Abstract
Conclusions
The middle ear (ME) surface area/volume ratio (SA/V) is greater than that of the tympanum. The rate of ME pressure decrease between Eustachian tube openings is proportional to the ME SA/V. This analysis showed that the MACS will not function as a ME gas reserve under the assumed conditions, but could, if the blood perfusion/surface area is much greater for the tympanum than the MACS and is lesser for greater MACS volumes.
Objective
Measure the surface and volumes for the MACS and tympanum and evaluate if the MACS could function as a ME gas reserve.
Methods
Twenty adult subjects with a wide range of MACS volumes had a CT scan of their MEs. Using Image J software, the left and right surface areas and volumes of the tympanum and MACS were reconstructed. These data were entered into a simple perfusion-limited model of transmucosal gas exchange between ME and mucosal blood. The model predicted that the MACS would function as a ME gas reserve if the SA/V for the ME is less that that for the tympanum, or equivalently, if the tympanum SA/V divided by the ME SA/V is less than a critical value of 1.
Results
Both MACS and tympanum surface areas were linearly related to their volumes. MACS surface area and volume and the ME SA/V were significantly greater than those for the tympanum. Solving the model equation using the measured values yielded a critical value of 1.4 which was significantly greater than 1.
Keywords: Adults, Mastoid, Volume, Surface Area
INTRODUCTION
During periods between openings of the Eustachian tube (ET), the normal middle ear (ME) is a closed, relatively non-collapsible (with the exception of tympanic membrane displacements), gas-filled, bony cavity located within the petrous portion of the temporal bone. The ME can be anatomically and functionally subdivided into two communicating airspaces, the tympanum and the mastoid air-cell system (MACS). The tympanum is essentially a single, large air-cell that contains the ME ossicles which couple movements of the tympanic membrane to those of the oval window and, thus, functions as the peripheral transducer organ for hearing. In contrast, the MACS is a multiply partitioned, cellular, air-space that increases ME volume, but does not participate directly in sound transduction. Both the MACS and the tympanum are lined by a mucosa that embeds a network of blood vessels that that can serve as the source/sink for gas exchange between the ME and local mucosal blood. While the function of the MACS is debated [1], numerous studies show that MACS volume is indirectly related to the predisposition of the ME to certain pathological conditions including cholesteatoma and otitis media [2–6]. One hypothesis advanced to explain this relationship is that the MACS functions as a ME gas reserve such that MEs with larger MACS require less frequent ET openings to maintain near-ambient total pressure [1].
For normal tympanum function, total ME gas pressure needs to be maintained at near-ambient levels. However, both the tympanum and MACS passively exchange gases with the local blood across their respective mucosa [7–9]. While the O2, CO2 and H2O partial-pressures in the ME and blood are in approximate equilibrium, at ambient ME pressure, the ME to blood N2 partial-pressure gradient approximates 5.8 kPa [10]. This gradient drives the progressive loss of N2 from the ME which results in decreasing ME pressure. At an ambient-ME pressure difference of approximately 2.5 kPa, the ME mucosal capillaries become permeable to fluids and an effusion develops in the normally air-filled ME, a pathological condition not different from the clinical presentation of otitis media with effusion [11]. For MEs with good ET function, this is prevented by the transient, muscle assisted openings of the ET which resupply gas to the ME and, thus, re-establishes near-ambient ME pressure.
The MACS will function as a gas reserve for the ME by slowing the rate of physiological ME pressure decrease if the rate of change in the ME partial-pressure of N2 is less for MEs with a developed MACS and if that effect is accentuated by larger MACS volumes [1]. If valid, the slower rate of ME pressure decrease for larger MACS volumes will decrease the required number and/or efficiency of ET openings to preserve near ambient ME pressures and prevent ME pathology in ears with constitutionally marginal or even poor ET function.
In experiments using primates, transmucosal N2 exchange was shown to be perfusion-limited [12]. Consequently, the rate of N2 exchange across the ME mucosa is proportional to mucosal blood flow/volume which, in turn, is a function of the surface area available for exchange/volume [1]. In this study, we measured the surface area and volume of the MACS and tympanum from CT scans of adults with a wide range of MACS volumes. Using a simple mathematical model of perfusion-limited transmucosal N2 exchange and these data, we tested the hypothesis that the MACS could function as a ME gas reserve.
METHODS
Protocol
The goals of this study were to measure the MACS and tympanum surface areas and volumes for a wide range of MACS volumes and to use these measures to determine if the MACS could function as a gas reserve for the ME. To that end, 28 adult subjects with and without a history of OM in childhood were recruited by advertisement, informed of the risks and benefits of study participation, and signed an institutionally approved informed consent. These subjects were then screened for entry by bilateral pneumatic otoscopy and tympanometry to document disease-free MEs and by taking bilateral ME x-ray’s in Schuller projection and measuring the total pneumatized area for each MACS. Using these areas as estimates of MACS volume, the first 20 subjects with pneumatized areas evenly distributed across the expected range of values were selected for CT scanning (left ear: average=7.8±4.1, range=1.8–16.4 cm2; right ear: average=8.3±4.0, range=2.7–18.0 cm2; r=0.90 for the left vs. right pneumatized areas, p<.01). Eight subjects had pneumatized areas similar or identical to previously enrolled subjects and by protocol (which limited CT scanning to 20 subjects) were discontinued from study participation.
These 20 subjects then had a CT scan of the ME in the transverse plane at a resolution of .031 mm/pixel and a slice thickness of 0.63 mm using a GE LightSpeed VCT system (General Electric Health Care). From each CT scan, a set of transverse images through the bilateral MACS regions (superior to inferior) at 0.25 cm intervals were selected for study. For the tympanum, every image was used for the reconstruction (i.e. interval=.63 mm). Using Image J software (http://rsbweb.nih.gov/ij/), these sections were imported, and the left and right MACS and tympanums were identified, segmented out and analyzed. For each MACS and tympanum section, the perimeter and area of all air-cells were highlighted, measured and summed across images. These sums were multiplied by the section interval to yield MACS and tympanum surface area (cm2) and volume (ml). This procedure is essentially identical to that used previously to measure MACS surface area and volume in adult subjects with normal MACS volumes [13]. The protocol was approved by the Institutional Review Board at the University of Pittsburgh.
Model Description
The total ME pressure is defined by the ideal gas law or:
| 1 |
where PME is total ME pressure, R is the gas constant, T ME is ME temperature, a constant, NM and NT are the total gas moles in the MACS and tympanum, and VM and VT are the volumes of the MACS and tympanum (constants). Differentiating this equation on time results in:
| 2 |
where δ indicates change and t is time. Recognizing that the rate of change in ME pressure is equal to the rate of transmucosal N2 exchange [10, 12], the equation can be rewritten as:
| 3 |
where the subscript indicates N2 partial-pressure and N2 moles. Now, if the MACS is completely sclerotic (i.e.VM=0), the equation reduces to:
| 4 |
and for the most-simple case of open N2 exchange between the MACS and tympanum [14], the rate of change in ME N2 partial-pressure will be slowed if the following inequality is satisfied:
| 5 |
or the rate of change in ME N2 partial-pressure for an ear with a mastoid is less than that for the tympanum. Dividing both sides of the equation by the constants R and TME and substituting the terms of the Fick perfusion equation [15] for δNN2/dt yields:
| 6 |
where SN2B is the N2 solubility in blood, a constant, PN2B is the N2 blood partial-pressure, a constant, and QTand QM are the mucosal blood perfusion for tympanum and MACS, respectively. Under the assumption of open communication between the tympanum and MACS, PN2M=PN2T=PN2ME or the partial-pressure of N2 in MACS and tympanum is equal to that of the ME. Therefore, (PN2M-PN2B)=(PN2T-PN2B)=(PN2ME-PN2B). Substituting these results in equation 6 yields:
| 7 |
and by dividing out the constants, equation 7 reduces to:
| 8 |
The mucosal blood perfusions are proportional to the respective surface areas, or:
| 9 |
where AM and AT are the surface areas and b and c are constants of proportionality defined by QM/AM and QT/AT for the MACS and tympanum, respectively. As a first approximation, b is assumed to equal to c, and the equation reduces to:
| 10 |
Multiplying both sides of equation by VT and dividing by AT yields:
| 11 |
or the N2 exchange rate for the MACS±tympanum multiplied by the inverse of that for the tympanum is a critical ratio for MACS functioning as a ME gas reserve. Now, recognizing that larger MACS volumes are associated with lesser ME disease [2–6], under the defined hypothesis, increasing MACS volumes should be associated with a decreasing critical ratio as defined in equation 11.
Note that the derivation of equation 9 is also applicable to diffusion-limited gas exchange where the relevant equation becomes:
| 9a |
where HM and HT are the thicknesses of the MACS and tympanum mucosa between the air-mucosa boundary and the mucosal blood.
Analysis
All data were entered into Microsoft Excel for analysis. Linear regression was used to determine the relationships between the values of selected variables. Between-group/ear comparisons were done using a paired or two-group Student’s t test as applicable. Throughout, the format average±standard deviation is used to summarize the data.
RESULTS
The study population consisted of 14 females and 6 males, aged 26.3±6.4 (range=20.4 to 40.5) years. All subjects were Caucasian. Six (30%) subjects reported a history of otitis media during childhood. For the 20 subjects, the right and left MACS volume (r=.81), surface area (r=87) and SA/V (r=.75) were highly correlated as were the right and left tympanum volume (r=.87), surface area, (r=66) and SA/V (r=.86), all correlations were significant at p<.001. Thus, the values for the right and left ears of these variables are not independent and potentially represent redundant information. Consequently the reported comparisons were done for the right and left ears, separately.
Table I reports the average and standard deviations for the left and right MACS and tympanum volumes, surface areas and SA/Vs for all subjects. Also reported are the absolute values of a paired Students t test and probability levels for left-right comparisons and those for a 2-group Student’s t test for the MACS-tympanum comparisons. None of the left-right comparisons were statistically significant, but all of the MACS-tympanum comparisons were significant, with the MACS having larger values than the tympanum.
TABLE.
Average (Avg) and Standard Deviation (Std) for the MACS and Tympanum Volume, Surface Area and the SA/V, and the Paired Student’s t (t) and Probability Level (P) for Left-Right Comparisons and the Students t and Probability Levels for the left and right MACS-Tympanum Comparisons
| Left vs Right | MACS vs Tympanum | |||||||
|---|---|---|---|---|---|---|---|---|
| Avg | Std | t | p | t | p | |||
| Volume | MACS | Left | 5.45 | 4.92 | 0.02 | 0.98 | 4.35 | <.001 |
| MACS | Right | 5.46 | 3.58 | 5.98 | <.001 | |||
| Tympanum | Left | 0.66 | 0.11 | 0.53 | 0.60 | |||
| Tympanum | Right | 0.67 | 0.13 | |||||
| Surface Area | MACS | Left | 82.77 | 59.76 | 0.97 | 0.37 | 5.63 | <.001 |
| MACS | Right | 88.91 | 60.25 | 6.02 | <.001 | |||
| Tympanum | Left | 7.59 | 1.17 | 0.88 | 0.39 | |||
| Tympanum | Right | 7.80 | 1.37 | |||||
| SA/V ratio | MACS | Left | 17.37 | 4.78 | 0.94 | 0.36 | 5.26 | <.001 |
| MACS | Right | 16.70 | 3.71 | 5.47 | <.001 | |||
| Tympanum | Left | 11.57 | 1.24 | 1.33 | 0.20 | |||
| Tympanum | Right | 11.80 | 1.51 | |||||
For both tympanum and MACS, the left and right surface areas were a direct linear function of their respective volumes (MACS: slope=7.20±2.37,r=.59, p=.006 for the left ear and slope=17.91±1.33, r=.95, p<.001 for the right ear; Tympanum: slope=8.40±1.50, r=.80, p<.001 for the left ear and slope=8.42±1.59, r=.99, p<.001 for the right ear). These relationships are displayed in figure 1 for the MACS (a) and tympanum (b), respectively. Note that the value for the left MACS of one subject (#13, 40 year old, male without a history of otitis media), but not for the other MACS or either tympanum, was a significant outlier with respect to the linear relationship characteristic of all other MACS. That MACS was also an outlier for the otherwise linear relationships for the paired values of the other variables. Visual examination of the CT scans for that ear showed an abnormally high distribution of large air-cells throughout the MACS, a geometry that was atypical of all other MACS studied. Consequently, the data for that MACS were not included in the calculations of the derived variables described below.
Figure 1.
The MACS (a) and tympanum (b) surface areas as a linear function of their respective volumes for the left (open squares) and right (closed circles) ears. Note the outlier for the one left MACS at volume=21.4 and surface area=69.1.
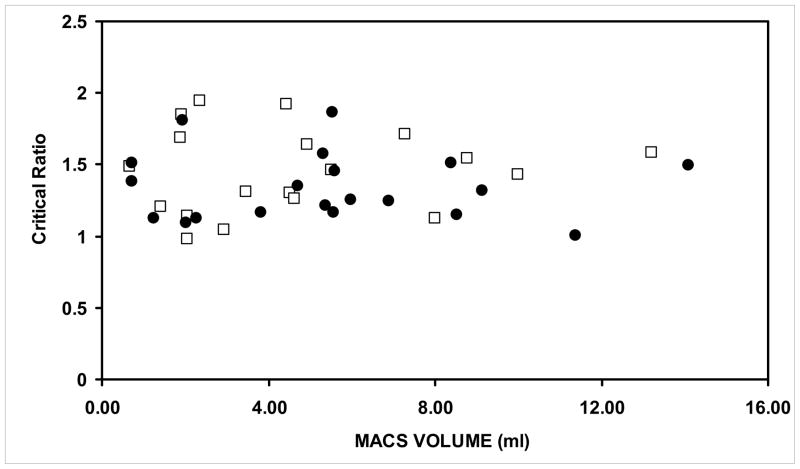
The left-right values for ME SA/V (r=.79), tympanum SA/V (r=.81) and the critical value (r=.71) were directly related and all correlation coefficients were statistically significant (P<.001). The average values of the ME SA/Vs were 16.99±2.87 and 15.76±2.99, of the SA/Vs for the tympanum were 11.57±1.24 and 11.8±1.52, and of the critical values were 1.46±.29 and 1.34±.23 for the left and right ears, respectively. The left and right SA/Vs for the ME were significantly greater than the left and right SA/Vs for the tympanums, t=7.72, p<.001 and t=5.29, p<.001, respectively. As shown in figure 2, the right and left critical values were significantly greater than 1 (t=6.78, p<.001 and t=6.50, p<.001) and independent of their respective MACS volumes. Correlation coefficients between the left and right ear critical values and respective MACS volumes were: r=.09, p=.701 and r=−.052, p=.825, respectively.
Figure 2.
The left (open squares) and right (closed circles) critical values as a function of MAC volume.
DISCUSSION
In this study, the surface area and volume of the MACS and tympanum were measured from CT scans over a wide range of MACS volumes using methods previously described for the MACS [13]. Using a simple mathematical model of perfusion-limited transmucosal N2 exchange and these data, we tested the hypothesis that the MACS could function as a ME gas reserve. The developed model requires that N2 is the only gas not in equilibrium between ME and mucosal blood [10]; transmucosal N2 (and other inert gases) exchange between the ME and local mucosal blood is perfusion-limited [9, 12] and the MACS and tympanum are in open communication in the air-phase [14]. All of these requirements are supported by experimental data. For the most-simple model used to test the gas reserve function of the MACS, it was assumed that the blood perfusion/surface area of mucosa is similar for all compartments of the ME, though this has not been validated by experiment.
That model showed that the MACS will function as a ME gas reserve if the transmucosal N2 exchange rate/volume is greater for the tympanum than for the ME. As shown, this relationship can be expressed as the ratio of the SA/V for the tympanum to that of the ME. A critical value of that ratio was determined to be 1 with all ratios <1 supporting a ME gas reserve function for the MACS and all values greater than 1 indicating that the MACS acts as a gas sink (i.e. increases the rate of ME pressure decrease). The MACS function as a ME gas reserve was not supported by the data, as measurement of that ratio was approximately 1.4. A second requirement for that function is that the ratio decreases with increasing MACS volume which was also unsupported by the data. Consequently, for this simple model of perfusion-limited gas exchange and the relevant measures made in this study, we reject our hypothesis that the MACS could function as a ME gas reserve. It should be noted that this result is also consistent with a diffusion-limitation for transmucosal N2 exchange (see equation 9b).
However, the results for one study of humans breathing gas mixtures containing high concentrations of the inert gas, N2O, reported a curvilinear inverse relationship between the rate of ME pressure increase caused by blood to ME gas transfers and mastoid volume [8], a result predicted by mathematical modeling of the MACS as a ME gas reserve [1]. In contrast, an experimental study on monkeys showed that the rate of transmucosal inert gas exchange was not affected by physical blockage of the mastoid Antrim [16]. The reason for these contrasting results is unknown but a reconstruction of ME geometry for the monkey and other primates is ongoing.
A limitation of the developed model is that it ignored the contribution of regional blood perfusion/surface area between and within compartments (i.e. assumption that the proportionality constants b and c are equal). Consequently, the MACS could still function as a gas reserve if the blood perfusion/surface area is much greater for the tympanum than for the MACS and if that rate is an inverse function of MACS volume. To date, these measurements have not been made. A second limitation is that all persons studied were adults while otitis media is a disease of children. The growth and development of the MACS has not been studied with respect to its geometry and it is possible that younger children may have a different MACS geometry that is more favorable to its possible function as a ME gas reserve. This is currently being studied.
In summary, tympanum, MACS and total ME geometry are characterized by a SA/V that is independent of volume and that the SA/V for the ME is greater than that for the tympanum. This result suggests that the MACS acts as a gas sink with a developed MACS increasing the rate of change in ME pressure between ET openings, a result inconsistent with the MACS functioning as ME gas reserve. This result depends on the blood perfusion/surface area for the two compartments, parameters that are currently unknown. Also unknown are the changes in MACS geometry during growth and development and these areas are priority goals for future research in this area of study.
Acknowledgments
Supported in part by a grant from the National Institutes of Health (P50 DC007667)
This work was supported in part by a grant from the National Institutes of Health (P50 DC007667). The investigators thank the personnel of the Radiology Department at the Children’s Hospital of Pittsburgh for their assistance in performing the x-ray and CT procedures and Ms. Julianne Banks for her assistance with subject recruitment and scheduling.
References
- 1.Doyle WJ. The mastoid as a functional rate-limiter of middle ear pressure change. Int J Pediatr Otorhinolaryngol. 2007 Mar;71(3):393–402. doi: 10.1016/j.ijporl.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sade J, Fuchs C. A comparison of mastoid pneumatization in adults and children with cholesteatoma. Eur Arch Otorhinolaryngol. 1994;251(4):191–195. doi: 10.1007/BF00628421. [DOI] [PubMed] [Google Scholar]
- 3.Sade J, Fuchs C. Secretory otitis media in adults: I. The role of mastoid pneumatizationas a risk factor. Ann Otol Rhinol Laryngol. 1996 Aug;105(8):643–647. doi: 10.1177/000348949610500810. [DOI] [PubMed] [Google Scholar]
- 4.Sade J, Fuchs C. Secretory otitis media in adults: II. The role of mastoid pneumatization as a prognostic factor. Ann Otol Rhinol Laryngol. 1997 Jan;106(1):37–40. doi: 10.1177/000348949710600107. [DOI] [PubMed] [Google Scholar]
- 5.Lesinskas E. Factors affecting the results of nonsurgical treatment of secretory otitis media in adults. Auris Nasus Larynx. 2003 Feb;30(1):7–14. doi: 10.1016/s0385-8146(02)00100-1. [DOI] [PubMed] [Google Scholar]
- 6.Valtonen HJ, Dietz A, Qvarnberg YH, Nuutinen J. Development of mastoid air cell system in children treated with ventilation tubes for early-onset otitis media: a prospective radiographic 5-year follow-up study. Laryngoscope. 2005 Feb;115(2):268–273. doi: 10.1097/01.mlg.0000154731.08410.b8. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H, Honjo I, Naito Y, et al. Gas exchange function through the mastoid mucosa in ears after surgery. Laryngoscope. 1997 Aug;107(8):1117–1121. doi: 10.1097/00005537-199708000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Elam M, Harell M, Luntz M, Fuchs C, Sade J. Middle ear pressure variations during 50% N2O anesthesia as a function of mastoid pneumatization. Am J Otol. 1998 Nov;19(6):709–711. [PubMed] [Google Scholar]
- 9.Doyle WJ. Mucosal surface area determines the middle ear pressure response following establishment of sniff-induced underpressures. Acta Otolaryngol. 1999;119(6):695–702. doi: 10.1080/00016489950180658. [DOI] [PubMed] [Google Scholar]
- 10.Hergils L, Magnuson B. Human middle ear gas composition studied by mass spectrometry. Acta Otolaryngol. 1990 Jul-Aug;110(1–2):92–99. doi: 10.3109/00016489009122520. [DOI] [PubMed] [Google Scholar]
- 11.Alper CM, Tabari R, Seroky JT, Doyle WJ. Magnetic resonance imaging of the development of otitis media with effusion caused by functional obstruction of the eustachian tube. Ann Otol Rhinol Laryngol. 1997 May;106(5):422–431. doi: 10.1177/000348949710600511. [DOI] [PubMed] [Google Scholar]
- 12.Doyle WJ, Seroky JT, Alper CM. Gas exchange across the middle ear mucosa in monkeys. Estimation of exchange rate. Arch Otolaryngol Head Neck Surg. 1995 Aug;121(8):887–892. doi: 10.1001/archotol.1995.01890080055011. [DOI] [PubMed] [Google Scholar]
- 13.Park MS, Yoo SH, Lee DH. Measurement of surface area in human mastoid air cell system. J Laryngol Otol. 2000 Feb;114(2):93–96. doi: 10.1258/0022215001904969. [DOI] [PubMed] [Google Scholar]
- 14.Raveh E, Sade J, Mover-Lev H, Guney S. Mastoid buffering properties: I. Gas partial-pressures. Ann Otol Rhinol Laryngol. 1999 Aug;108(8):750–755. doi: 10.1177/000348949910800807. [DOI] [PubMed] [Google Scholar]
- 15.Ranade A, Lambertsen CJ, Noordergraaf A. Inert gas exchange in the middle ear. Acta Otolaryngol Suppl. 1980;371:1–23. [PubMed] [Google Scholar]
- 16.Doyle WJ, Alper CM, Banks JM, Swarts JD. Rate of nitrous oxide exchange across the middle ear mucosa in monkeys before and after blockage of the mastoid antrum. Otolaryngol Head Neck Surg. 2003 May;128(5):732–741. doi: 10.1016/S0194-59980223309-4. [DOI] [PubMed] [Google Scholar]