TO THE EDITOR
Genes regulating development and function of skin and hair often play critical roles in other organs. Accordingly, understanding the mechanisms underlying hair follicle (HF) morphogenesis and cycling may provide insight into disease processes of relevance for various organs. The hair interior defect mutation (hid) arose spontaneously in AKR/J mice (Giehl et al., 2009; Trigg, 1972). While AKR/J-hid/hid mice appear superficially normal at all ages, microscopic analysis of internal hair morphology revealed changes in the cortico-medullary boundary, a lesion that was proposed to be a failure of medullary cells to change shape during their early differentiation (Trigg, 1972) . Subsequent ultrastructural studies found hid/hid hairs were deficient in projections of cortex cells (Rice et al., 2009) which normally have a characteristic tonofilament pattern (Morioka, 2005) into adjoining medulla cells. Proteomic analyses further revealed the hid/hid hairs were low in trichohyalin (Rice et al., 2009).
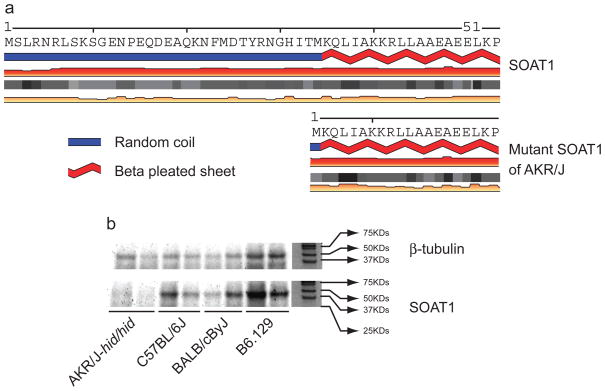
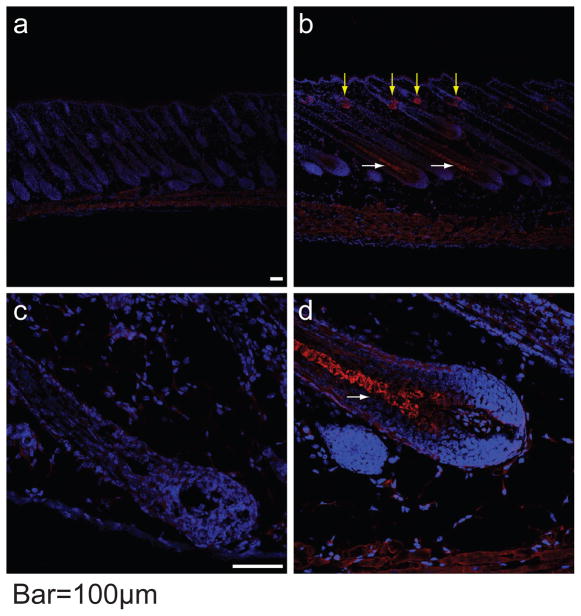
Having mapped the hid locus to mouse Chr. 1 (Giehl et al., 2009), we generated an additional 260 AKRCByJF2 mice yielding 14.62% (38 mice) affected progeny (<25% expected), and suggesting one or more modifier genes. Crosses between AKR/J-hid/hid and C3H/HeJ, C57BL/6J, FVB/NJ, and CAST/EiJ yielded 1289 F2 progeny at the expected 25% frequency. Genotyping reduced the hid locus to 0.74 Mb containing 9 genes. Sequencing of the corresponding cDNAs revealed only synonymous SNPs, with the exception of Soat1, which had a 118 bp deletion that included exon 2 and ranged from nucleotides 30–88 of the Soat1 ORF. Genomically, we confirmed a 6.8 kb deletion using whole genome re-sequencing data (http://www.sanger.ac.uk/modelorgs/mousegenomes/). This mutation results in a 33 amino acid (AA) truncation at the N-terminus of the SOAT1 protein consisting of 540 AAs. Because no structure of any member of the SOAT family was confirmed, tertiary structural changes caused by this mutation could not be predicted based on a congenerous model. Based on analysis by SABLE (http://sable.cchmc.org/), the secondary structure of the truncated N-terminus forms a random coil, which is not predicted to markedly change the remaining structure including beta pleated sheets and coils of currently undefined function (Figure 1a). Western blots of dorsal skin from 5 day old male AKR/J-hid/hid mice and C57BL/6J, BALB/cByJ and B6.129 +/+ controls revealed loss of SOAT1 expression in hid/hid mice only (Figure 1b). Strong fluorescence signals for SOAT1 were seen in the pre-medulla region of hair follicles and sebaceous glands from BALB/cByJ +/+ mouse but not for AKR/J-hid/hid (Figure 2; for details see Supplemental Material).
Figure 1. Putative expression of SOAT1 protein.
Truncated N-terminal and the secondary structure of SOAT1 protein of AKR/J-hid/hid (a). SOAT1 is not expressed in the dorsal skin of AKR-hid/hid mutant mice but is in C57BL/6J, BALB/cByJ, and B6.129 wild type mice (b).
Figure 2. Immunofluorescence localizing SOAT1 protein in hair follicles.
Yellow arrows indicate the positive signal in sebaceous glands and white arrows identify strong signals in the premedulla in the bulb of BALB/cByJ +/+ (b, d) but not in AKR/J-hid/hid mutant mice (a, c). The white dotted line outlines epidermis and hair follicles. Red = SOAT1, Blue = DAPI. Bar = 100 um
All AKR/J mice have the hid (Trigg, 1972) and adrenocortical lipid depletion (ald) (Arnesen, 1963) mutations. Acyl-CoA:cholesterol acyltransferase (Acat, currently sterol O-acyltransferase 1, Soat1) null mice also had the ald phenotype indicating that the ald resulted from a Soat1 mutation (Meiner et al., 1998). However, no hair abnormalities were reported in 2 Soat1 null mutations (Meiner et al., 1996; Yagyu et al., 2000). Our study confirmed that hid was due to the same Soat1 mutation causing ald and that SOAT1 protein was present within the premedulla, sebaceous glands, and hair interior of +/+ but not hid/hid mice.
Meiner et al. found a 118 bp deletion in the Soat1 mRNA from AKR/J mice (Meiner et al., 1998). Our data confirm this result and show that the underlying cause of the deletion in the mRNA is a 6.8 genomic deletion encompassing exon 2 and large portions of introns 1 and 2. Quantitative Real Time PCR analyses showed that truncated Soat1 mRNA expression was present but reduced in skin from male hid/hid pups while the SOAT1 protein was absent in hid/hid HFs. Robust expression of SOAT1 in the pre-medulla and hair interior region of the normal HF and shaft suggests that SOAT1 plays an important role in normal hair formation. Mutations in Soat1 affect the adrenal gland resulting in the ald mutant phenotype demonstrating the importance of SOAT1 in lipid metabolism. SOAT1 was strongly expressed in +/+ mouse sebaceous glands but not those of the hid mutant mice, again because of its known role in lipid metabolism. Failure to find changes in hid sebaceous glands suggests that subtle changes in structure or lipids may also be part of the phenotype, warranting future investigation, especially of surface lipids and epidermal hydration. These are important aspects of stearoyl-Coenzyme A desaturase 1 (Scd1), the gene mutated in the asebia mouse (Fluhr et al., 2003; Sundberg et al., 2000; Zheng et al., 1999). Lipid metabolism is not currently considered a primary function of cells within the hair shaft. This is an overlooked area, especially since changes are not obviously detrimental to health. Hofmann described a large and diverse superfamily of membrane-associated acyltransferases of which SOAT1 is an important member (Hofmann, 2000). All member proteins harbor several membrane-spanning regions, typically between 8 and 10, and share a region of detectable sequence similarity (AA 410 to 450 in SOAT1). This superfamily is implicated in WNT signaling, an important pathway in HF development (Ito et al., 2007). The primary function of SOAT1 is in the maintenance of ratios of free cholesterol and cholesterol esters in cells. One possibility is that the amino-terminal deletion in the SOAT1 protein affects protein-protein interactions or the localization of the SOAT1 protein in cell membranes and that these alterations influence the connection between cells or the cytoskeleton itself. Kazantseva et al. identified lipase H (LIPH), a phospholipase thought to regulate the production of lipid signaling molecules, in families with an inherited deficiency in hair growth (Kazantseva et al., 2006). Medulla cells arranged along hair shafts are separated by either an air space or more likely by lipids (Wagner et al., 2007), which may explain why mutations in Soat1 result in the hid phenotype.
While often dismissed as simply cosmetic, hair changes may reflect more serious and life threatening systemic effects, as in the case of hid mutant mice which have an adrenal gland abnormality as well as propensity for developing atherosclerosis (Meiner et al., 1996; Meiner et al., 1998; Yagyu et al., 2000). Taken together, groups of mutant mice with somewhat similar phenotypes provide powerful tools to unlock new genetic networks, as appears to be the case here.
Supplementary Material
Acknowledgments
This work was supported by the NIH (AR053639), NNSF, China (30670231, 2009F80022), NAAF (NAAF), and NAHRS. Short read sequence data courtesy of Mouse Genomes Project (http://www.sanger.ac.uk/mousegenomes/).
Footnotes
Conflict of interest: Dr. Sundberg has a research contract with the Procter and Gamble Company for work on hair biology unrelated to this topic.
References
- Arnesen K. The cytology of the adrenal cortex in mice with spontaneous adrenocortical lipid depletion. Acta Pathol Microbiol Scand. 1963;58:212–8. doi: 10.1111/apm.1964.60.4.487. [DOI] [PubMed] [Google Scholar]
- Fluhr JW, Mao-Qiang M, Brown BE, Wertz PW, Crumrine D, Sundberg JP, et al. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol. 2003;120:728–37. doi: 10.1046/j.1523-1747.2003.12134.x. [DOI] [PubMed] [Google Scholar]
- Giehl KA, Potter CS, Wu B, Silva KA, Rowe L, Awgulewitsch A, et al. Hair interior defect (hid) in AKR/J mice maps to mouse Chromosome 1. Clin Exp Dermatol. 2009;34:509–17. doi: 10.1111/j.1365-2230.2008.03135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem Sci. 2000;25:111–2. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Miller SE, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–21. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Kazantseva A, Goltsov A, Zinchenko R, Grigorenko AP, Abrukova AV, Moliaka YK, et al. Human hair growth deficiency is linked to a genetic defect in the phospholipase gene LIPH. Science. 2006;314:982–5. doi: 10.1126/science.1133276. [DOI] [PubMed] [Google Scholar]
- Meiner VL, Cases S, Myers HMER, Sande S, Bellosta Schambelan M, et al. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc Natl Acad Sci U S A. 1996;93:14041–6. doi: 10.1073/pnas.93.24.14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiner VL, Welch CL, Cases S, Myers HM, Sande E, Lusis AJ, et al. Adrenocorical lipid depletion gene (ald) in AKR mice is associated with an acyl-coA:cholesterol acyltransferase (ACAT) mutation. J Biol Chem. 1998;273:1064–9. doi: 10.1074/jbc.273.2.1064. [DOI] [PubMed] [Google Scholar]
- Morioka K. An atlas. Springer-Verlag; Tokyo: 2005. Hair follicle. Differentiation under the electron microscope. [Google Scholar]
- Rice RH, Rocke DM, Tsai HS, Silva KA, Lee YJ, Sundberg JP. Distinguishing mouse strains by proteomic analysis of pelage hair. J Invest Dermatol. 2009;129:2120–5. doi: 10.1038/jid.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg JP, Boggess D, Sundberg BA, Eilersten K, Parimoo S, Filippi M, et al. Asebia-2J (Scdab-2J): A new allele and a model for scarring alopecia. Am J Pathol. 2000;156:2067–75. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigg MJ. Hair growth in mouse mutants affecting coat texture. J Zool, Lond. 1972;168:165–98. [Google Scholar]
- Wagner RdC, Kiyohara PK, Silveira M, Joekes I. Electron microscopic observations of human hair medulla. J Microsc. 2007;226:54–63. doi: 10.1111/j.1365-2818.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- Yagyu H, kitamine T, Osuga J-I, Tozawa R-I, Chen Z, Kaji Y, et al. Absence of ACAT-1 attenuates atherosclerosis but causes dry eye and cutaneous xanthomatosis in mice with congenital hyperlipidemia. J Biol Chem. 2000;275:21324–30. doi: 10.1074/jbc.M002541200. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Eilersten K, Ge L, Zhang L, Sundberg JP, Prouty S, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nature Genetics. 1999;23:268–70. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.