Abstract
Objective
We assessed whether differing autoantibody screening criteria for type 1 diabetes (T1D) prevention trials result in different baseline metabolic profiles of those who screen positive.
Methods
Diabetes Prevention Trial-Type 1 (DPT-1) participants were screened for islet cell autoantibodies (ICA), whereas TrialNet Natural History Study (TNNHS) participants were screened for biochemical autoantibodies. In both studies, those determined to be autoantibody positive underwent baseline oral glucose tolerance tests (OGTTs) in which glucose and C-peptide were measured.
Results
The percentage of those with an OGTT in the diabetic range was higher among the DPT-1 participants (10.0% of 956 vs. 6.4% of 645, p<0.01). In a logistic regression analysis with adjustments for age and gender, the difference persisted (p<0.01). Among those in the non-diabetic range (n=860 for DPT-1 and n=604 for the TNNHS), glucose levels were similar at all time points, except for higher fasting glucose levels in the TNNHS participants (p<0.001). There was a higher percentage of impaired fasting glucose in the TNNHS participants (10.9% vs. 6.7%, p<0.01); however, with adjustments for age and gender, there was no longer a significant difference. There was no significant difference in the percentages with IGT. C-peptide levels were much lower in the DPT-1 cohort at all OGTT time points (p<0.001 for all).
Discussion
Differing criteria for autoantibody screening can result in marked differences in the baseline metabolic profiles of prospective participants of T1D prevention trials.
Keywords: Type 1 Diabetes, Prevention, Trials, Glucose, C-peptide
INTRODUCTION
Pancreatic islet autoantibodies are associated with type 1 diabetes (T1D) at its diagnosis and are highly predictive of that disorder (1-10). Since autoantibodies are predictive of T1D, they have been used as a basis for identifying potential candidates for prevention trials. In the parenteral and oral insulin prevention trials of the Diabetes Prevention Trial-Type 1 (DPT-1) (11,12) and in the European Nicotinamide Diabetes Intervention Trial (ENDIT) (13), a positive test for islet cell autoantibodies (ICA) was a prerequisite for trial entry. However, the TrialNet Natural History Study (TNNHS) (14), the conduit for TrialNet prevention trials, has used biochemical autoantibody positivity [glutamic acid decarboxylase 65 (GAD65), insulin associated antigen-2 (ICA512), and/or insulin (micro IAA or mIAA)], as a prerequisite for those trials.
Since autoantibody positive participants have subsequently undergone oral glucose tolerance tests (OGTTs) in both DPT-1 and TNNHS, we have compared those studies to assess whether different screening criteria lead to differing metabolic profiles. Such information should lead to improved specificity in identifying appropriate participants for prevention trials.
METHODS
Subjects
DPT-1
DPT-1 screened 97,272 relatives of T1D patients with ICA for possible entry into prevention trials. Participants who were ICA positive at the first screening were invited back for confirmation. If ICA positivity was confirmed, baseline OGTTs were performed to assess whether they qualified for entry into the parenteral insulin or oral insulin trials. Individuals who had OGTTs and did not enter the trials, either did not qualify or chose not to participate in the trials. The procedures were approved by human subjects committees in accordance with the Declaration of Helsinki.
TNNHS
At the time of this analysis, 31,889 relatives of T1D patients have been screened with biochemical autoantibodies for follow-up in the TNNHS and possible entry into a prevention trial. Those who had one positive biochemical autoantibody at the first screening were required to have confirmation of that autoantibody in order to have the baseline OGTT performed. There was subsequent testing for the presence of ICA only if a biochemical autoantibody was present upon the initial test. In very rare instances an OGTT was performed in an individual who was confirmed ICA positive, but not ultimately confirmed to be positive for a biochemical autoantibody. Those who had two positive biochemical autoantibodies at the first screening had the choice of either being tested for confirmation prior to having an OGTT or proceeding to have an OGTT with the provision that they would have confirmatory testing at the time of the OGTT. The procedures were approved by human subjects committees in accordance with the Declaration of Helsinki.
Metabolic Measurements
In both DPT-1 and TNNHS, glucose was measured by the glucose oxidase method. In DPT-1 C-peptide was measured by radioimmunoassay (RIA). C-peptide was measured by the TOSOH assay for TNNHS. In a prior analysis, 564 individuals had C-peptide measurements by both assays (r=0.961; TOSOH=0.96RAI+0.1).
Autoantibody Measurements
An immunofluorescence assay was used to measure ICA on frozen sections of blood type O human pancreas in the DPT-1 ICA Core Laboratory (Gainesville, FL, February 1994 to September 1997 and January 1999 to October 2003; New Orleans, LA, September 1997 to January, 1999), and in the TrialNet Core Screening Laboratory (Gainesville, FL). If ICA values were 10 or more Juvenile Diabetes Foundation (JDF) units, they were considered positive. Measurements of GAD65 and ICA512 were performed at the Barbara Davis Center (Denver, CO). Measurements of mIAA were performed at the Barbara Davis Center and the Joslin Diabetes Center (Boston, MA). The upper limits of normal were 0.032 for GAD65, 0.049 for ICA512, and 0.01 (Denver) and 0.02 (Boston) for mIAA.
Analyses
T-tests and chi-square tests were utilized for simple comparisons. Logistic regression and analysis of covariance were performed to assess associations with adjustments for other variables. Glucose tolerance abnormalities were defined as: diabetic=fasting glucose ≥126 and/or 2-hr glucose ≥200; impaired fasting glucose (IFG)=fasting glucose value 100-125 mg/dl; impaired glucose tolerance (IGT)=2-hr glucose value 140-199 mg/dl. A small number of individuals were not included in the C-peptide analyses due to missing values. The p-values are 2-sided.
RESULTS
The numbers of DPT-1 and TNNHS participants included in the analysis were 956 and 645, respectively. TNNHS participants were significantly older (mean±SD: 18.7±14.2 years vs. 12.3±10.0 years, p<0.001) with an age range of 1-45 years for both studies. There was a higher proportion of females in the TNNHS (56.3% vs. 44.6%, p<0.001). Due to the procedures that were required for entry and the timing of the actual initiation of the trials in DPT-1, there was a longer lag time between initial autoantibody positivity and the performance of the OGTTs in DPT-1 than in the TNNHS (medians: 401days vs. 140 days, p<0.001). In DPT-1, the body mass index (BMI) was only measured in those who entered the trials and it was not obtained from some in the TNNHS. However, among those with measurements, for those <13 years, there was not a significant difference between the DPT-1 (18.2±3.7 kg/m2; n=513) and TNNHS participants (17.8±3.6 kg/m2; n=324). For those ≥13 years, the BMI was significantly higher (p<0.001) in the TNNHS participants (26.7±6.1 kg/m2; n=310) than in the DPT-1 participants (24.1±6.1 kg/m2; n=198).
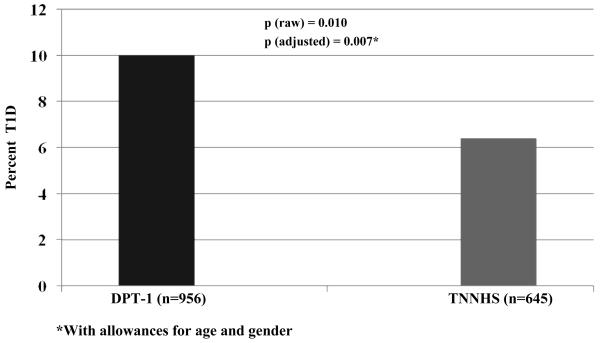
Figure 1 shows the percentages of the baseline OGTTs that were in the diabetic range for the two cohorts; it was appreciably higher in the DPT-1 participants (p<0.01). The higher percentage of diabetic range OGTTs in the DPT-1 cohort persisted (p<0.01) in a logistic regression analysis adjusted for the differences in age and gender between studies. There were no significant associations of diabetic range OGTTs with either age or gender. Among DPT-1 participants, in a logistic regression analysis adjusted for age and gender, there was an inverse association (p<0.01) between diabetic range OGTTs and the interval (log transformed) from initial ICA positivity to the OGTT.
Figure 1.
This shows the percentage of individuals whose baseline OGTTs were in the diabetic range for DPT-1 and the TNNHS. The percentage was higher among the DPT-1 participants.
Glucose levels from non-diabetic range OGTTs were compared between the DPT-1 and TNNHS participants. Fasting glucose levels were significantly, but modestly higher in the TNNHS participants with adjustments for age and gender in an analysis of covariance (difference±SE:1.6±0.5 mg/dl, p<0.001). The differences at all other time points during the OGTT were not significant. Glucose levels were significantly positively associated with age in the fasting state (p<0.001), and at 30 and 60 minutes (p<0.01 for both). Males had significantly higher glucose levels in the fasting state (p<0.001) and at 30 minutes (p<0.05).
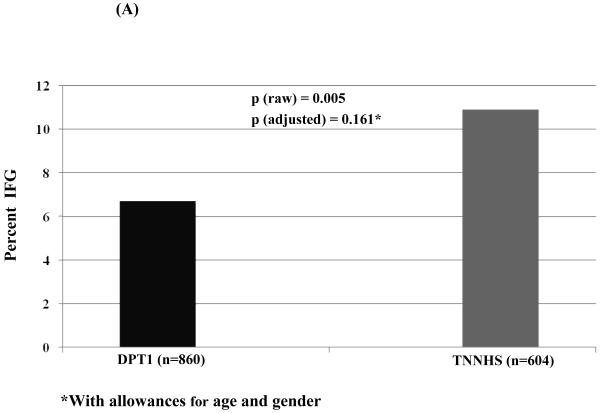
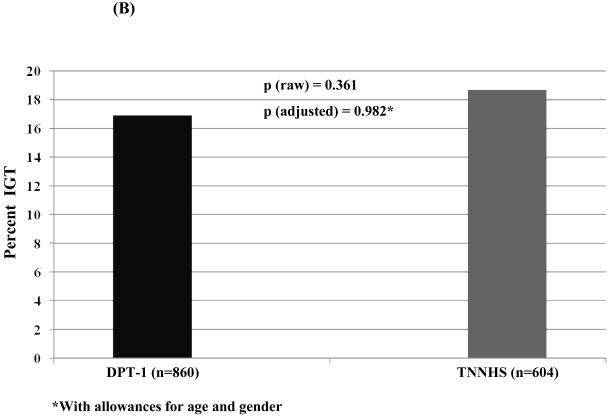
Among those with OGTTs in the non-diabetic range (Figure 2), the percentage with IFG (A) was higher in the TNNHS participants (p<0.01). However, in a logistic regression analysis adjusted for age and gender, the difference in the percentage with IFG was no longer significant (p=0.161). The percentage with IGT was slightly higher in the TNNHS participants, but not significantly in either the raw or adjusted analyses (B).
Figure 2.
This shows the percentage of individuals whose baseline OGTTs were classified as IFG (A) and IGT (B) for DPT-1 and the TNNHS. Those with glucose levels in the diabetic range were excluded. Although the percentage was higher in the raw data for the TNNHS (p<0.01), the difference was not significant with adjustments for age and gender. There was little difference in the percentage with IGT.
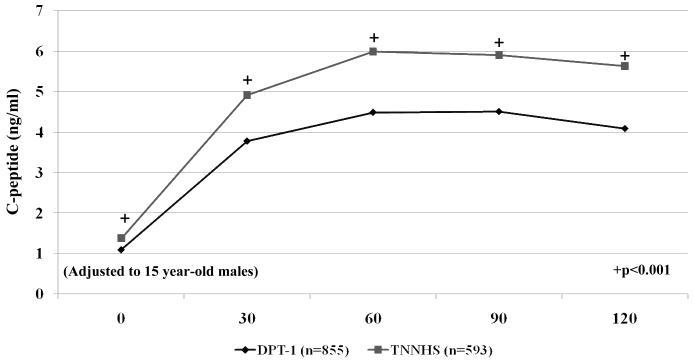
C-peptide values of the OGTT time points were also compared between DPT-1 and TNNHS participants among those with a non-diabetic OGTT. Both in the aggregate and when age was stratified to those <13 years and those ≥13 years, C-peptide values were significantly lower at all OGTT time points (p<0.001) in the DPT-1 participants (Figure 3). C-peptide values were significantly positively associated with age at all time points (p<0.001). Females had higher C-peptide levels at all time points, especially from 60-120 minutes (p< 0.001). With adjustments for age and gender, C-peptide values were markedly lower (p<0.001) in the DPT-1 participants at each time point.
Figure 3.
This shows C-peptide levels according to time points after the oral glucose challenge for participants in DPT-1 and the TNNHS. Those with glucose levels in the diabetic range were excluded. C-peptide levels were substantially lower for those in DPT-1 at every time point. For purposes of display the values for each time point were adjusted to males 15 years of age. They were calculated using coefficients derived from an analysis of covariance that included study (DPT-1 vs. the TNNHS), age, and gender as covariates.
DISCUSSION
The data show that there were appreciable metabolic differences between the DPT-1 and TNNHS cohorts in their baseline OGTTs. DPT-1 participants had a higher percentage of OGTTs in the diabetic range. Also, although glucose levels were similar within the non-diabetic range, those in DPT-1 had much lower C-peptide values at all time points. These differences persisted with adjustments for age and gender. Moreover, in the raw data, IFG was more common in the TNNHS participants, and in an analysis of covariance, there was a small but significant elevation of the fasting glucose values in that same group. There are no previous reports that have compared studies with regard to metabolic outcomes of autoantibody screening.
The findings of a higher prevalence of diabetic OGTTs and markedly lower C-peptide levels in the DPT-1 cohort suggest that DPT-1 screening yielded individuals more at risk for T1D than did TNNHS screening. However, the C-peptide differences must be interpreted cautiously. The combination of higher fasting glucose and C-peptide levels could be interpreted to mean that the TNNHS cohort tended to be more insulin resistant than the DPT-1 cohort. Thus, one cannot necessarily attribute the differences in C-peptide entirely to differences in β-cell function. Also, despite the strong correlation with little deviation from the line of identity, it is still possible that the different C-peptide assays could account for some of the difference in C-peptide levels.
It appears likely that the differences in autoantibody screening criteria at least partially explained the metabolic differences, but other factors could also have influenced the differences. Although the cutoffs for autoantibody positivity in both studies were based on available information regarding sensitivities and specificities, they were still arbitrary to some extent. Also, confirmation of positivity was required for the performance of an OGTT in all DPT-1 participants, whereas in the TNNHS, OGTTs were permitted without confirmation in those who had two positive autoantibodies at the initial screen; however, almost all ultimately had confirmation of at least one autoantibody. The longer interval between initial autoantibody positivity and the OGTTs in the DPT-1 cohort does not explain the appreciable metabolic differences, since there was actually an inverse association between the diabetic range OGTTs and that interval in DPT-1. The similarity of the BMI values between the DPT-1 and TNNHS participants <13 years suggests that the marked C-peptide differences were not related to adiposity among those individuals. Among those ≥13 years, the higher BMI values in the TNNHS cohort could possibly have contributed to the C-peptide differences in that age group, however. Since T1D was previously found to be associated with BMI in DPT-1 (15), it will be important to further assess how adiposity measures might increase screening accuracy.
There were sizeable influences of age and gender on glucose, and especially C-peptide levels, among those with non-diabetic OGTTs. The strong positive association between C-peptide levels and age, and the higher C-peptide levels in females could possibly be explained by insulin resistance. However, data in normal adolescents suggest that associations of insulin resistance with age and gender are complex (16). Moreover, these associations might not be the same in autoantibody positive relatives of T1D patients (17).
The findings in this study show that differing autoantibody screening methodologies for T1D prevention trials can yield substantial metabolic differences. The metabolic profiles that result from autoantibody screening should be evaluated on an ongoing basis in order to refine participant selection for T1D prevention trials.
ACKNOWLEDGEMENTS
TrialNet is funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, and General Clinical Research Centers Program; the Juvenile Diabetes Research Foundation International; and the ADA.
Contributor Information
Jay M. Sosenko, Division of Endocrinology, University of Miami, Miami, FL 33101.
Jeffrey Mahon, Department of Epidemiology and Biostatistics, University of Western Ontario, Ontario, Canada N6A5K8.
Lisa Rafkin, Division of Endocrinology, University of Miami, Miami, FL 33101.
John M. Lachin, The Biostatistics Center, George Washington University, Rockville, Maryland 20852.
Heidi Krause-Steinrauf, The Biostatistics Center, George Washington University, Rockville, Maryland 20852.
Jeffrey P. Krischer, Division of Informatics and Biostatistics, University of South Florida, Tampa, Florida 33612.
David Cuthbertson, Pediatrics Epidemiology Center, University of South Florida, Tampa, Florida 33612.
Jerry P. Palmer, Division of Endocrinology/ Metabolism, VA Puget Sound Health Care System and the University of Washington, Seattle, Washington 98109.
Clinton Thompson, The Biostatistics Center, George Washington University, Rockville, Maryland 20852.
Carla J. Greenbaum, Benaroya Research Institute, Seattle, Washington 98101.
Jay S. Skyler, Diabetes Prevention Trial-Type 1 Diabetes (DPT-1) and Type 1 Diabetes TrialNet Study Groups, Division of Endocrinology, University of Miami, Miami, FL 33101.
REFERENCES
- (1).Bottazzo GF, Florin-Christensen A, Doniach D. Islet cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–1282. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- (2).Lendrum R, Walker G, Cudworth AG, et al. Islet-cell antibodies in diabetes mellitus. Lancet. 1976;2:1273–1276. doi: 10.1016/s0140-6736(76)92033-x. [DOI] [PubMed] [Google Scholar]
- (3).Gorsuch AN, Spencer KM, Lister J, et al. Evidence for a long prediabetic period in Type I (insulin dependent) diabetes mellitus. Lancet. 1981;2:1363–1365. doi: 10.1016/s0140-6736(81)92795-1. [DOI] [PubMed] [Google Scholar]
- (4).Palmer JP, Asplin CM, Clemons P, et al. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- (5).Wilkin T, Armitage M, Casey C, et al. Value of insulin autoantibodies as serum markers for insulin- dependent diabetes mellitus. Lancet. 1985:480–481. doi: 10.1016/s0140-6736(85)92086-0. [DOI] [PubMed] [Google Scholar]
- (6).Baekkeskov S, Landin M, Kristensen JK, et al. Antibodies to a 64,000 Mr human islet cell antigen precede the clinical onset of insulin-dependent diabetes. J Clin Invest. 1987;79(3):926–934. doi: 10.1172/JCI112903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Christie MR, Vohra G, Champagne P, Daneman D, Delovitch TL. Distinct antibody specificities to a 64-kD islet cell antigen in type 1 diabetes as revealed by trypsin treatment. J Exp Med. 1990;172(3):789–794. doi: 10.1084/jem.172.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Payton MA, Hawkes CJ, Christie MR. Relationship of 37,000 and 40,000-M triptic fragment of islet antigens in insulin dependent diabetes to the protein tyrosine phosphatase-like molecule IA-2(ICA 512) J Clin Invest. 1995;96(3):1506–1511. doi: 10.1172/JCI118188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Verge CF, Gianani R, Kawasaki E, et al. Number of autoantibodies (against insulin, GAD or ICA512/IA2) rather than particular autoantibody specificities determines risk of type I diabetes. J Autoimmun. 1996;9(3):379–383. doi: 10.1006/jaut.1996.0051. [DOI] [PubMed] [Google Scholar]
- (10).Kulmala P, Savola K, Petersen JS, et al. The Childhood Diabetes in Finland Study Group Prediction of insulin-dependent diabetes mellitus in siblings of children with diabetes. A population-based study. J Clin Invest. 1998;101(2):327–336. doi: 10.1172/JCI119879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Diabetes Prevention Trial-Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- (12).Diabetes Prevention Trial-Type 1 Diabetes Study Group Effects of insulin in relatives of patients with type 1 diabetes. Diabetes Care. 2005;28:1068–1076. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- (13).Gale EA, Bingley PJ, Emmett CL, et al. A randomized controlled trial of intervention before the onset of type 1. Lancet. 2004;363:925–931. doi: 10.1016/S0140-6736(04)15786-3. [DOI] [PubMed] [Google Scholar]
- (14).Mahon JL, Sosenko JM, Rafkin-Mervis L, et al. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10(2):97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- (15).Sosenko JM, Krischer JP, Palmer JP, Mahon J, Cowie C, Greenbaum CJ, Cuthbertson D, Lachin JM, Skyler JS, Diabetes Prevention Trial -Type 1 Study Group A risk score for type 1 diabetes derived from autoantibody positive participants in The Diabetes Prevention Trial-Type 1. Diabetes Care. 2008;31:528–533. doi: 10.2337/dc07-1459. MD. [DOI] [PubMed] [Google Scholar]
- (16).Moran A, Jacobs DR, Jr, Steinberger J, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. 2008;117(18):2361–2368. doi: 10.1161/CIRCULATIONAHA.107.704569. [DOI] [PubMed] [Google Scholar]
- (17).Ma X, Becker D, Arena V, Vicini P, Greenbaum C. The effect of age on insulin sensitivity and insulin secretion in first-degree relatives of Type 1 Diabetic Patients: a population analysis. J Clin Endocrinol Metab. 2009;94(7):2446–2451. doi: 10.1210/jc.2008-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]