated with cIg (Figure 5C). Of note, the overall ability of TIL from d21 tumors to produce IFNγ after in vitro stimulation was significantly reduced compared to TIL from d14 tumors. When compared as a proportion of the total OVA257-specific CD8+ T cell population (defined by MHC-tetramer staining (Figure 4)), 50% of the cIg treated OVA257-specific CD8+ T cells could produce IFNγ after coculture with OVA257-pulsed LB15.13. In contrast, 80% of OVA257-specific CD8+ T cells from tumors treated with anti-CD27 could produce IFNγ after OVA257 stimulation. By d21, 25% of the anti-CD27 CD8+ T cells could still produce IFNγ after OVA257 stimulation, compared to only 10% of cIg treated CD8+ T cells. Therefore, treatment with anti-CD27 maintains a higher proportion of IFNγ-expressing cells within the OVA257-specific CD8+ T cell population (Figure 5B).
Figure 5. CD27 stimulation maintains IFNγ-production by effector TIL.
(A). Isolated TIL from cIg (left dot plots) or anti-CD27 (right dot plots) treated mice bearing d14 s.c. B16cOVA tumors were cocultured in vitro with B16cOVA, surface stained for CD8 expression, and assessed for IFNγ production. Numbers in the regions describe the frequency of CD8+ T cells and CD8+ IFNγ+ T cells. Dot plots show duplicate representative tumors from a single experiment of 3 performed. (B). Summary of the frequency of IFNγ-producing CD8+ T cells from d14 or d21 tumors from cIg or anti-CD27 treated mice after in vitro coculture with B16cOVA or OVA257-pulsed LB15.13 cells (left chart) or PMA/ionomycin (right chart). (C). Partial restoration of TIL function by IL-2. Proportion of OVA257-tetramer+ CD8+ T cells from TIL of cIg or anti-CD27 treated tumors that produce IFNγ either after direct ex vivo isolation, or after 5d of coculture with OVA257 and IL-2. *p<0.05 compared to proportion of cells secreting IFNγ directly ex vivo; **p<0.01, comparing direct ex vivo production of IFNγ by TIL from cIg and anti-CD27 treated tumors. (D). Geometric mean fluorescence of IFNγ from CD8+ T cells derived from d14 tumors after coculture with B16cOVA. (E). Expression of IFNγ by NK1.1+ cells isolated from d14 tumors after coculture with B16cOVA. For B, D and E: *p<0.05; **p<0.01 compared to cIg treated mice at the respective time points. Assays in B-E were repeated 2-3 times.
In addition to the effects of anti-CD27 on CD8+ T cells, twice the frequency of NK1.1+ cells from anti-CD27 treated tumors secreted IFNγ after coculture with B16cOVA as compared to NK1.1+ cells from tumors of cIg treated mice (Figure 5D). No CD4+ T cells secreted IFNγ after coculture with B16cOVA, and little IFNγ-secretion was observed in cultures with OVA323 pulsed LB15.13 cells, or after PMA/Ionomycin treatment (not shown). Therefore, while the frequency of both OVA257-specific CD8+ T cells and NK1.1+ cells is equivalent in d14 tumors in cIg and anti-CD27 treated mice, anti-CD27 treatment augments or sustains IFNγ-production by tumor infiltrating CD8+ T cells and NK1.1+ cells.
CD27-mediated costimulation reduces the proportion of FoxP3+ CD4+ T cells in tumor
The analysis of CD27 expression by TIL had revealed that FoxP3-expressing CD4+ T cells (Treg) strongly expressed CD27 (Figure 3). As Treg can play an important role in constraining the immune response against B16 melanomas (39), and could explain the reduction in IFNγ production by CD8+ TIL, we assessed whether anti-CD27 treatment had any effect on Treg frequency or function. Lymphocytes isolated from d14 tumors (7d after initiation of anti-CD27) contained ~2-fold lower number of FoxP3+ cells/mm3 tumor (Table 1). However, no difference in suppressor activity, on a per cell basis, was found when enriched CD4+CD25+ T cells from cIg or anti-CD27 treated mice were assessed in a standard in vitro proliferation assays (not shown). Further, specific depletion of Treg by anti-CD25 treatment prior to tumor implantation did not alter the kinetics of tumor outgrowth for either control or anti-CD27 treated mice (Figure 6A). Thus, anti-CD27 treatment reduces the frequency of FoxP3-expressing cells within tumors, resulting in a ~9-fold increase in the ratio of OVA257-specific CD8+ T cells: FoxP3+CD4+ T cells, but the therapeutic efficacy of anti-CD27 is independent of Tregs.
Table 1.
Ratio of OVA257-tetramer+ CD8+ T cells: FoxP3+ CD4+ T cells in d14 tumors
| Treatment | #FoxP3+ cells/ mm3 tumor |
#OVA257-tetramer+ cells/ mm3 tumor |
Ratio |
|---|---|---|---|
| cIg (n=10) | 140+/−44 | 122+/−84 | 0.9 |
| Anti-CD27 (n=12) | 76+/−87 | 600+/−167 | 7.8 |
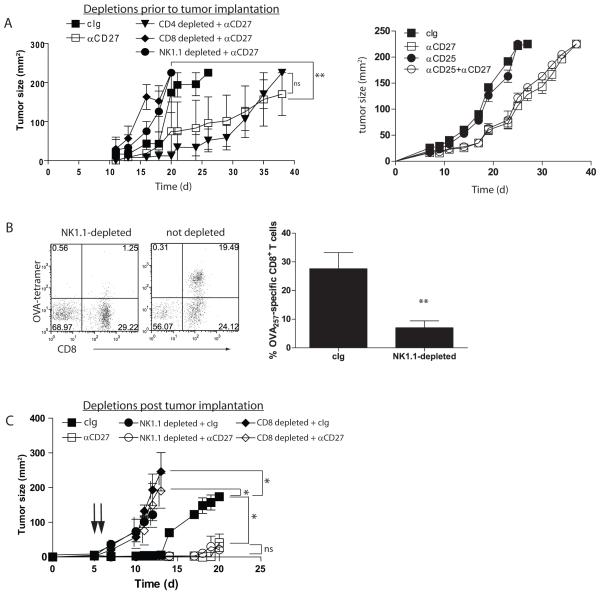
Figure 6. CD27-mediated control of s.c. tumor is dependent upon NK1.1+ cells and CD8+ T cells.
(A). Cohorts of mice (n=5) were depleted (7d, 4d, 1d prior to tumor implantation) of NK1.1-, CD4- or CD8-expressing cells, or treated with cIg, and implanted with 4×105 B16cOVA. Treatment with anti-CD27 (100 μg every 3-4d) began 7d after implantation. Tumor outgrowth was measured over time; **p<0.01, comparing either NK1.1 or CD8 depleted, anti-CD27 treated mice to control depleted anti-CD27 treated mice. Data is from one of two similar experiments. (B). Dot plots showing representative CD8+ OVA257 tetramer staining of TIL from d21 tumors from NK1.1-depleted or undepleted mice treated with anti-CD27. The numbers in the quadrants describe the percentage of the population expressing each marker. Chart shows mean+/−SEM for OVA257- tetramer+CD8+ T cells in tumors from cIg or αNK1.1 treated mice (n=5 each). **p<0.01. (C). Tumors were established as in (A) for 5d. Mice received depleting antibodies specific for NK1.1 (circles) or CD8 (diamonds) on d6 and d7, and treatment with cIg (filled symbols) or anti-CD27 (open symbols) began on d7. Tumor outgrowth was monitored over time. *p<0.05 for indicated d14 comparisons. ns= not significant. Data is from one of two similar experiments. Arrows indicate antibody infusions.
Control of tumor by anti-CD27 is lost in the absence of CD8+ T cells and NK1.1+ cells, but not CD4+ T cells
To distinguish the contribution of each of the lymphocyte subsets in anti-CD27-mediated control of tumor, cohorts of mice were depleted of CD8+, CD4+ or NK1.1+ cells. Eliminating CD4+ T cells had no significant impact on the ability of anti-CD27 to augment control of tumor outgrowth. In contrast, depletion of either NK1.1- or CD8-expressing cells completely abrogated control (Figure 6A). Notably, depletion of NK1.1+ results in a few OVA257-specific CD8+ T cells in the resulting tumors (Figure 6B). As both NK1.1+ and CD8+ cells could play a critical role in controlling early stages of tumor engraftment, we determined the impact of the NK1.1+ or CD8+ lymphoctyes on anti-CD27-mediated control of tumor during the treatment phase. Tumors were allowed to establish for 5d, then CD8- or NK1.1-expressing cells were depleted (d6 and d7), followed by the initiation of anti-CD27 treatment on d7. When subset deletion was performed after tumor was established, anti-CD27-mediated control of tumor was completely lost in the absence of CD8+ T cells. In contrast, mice depleted of NK1.1+ cells and treated with anti-CD27 controlled tumor outgrowth to the same extent as undepleted mice treated with cIg (Figure 6C). Thus, the primary effect of anti-CD27 is via CD8+ T cell immunity, and NK1.1+ cells play a critical role in controlling the early establishment of tumor but are dispensable for anti-CD27 mediated control of established tumor.
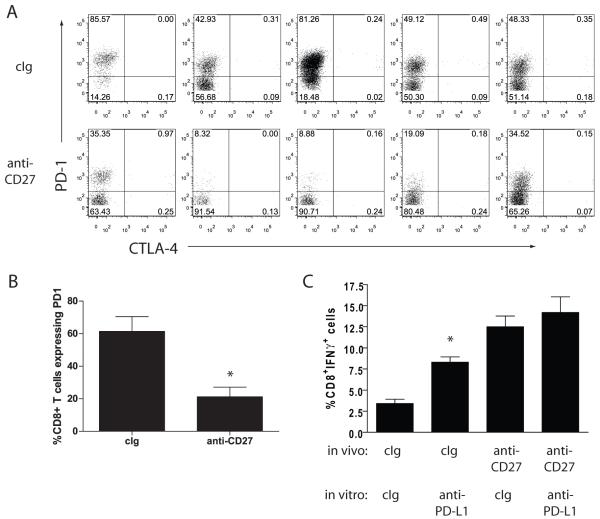
Tumor control by anti-CD27 treatment correlates with lower levels of PD-1 expression on CD8+ TIL
We had previously observed the upregulation of PD-1 on CD8+ T cells in B16 melanomas, and have found that B16 express PD-L1 (unpublished results). As PD-1 expression has been associated with dysfunction in TIL (8), we examined whether anti-CD27 treatment influenced PD-1 expression in CD8+ TIL. Lymphocytes were isolated from d14 tumors from cIg or anti-CD27 treated mice, and stained for the expression of PD-1. The level of PD-1 expression on CD8+ T cell TIL from anti-CD27 treated tumors was consistently lower than that found for cIg treated mice (Figure 7A). Treatment of short term cultures of B16cOVA with TIL isolated from cIg treated d14 tumors with anti-PD-L1 resulted in a significant increase in the proportion of CD8+ T cells that secreted IFNγ (Figure 7B). In contrast, the production of IFNγ by anti-CD27 treated TIL was not significantly increased by the in vitro anti-PD-L1 treatment. Thus, the therapeutic effect of anti-CD27 on B16cOVA strongly correlates with preventing the expression of PD-1 on CD8+ TIL.
Figure 7. Anti-CD27 treatment prevents PD-1 mediated block of effector activity.
(A). TIL from d14 s.c. B16cOVA tumors treated with cIg (upper plots) or anti-CD27 (right plot) (n=5 each) were isolated and stained for the expression of CD8 and PD-1. Data is summarized for this experiment in (B); *p<0.01. (C). TIL isolated from cIg or anti-CD27 treated tumors were cocultured with B16cOVA treated with either cIg or anti-PD-L1. 5h later, cells were stained for the surface expression of CD8 and the production of IFNγ by intracellular staining. * p<0.05 comparing IFNγ-production from cIg TIL cultured in vitro with anti-PD-L1 or cIg. Data represents one of 3 similar experiments.
Discussion
The studies presented here demonstrate that treatment of established solid melanomas with anti-CD27 is therapeutically effective in a preclinical model. Treatment with anti-CD27 has two major effects on CD8+ TIL. First, anti-CD27 treatment resulted in the maintenance of the CD8+ T cells that are specific for OVA257, the immunodominant epitope in B16cOVA. The basis for this increase is currently uncertain, but is consistent with the well established function of CD27 signaling in the prevention of activation-induced cell death (24). Thus, the increase in tumor-specific CD8+ T cells might be a consequence of increased CD8+ T cell survival in the tumor microenvironment. Alternatively, recent studies in our lab suggest that CD70-CD27 costimulation can increase the rate of CD8+ T cell division after encountering tumor antigen (Drew Roberts, unpublished data), suggesting that in environments with low inflammation costimulation of CD27 augments the activation and expansion of naïve CD8+ T cells.
In addition to maintaining the presence of OVA257-specific CD8+ T cells within tumor, targeting CD27 supported in the proportion of OVA257-specific CD8+ T cells that could produce IFNγ after stimulation with their cognate antigen. This data is consistent with that from tumor-control studies performed in CD70-transgenic mice (4), confirming the ability of CD27 stimulation to influence the effector status of CD8+ T cells in the tumor microenvironment. Multiple hypotheses exist to explain the reduction in IFNγ-production in TIL, ranging from the effects of regulatory populations such Treg and myeloid-derived suppressor cells (MDSC), to the induction of functional exhaustion due to chronic antigen exposure. In the studies presented here, depletion of CD4+ T cells has a neutral effect on CD27-mediated therapy, and did not modulate IFNγ-production by CD8+ T cells (not shown). Further, removal of Treg prior to treatment had no discernable effect on anti-CD27 mediated therapy, suggesting that anti-CD27 is not working by modulating Treg function. Additionally, we have not observed significant association of MDSC with B16 melanomas. In contrast, we find a close association with CD8+ TIL effector function with PD-1 expression, mimicking recent observations in human melanomas (1). Blockade of PD-L1 restored a considerable proportion of the effector activity of CD8+ T cells, as previously seen with other tumor studies using adoptively transferred T cells (8), and chronic viral infections (7). Coculture of TIL with IL-2 and OVA257-pulsed APC was also able to partially restore the ability of the TIL to secrete IFNγ. Further, the competent cytokine production by TIL after PMA/ionomycin stimulation, which would bypass PD-1 mediated inhibition, is consistent with a defect in receptor-mediated signaling. PD-1/PD-L1 blockade and IL-2 stimulation have been shown to impede the generation of both functional anergy and exhaustion in CD8+ T cells (9;11), defining which process is responsible for the defects in TIL in progressing tumors will depend on further molecular characterization. Further studies will also be necessary to define a direct link between CD27 stimulation and PD-1 expression; however, it should be noted that CD8+ T cell activation in the absence of CD4+ T cell help, and thus CD27 stimulation, has been shown to result in the upregulation of PD-1 (19), suggesting that anti-CD27 therapy directly leads to modulation of PD-1 expression on CD8+ TIL.
The enhanced control of tumor outgrowth obtained with anti-CD27 treatment was found to be dependent upon both CD8+ T cells and NK1.1-expressing cells, and in the absence of NK1.1+ cells the recruitment of OVA257-specific CD8+ T cells into the tumor was severely curtailed. This is consistent with our previous observations that CD27-mediated stimulation of NK cells induced tumor-specific T cell responses (31). The present studies do not allow us to discriminate whether the effectiveness of anti-CD27 is due to NK or NKT cells, but it should be noted that some therapeutic activity of anti-CD27 was evident in RAG2-deficient mice, implying a role for NK cells. CD27 expression on NK cells is associated with a subset of mature NK cells that resides in lymphoid tissue, and identifies a population that is less functionally constrained by inhibitory receptors (23). In vitro stimulation of CD27 on NK cells results in enhanced NK cell proliferation and IFNγ secretion (40). We are currently defining whether CD27-mediated stimulation further alters either inhibitory or stimulatory receptors on NK cells in the tumor environment. Pertaining to this, preliminary studies (not shown) indicate that while CD27-stimulation does not alter perforin expression by NK cells, it does increase their ability to lyse B16cOVA cells in vitro, implicating enhanced recognition of the tumor. It is also quite tenable that stimulating CD27 on NK cells results in NK-mediated stimulation of DC, increasing the cross-presentation of tumor antigen by DC and activation of naïve tumor-specific CD8+ T cells. Indeed, removal of NK cells prior to tumor challenge reduces the activation of adoptively transferred OT-I CD8+ T cells (NF and TNJB; unpublished results). Such an outcome would explain why few OVA257-specific CD8+ T cells are found in tumors of NK1.1-depleted mice. Taken together, these data emphasize the importance of the interplay between innate and adaptive immunity in effective tumor control, and CD27-mediated costimulation may provide an avenue to simultaneously recruit both arms against tumor outgrowth.
In contrast to NK and CD8+ T cells, CD4+ T cells were dispensable for CD27-mediated tumor control. Recent work by Soares et al (37), and studies in our laboratory (Mancuso, S et al, manuscript in preparation), indicate that CD27-mediated costimulation supports Th1 differentiation. Furthermore, it has recently been shown that targeting OX40 on regulatory T cells abrogates their suppressive activity. Therefore we had hypothesized that anti-CD27 treatment would induce beneficial, IFNγ-secreting CD4+ T cells that would be supportive of tumor control, perhaps at the expense of recruitment/conversion of regulatory T cells. Clearly the data presented here are not consistent with this hypothesis. The CD4+ T cells found in the tumors of anti-CD27 treated mice did not secrete IFNγ after restimulation with OVA323 or PMA/ionomycin, and the regulatory T cell population, while significantly reduced in number (substantially altering the Treg:OVA257 CD8+ T cell ratio), was not functionally impeded. These data make the interesting point that the endogenous helper CD4+ T cell populations found in these tumors are not TH1 polarized, and that CD27 stimulation is insufficient, in the context of established tumor, to drive TH1 polarization. Interestingly, while removal of Treg prior to tumor implantation had little impact on the effectiveness of anti-CD27 therapy, we did note a slight increase in the proportion of CD4+ T cells that could produce IFNγ after in vitro stimulation, suggesting Treg may constrain the development or functional activity of TH1- polarized CD4+ TIL. However, the overall implication of the independence of anti-CD27 therapy from CD4+ T cells is that anti-CD27 treatment has a sufficiently potent effect on CD8+ T cells as to override the support or suppression normally found with tumor-associated CD4+ T cells.
Taken together, these data strongly support the development of therapeutic strategies that target the CD70-CD27 costimulatory pathway. Though not directly compared in this study, anti-CD27 appears to be at least as effective as monotherapies targeting 4-1BB (CD137), OX40 (CD134) and GITR in treating established B16F1 melanoma (12;26;32). The constitutive expression of CD27 both on naïve T cells and a large proportion of the TIL, which contrasts with the necessity for activation induced expression of 4-1BB, OX40 and GITR, may account for the difference (14). While anti-CD27 treatment shows considerable promise as a monotherapy, tumors did ultimately grow out in the treated mice. In many instances the efficacy of targeting TNF superfamily receptors can be enhanced by concomitant vaccination or strategies to induce tumor destruction (26;45), or indeed, coordinated targeting of TNF superfamily receptors (21). Thus, complete therapeutic effectiveness may be obtained by inclusion of a vaccination regimen that augments the acute expansion of tumor-specific effector CD8+ T cells, or by co-targeting the inhibitory molecules, such as PD-1, that are found on exhausted TIL.
Acknowledgments
Supported by National Cancer Institute (CA115882), the Cancer Research Institute /Libby Bartnick Memorial Investigator Award and the University of Virginia Cancer Center Support Grant P30 CA44579 (all to TNJB).
Abbreviations used
- DC
dendritic cells
- TLR
toll-like receptors
- cIg
control immunoglobulin
- TDLN
tumor draining lymph node
- TIL
tumor infiltrating lymphocyte
Footnotes
Financial Disclosure: All authors have declared there are no financial conflicts of interest in regards to this work.
Reference List
- 1.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahonen CL, Wasiuk A, Fuse S, et al. Enhanced efficacy and reduced toxicity of multifactorial adjuvants compared with unitary adjuvants as cancer vaccines. Blood. 2008;111:3116–3125. doi: 10.1182/blood-2007-09-114371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 4.Arens R, Schepers K, Nolte MA, et al. Tumor Rejection Induced by CD70-mediated Quantitative and Qualitative Effects on Effector CD8+ T Cell Formation. J Exp Med. 2004;199:1595–1605. doi: 10.1084/jem.20031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assudani D, Cho HI, DeVito N, et al. In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells. Cancer Res. 2008;68:9892–9899. doi: 10.1158/0008-5472.CAN-08-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett SR, Carbone FR, Karamalis F, et al. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [see comments] [DOI] [PubMed] [Google Scholar]
- 7.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blank C, Brown I, Peterson AC, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 9.Blattman JN, Grayson JM, Wherry EJ, et al. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 10.Bullock TN, Yagita H. Induction of CD70 on dendritic cells through CD40 or TLR stimulation contributes to the development of CD8+ T cell responses in the absence of CD4+ T cells. J Immunol. 2005;174:710–717. doi: 10.4049/jimmunol.174.2.710. [DOI] [PubMed] [Google Scholar]
- 11.Chikuma S, Terawaki S, Hayashi T, et al. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol. 2009;182:6682–6689. doi: 10.4049/jimmunol.0900080. [DOI] [PubMed] [Google Scholar]
- 12.Cohen AD, Diab A, Perales MA, et al. Agonist anti-GITR antibody enhances vaccine-induced CD8(+) T-cell responses and tumor immunity. Cancer Res. 2006;66:4904–4912. doi: 10.1158/0008-5472.CAN-05-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cote AL, Usherwood EJ, Turk MJ. Tumor-specific T-cell memory: clearing the regulatory T-cell hurdle. Cancer Res. 2008;68:1614–1617. doi: 10.1158/0008-5472.CAN-07-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 15.Curiel TJ, Wei S, Dong H, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 16.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells In vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French RR, Taraban VY, Crowther GR, et al. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–4815. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 19.Fuse S, Tsai CY, Molloy MJ, et al. Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1. J Immunol. 2009;182:4244–4254. doi: 10.4049/jimmunol.0802041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 21.Gray JC, French RR, James S, et al. Optimising anti-tumour CD8 T-cell responses using combinations of immunomodulatory antibodies. Eur J Immunol. 2008;38:2499–2511. doi: 10.1002/eji.200838208. [DOI] [PubMed] [Google Scholar]
- 22.Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 24.Hendriks J, Gravestein LA, Tesselaar K, et al. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 25.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang AY, Golumbek P, Ahmadzadeh M, et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 28.Hwang ML, Lukens JR, Bullock TN. Cognate Memory CD4+ T Cells Generated with Dendritic Cell Priming Influence the Expansion, Trafficking, and Differentiation of Secondary CD8+ T Cells and Enhance Tumor Control. J Immunol. 2007;179:5829–5838. doi: 10.4049/jimmunol.179.9.5829. [DOI] [PubMed] [Google Scholar]
- 29.Keller AM, Schildknecht A, Xiao Y, et al. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Keller AM, Xiao Y, Peperzak V, et al. Costimulatory ligand CD70 allows induction of CD8+ T cell immunity by immature dendritic cells in a vaccination setting. Blood. 2009 doi: 10.1182/blood-2008-03-148007. [DOI] [PubMed] [Google Scholar]
- 31.Kelly JM, Darcy PK, Markby JL, et al. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat Immunol. 2002;3:83–90. doi: 10.1038/ni746. [DOI] [PubMed] [Google Scholar]
- 32.Miller RE, Jones J, Le T, et al. 4-1BB-specific monoclonal antibody promotes the generation of tumor-specific immune responses by direct activation of CD8 T cells in a CD40-dependent manner. J Immunol. 2002;169:1792–1800. doi: 10.4049/jimmunol.169.4.1792. [DOI] [PubMed] [Google Scholar]
- 33.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [see comments] [DOI] [PubMed] [Google Scholar]
- 34.Sanchez PJ, McWilliams JA, Haluszczak C, et al. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 35.Schoenberger SP, Toes RE, van der Voort EI, et al. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 36.Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 37.Soares H, Waechter H, Glaichenhaus N, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sumi T, Ishida W, Ojima A, et al. CD27 and CD70 do not play a critical role in the development of experimental allergic conjunctivitis in mice. Immunol Lett. 2008;119:91–96. doi: 10.1016/j.imlet.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic t lymphocyte-associated antigen 4 blockade and depletion of cd25(+) regulatory t cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic t lymphocyte responses. J Exp Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda K, Oshima H, Hayakawa Y, et al. CD27-mediated activation of murine NK cells. J Immunol. 2000;164:1741–1745. doi: 10.4049/jimmunol.164.4.1741. [DOI] [PubMed] [Google Scholar]
- 41.Taraban VY, Rowley TF, Al Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- 42.Van Oosterwijk MF, Juwana H, Arens R, et al. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int Immunol. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 43.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 44.Wells JW, Cowled CJ, Farzaneh F, et al. Combined triggering of dendritic cell receptors results in synergistic activation and potent cytotoxic immunity. J Immunol. 2008;181:3422–3431. doi: 10.4049/jimmunol.181.5.3422. [DOI] [PubMed] [Google Scholar]
- 45.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao Y, Peperzak V, Keller AM, et al. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J Immunol. 2008;181:1071–1082. doi: 10.4049/jimmunol.181.2.1071. [DOI] [PubMed] [Google Scholar]