Abstract
Activation of the corticotropin-releasing factor-1 (CRF-1) receptor in the anterolateral BNST (BSTal), a key subdivision of the extended amygdala, elicits opiate-seeking behavior exacerbated by stress. However, it is unknown whether the presence of CRF-1 affects expression of the mu-opioid receptor (μ-OR) in the many GABAergic BSTal neurons implicated in the stress response. We hypothesized that deletion of the CRF-1 receptor gene would alter the density and/or subcellular distribution of μ-ORs in GABAergic neurons of the BSTal. We used electron microscopy to quantitatively examine μ-OR immunogold and GABA immunoperoxidase labeling in the BSTal of CRFr-1 knockout (KO) compared to wildtype (WT) mice. To assess regional specificity, we examined μ-OR distribution in dorsal striatum. The μ-ORs in each region were predominantly localized in dendrites, many of which were GABA-immunoreactive. Significantly more cytoplasmic μ-OR gold particles per dendritic area were observed selectively in GABA-containing dendrites of the BSTal, but not of the dorsal striatum, in KO compared to WT mice. In both regions, however, significantly fewer GABA-immunoreactive axon terminals were present in KO compared to WT mice. Our results suggest that absence of CRF-1 results in enhanced expression and/or dendritic trafficking of μ-ORs in inhibitory BSTal neurons. They also suggest that expression of CRF-1 is a critical determinant of the availability of GABA in functionally diverse brain regions. These findings underscore the complex interplay between CRF, opioid and GABA systems in limbic and striatal regions, and have implications for the role of CRF-1 in influencing the pharmacological effects of opiates active at μ-ORs.
Keywords: stress, BNST, striatum, opiate, addiction, GABA, trafficking, immunocytochemistry
Introduction
Stress is a potent motivator of opiate seeking and relapse (Krueger, 1981; Kosten et al, 1986; Shaham & Stewart, 1995; Erb et al, 1996; Piazza & Le Moal, 1998; Shaham et al, 2000; Mayer et al, 2002; Grusser et al, 2007). These effects are mediated, in part, by the corticotropin-releasing factor-1 (CRF-1) receptor in the anterolateral bed nucleus of the stria terminalis (BSTal; Lu et al, 2000; Wang et al, 2006). The BSTal, comprised within the extended amygdala, is a complex limbic brain region implicated in many of the motivational aspects of opiate dependence (Alheid & Heimer, 1988; Koob, 2003; Veinante et al, 2003; Kash et al, 2008; Walker & Davis, 2008). In postsynaptic dendrites of the BSTal, the CRF-1 receptor is extensively co-expressed with the mu-opioid receptor (μ-OR; Jaferi & Pickel, 2009), the major receptor mediating the analgesic and rewarding effects of the opiates, morphine and heroin (Kieffer, 1999; Contet et al, 2004). However, it is not yet known whether the expression of the CRF-1 receptor is a determinant of the availability of the μ-OR in BSTal neurons, many of which contain gamma-aminobutyric acid (GABA; Sun & Cassell, 1993).
Intact GABAergic transmission in the extended amygdala is critical for normal inhibition of affective behaviors and conditioned associations, which, if over-expressed, could appear as pathological states such as anxiety disorders and addiction (Sanders and Shekhar, 1995; Fendt et al, 2003; Quirk & Gehlert, 2003; Sajdyk et al, 2008). Neurons containing the μ-OR express GABA in reward-associated limbic (Svingos et al, 1997; Steffensen et al, 2006) and antinociceptive brainstem regions (Kalyuzhny & Wessendorf, 1998). In line with this are findings that opiate activation of the μ-OR modulates GABA neurotransmission within the amygdala (Finnegan et al, 2005) and the mesocorticolimbic dopamine reward system (Steffensen et al, 2006). Opiate activation of the μ-OR also enhances the effect of GABAA receptor agonists on anxiolytic behavior (Sasaki et al, 2002). In turn, modulators of GABAergic transmission can influence the pharmacological and behavioral effects of opiates (Tejwani et al, 1993; Rattan and Sribanditmongkol, 1994; Sasaki et al, 2002; Cabral et al, 2009). Alterations in μ-OR expression is therefore of particular interest with regard to opiate addiction and affective behavior when occurring specifically in GABAergic neurons.
Given the anatomical and functional associations between the μ-OR and the CRF-1 receptor in the BSTal, we hypothesize that the availability of the μ-OR within subcellular compartments of GABAergic neurons of this region is CRF-1 receptor-dependent. To test this hypothesis, we used electron microscopy to quantitatively compare the immunogold labeling of the μ-OR in the BSTal of CRF-1 receptor (−/−) knockout (KO) and CRF-1 receptor (+/+) wildtype (WT) mice. The tissue used for analysis of μ-OR gold particle distributions was co-processed for immunoperoxidase labeling of GABA. To assess regional specificity, we conducted the equivalent analysis in the dorsal striatum (caudate-putamen nucleus) which, like the BSTal, is comprised mainly of GABAergic neurons that express the μ-OR (Aoki & Pickel, 1989; Wang et al, 1997), and of neurochemically-unidentified neurons that express the CRF-1 receptor (Justice et al, 2008). The dorsal striatum is essential for motor function, but is also prominently involved in learning of motor habits associated with addictive diseases (Reading et al, 1991; Everitt & Robbins, 2005; Grahn et al, 2008; White, 2009). The results have implications for the role of the CRF-1 receptor in regulation of μ-OR availability in inhibitory neurons of limbic and striatal brain regions.
Materials and Methods
Animals
All experiments were performed in accordance with the NIH regulations of animal care and were reviewed by the Institutional Animal Care and Use Committee of Weill Medical College of Cornell University. We used a total of eight adult, male mice of which four were CRF-1 receptor (−/−) KO mice and the rest were WT (+/+) littermates. CRF-1 receptor KO mice were purchased from the Jackson Laboratories (Bar Harbor, ME). To generate homozygous mice, the heterozygous littermates were crossed, and the litters were genotyped by standard polymerase chain reaction (PCR) procedures with tail DNA. Mice were group-housed in a quiet facility on a reverse 12-hour light/dark cycle and were given unlimited access to food and water. All efforts were made to minimize suffering and the number of mice used for experiments.
Tissue Preparation
Prior to perfusion, mice were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.). Their brain tissue was fixed by vascular perfusion through the left ventricle of the heart with sequential delivery of (1) 5 ml of heparin-saline (1000 U/ml), (2) 30 ml of 3.75% acrolein and 2% paraformaldehyde in 0.1M phosphate buffer (PB; pH 7.4), and (3) 100 ml of 2% paraformaldehyde in PB. Brains were removed from the cranium, post-fixed with 2% paraformaldehyde in 0.1M PB for 30min, and then transferred to PB. These brains were then cut coronally into 40 um sections on a Vibratome (Leica, Deerfield, IL). The coronal sections were collected through the rostrocaudal extent of the BNST and striatum based on the mouse brain atlas by Hof et al (2000). Rostrocaudally-matched pairs of sections from CRF-1 receptor KO and WT mice were simultaneously processed for immunogold–silver labeling of the μ-OR and immunoperoxidase labeling of GABA.
Antisera
For immunogold-silver labeling of the μ-OR, we used a polyclonal guinea pig antiserum directed against a synthetic peptide corresponding to amino acids 384-398 of the C-terminus of the cloned rat μ-OR (AB1774; Chemicon, Temecula, CA, USA). The specificity of this antiserum has previously been demonstrated by the absence of immunoreactivity in adsorption controls (Rodriguez et al., 2001; Drake and Milner, 2002; Chemicon, 2005). The guinea-pig μ-OR antiserum has also been shown to recognize proteins that are attenuated in μ-OR KO mice with deletions at distinct exons of the μ-OR gene including exon 1, exons 2/3 and exon 11 (Jaferi & Pickel, 2009).
For immunoperoxidase labeling of GABA, we used a polyclonal rat antiserum that was raised against GABA-glutaraldehyde-hemocyanin conjugates (Bayer & Pickel, 1991). Immunolabeling of this antiserum shows a concentration-dependent reduction following prior adsorption with GABA-bovine serum albumin (BSA) conjugates (Lauder et al, 1986). The cellular distribution of this antiserum in rat brain is comparable to that of the GABA-synthesizing enzyme, glutamic acid decarboxylase (GAD; Oertel et al, 1983; Ottersen & Storm-Mathisen, 1984).
Immunocytochemical Labeling
Brain sections from CRF-1 receptor KO and WT mice were co-processed for dual immunogold-silver and immunoperoxidase labeling of the μ-OR and GABA (Chan et al, 1990; Drake & Milner, 2002; Lane et al, 2008; Jaferi & Pickel, 2009; Jaferi et al, 2009). For this dual-labeling, the vibratome sections were placed in 1% sodium borohydride in 0.1 M PB for 30 minutes to remove excess aldehydes, and then washed thoroughly in 0.1 M PB. To enhance the penetration of tissue by immunoreagents, the sections were soaked in a cryoprotectant solution (25% sucrose and 3.5% glycerol in 0.05M PB) for 15 minutes, and rapidly freeze-thawed by sequential immersion in liquid chlorodifluoromethane (Freon, Refron Inc., NY), liquid nitrogen, and 0.1 M PB at room temperature. The sections were then rinsed in 0.1 M Tris-buffered saline (TBS). To reduce nonspecific binding of the antisera, the sections were incubated in 0.5% BSA in 0.1 M TBS, pH 7.6, for 30 minutes before placement in the primary antisera solution. The sections were incubated for 48 hours at 4°C in a solution containing both primary antisera (1:500 guinea pig anti-μ-OR for immunogold-silver labeling and 1:10,000 rat anti-GABA for immunoperoxidase labeling.) All antisera dilutions were prepared in 0.1% BSA in 0.1 M TBS. Incubations with primary as well as secondary antisera were done with shaking on a rotator (OS- 500; VWR, West Chester, PA).
For immunoperoxidase labeling of GABA, the sections were rinsed in 0.1 M TBS and then placed for 30 min in a 1:400 dilution of biotinylated anti-rat immunoglobulin (IgG). These were then washed in 0.1 M TBS and incubated for 30 min in avidin-biotin peroxidase complex (Vectastain Elite Kit, Vector Labs, Burlingame, CA). This incubation was followed by a wash in 0.1 M TBS, and reaction for 6 min in 0.022% 3,3′-diaminobenzidine (DAB, Aldrich, Milwaukee, WI) and 0.003% hydrogen peroxide in 0.1 M TBS.
For dual detection of antigens, the sections were rinsed in 0.01M PBS (pH 7.4) after the DAB reaction, and then blocked in 0.8% BSA and 0.1% gelatin in PBS for 10 minutes. The sections were subsequently incubated with a 1:50 dilution of anti-guinea pig IgG conjugated with one nm colloidal gold (Amersham, Arlington Heights, IL) for 2 hours at room temperature. The sections were washed several times with 0.1 M TBS. To aid in the binding of gold particles conjugated to IgG, the sections were placed in 2% glutaraldehyde for 10 minutes. Silver intensification was performed using the IntenS-EM kit (Amersham) with a 6 minute reaction in the silver solution at room temperature. The sections were post-fixed in 2% osmium tetroxide in 0.1 M PB for 60 minutes, washed in PB, and dehydrated by passing through an increasingly concentrated series of ethanol solutions, followed by 100% propylene oxide. The sections were kept overnight in a 1:1 mixture of propylene oxide and Epon (Electron Microscopy Sciences, Fort Washington, PA), and then transferred to 100% Epon for 2-3 hours before flat-embedding between sheets of Aclar plastic (Electron Microscopy Sciences, Fort Washington, PA). These flat-embedded sections were viewed with a light microscope to select immunolabeled portions of the dorsal area of the BSTal (Fig. 1A) and the dorsolateral striatum (Fig. 1B). These regions were identified in coronal sections of forebrain located at Bregma 0.2 mm in the mouse stereotaxic atlas (Hof et al, 2000). Ultra-thin sections of approximately 70nm thickness were cut from the superficial surface of the section using a diamond knife (Diatome, Fort Washington, PA) on an ultratome (NOVA LKV-Productor AB, Bromma, Sweden). The thin sections were collected on 400-mesh copper grids (Electron Microscopy Sciences), and counterstained with uranyl acetate and lead citrate (Reynolds, 1963). These sections on grids were rinsed and allowed to air dry before viewing at 60 kV with a Philips CM10 transmission electron microscope (FEI, Hillsboro, OR). Those areas showing immunolabeling of the μ-OR and/or GABA were then magnified and captured as digital images using AMT Advantage HR/HR-B CCD Camera System (Advanced Microscopy Techniques, Danvers, MA).
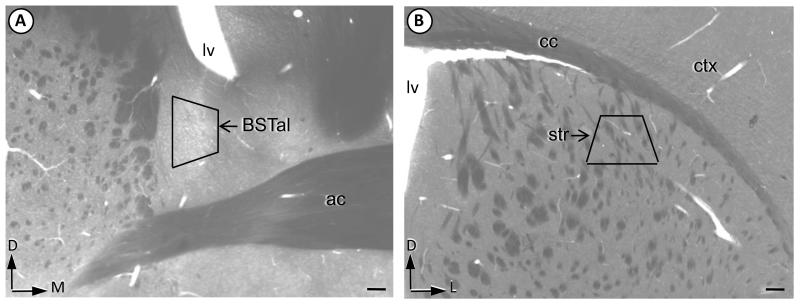
Fig. 1.
Brain regions sampled for electron microscopy. The BNST (A) and striatal (B) subregions sampled for electron microscopy are shown by the trapezoids in plastic-embedded coronal hemisections through the region of the anterior commissure (ac) in a mouse brain. These coronal sections were collected 0.2 mm anterior to Bregma (Hof et al, 2000). Corner arrows point dorsal (D), medial (M) or lateral (L) with respect to the brain surface. BSTal = anterolateral bed nucleus of the stria terminalis, Lv = lateral ventricle, cc = corpus callosum, str = dorsolateral striatum, ctx = somatosensory cortex. Scale bar = 100 μm.
Electron Microscopic Analysis
Ultra-thin sections of the BSTal from 16 vibratome sections (eight sections from four CRF-1 receptor KO mice, eight sections from four WT mice) were used for electron microscopic analysis. Micrographs containing μ-OR and GABA immunoreactivity at magnifications of 13,500 to 25,000 were analyzed in a total tissue area of 17,893 μm2 in the BSTal, of which equal tissue areas were examined from each mouse from WT and KO groups. This analysis was repeated in ultra-thin sections of the striatum from four KO and four WT mice. We only examined tissue at the surface of vibratome sections at the Epon-tissue interface in order to avoid differences in labeling due to differential penetration of reagents. All electron microscopic images were imported into an IBM computer, where Adobe Photoshop (version 7.0.1, Adobe Systems Inc., Mountain View, CA) was used only to sharpen and enhance contrast as needed. These images were then imported into PowerPoint (Microsoft Office, 2007), which was used to assemble figures and add lettering. We classified profiles containing μ-OR and/or GABA immunoreactivity as neuronal processes (dendrites, dendritic spines, axons and axon terminals) based on criteria described in Peters et al (1991). Immunoperoxidase labeling was regarded as positive when the electron-dense reaction product in individual profiles was greater than that seen in other morphologically similar profiles in the neuropil. Profiles were considered to be immunogold-labeled when they (1) contained one or more gold particles, and (2) were located in a neuropil in which no presumed extraneous particles were seen in an equally large area of the surrounding tissue. This approach was validated by examining spurious gold particles overlying myelin, a structure not known to express μ-ORs. For example, out of a total of 222 myelinated axons observed in 8,208 μm2 in the BSTal, none displayed μ-OR immunogold particles. We subsequently quantified the number of μ-OR- and/or GABA-labeled neuronal profiles as well as the density of the μ-OR gold–silver deposits in the plasmalemmal and cytoplasmic compartments of neurons. Gold particles in contact with any portion of the surface membrane were considered to be plasmalemmal while intracellular gold particles not in contact with the surface membrane were classified as cytoplasmic.
The quantification methods used for between-group comparisons of immunogold labeling were similar to those used in other studies (Glass et al, 2005; Lane et al, 2008; Jaferi et al, 2009). Morphological parameters that were used as indirect measures of dendritic size, including surface area (perimeter), cross-sectional area, and minor axis length of dendrites were measured using Microcomputer Imaging Device software (MCID, Imaging Research Inc., Ontario, Canada). The parameters used for statistical comparisons in the BSTal and striatum were: (1) the number of plasmalemmal μ-OR gold particles in a dendritic profile/dendritic perimeter, (2) the number of cytoplasmic μ-OR gold particles in a dendritic profile/dendritic cross-sectional area, (3) the number of total μ-OR gold particles in a dendritic profile/dendritic cross-sectional area, (4) the number of GABA immunoperoxidase-labeled dendritic profiles per neuropil area, (5) the number of GABA immunoperoxidase-labeled axonal profiles per neuropil area. Analyses #1-3 were conducted for all μ-OR-containing dendrites, and additionally for only those μ-OR-containing dendrites that were GABA-immunoreactive. Quantification of immunolabeled profiles was performed by an investigator who was blind to experimental groups (i.e., KO or WT.)
In order to compare similar structures across groups, cluster analysis by dendritic size was performed to statistically divide μ-OR-labeled dendrites from KO and WT groups using JMP 5.0.1 statistical software (SAS Institute Inc., NC, USA). This analysis was conducted because dendritic size is an indirect indicator of the distance of dendrites from somata, with the larger dendrites being more proximal and the smaller dendrites more distal from the somatic origins (Peters et al, 1991). There is, however, variation in dendritic size that may be attributed to their origin from neurons of different sizes and/or dendritic branching patterns.
Dendrites from mice within the same group can also display variations in immunoperoxidase labeling intensities (Bayer & Pickel, 1991; Lane et al, 2008). Dendritic populations that display distinct intensities in immunolabeling are also known to differ in their afferent inputs (Bayer & Pickel, 1991), and are differentially affected by drug administration (Lane et al, 2008), suggesting that these neurons may be transmitter- and/or function-specific. Data for immunolabeling were analyzed by individual dendrites, a method of analysis that has previously been used in electron microscopic studies of receptor or neurotransmitter distribution (Hara & Pickel, 2007; Lane et al, 2008). A within-groups analysis was carried out to assess whether significant variability exists between mice within each group. We found no significant intra-animal differences in the number of intensely- and lightly-labeled GABA dendrites or in the numbers of μ-OR gold particles. Data were analyzed by one-way Analysis of Variance (ANOVA), and differences in means were analyzed by Tukey’s post hoc test.
Results
Commonalities in μ-OR subcellular location and association with GABA in CRF-1 KO and WT
CRF-1 receptor KO and WT mice were similar in their neuronal distributions of μ-ORs and associations with GABA-immunoreactive neurons. In WT (Fig. 2A) and CRF-1 receptor KO (Fig. 2B) mice, μ-OR immunogold labeling in the BSTal was observed in many dendritic profiles (dendrites and dendritic spines). In addition, μ-OR immunogold was sparsely distributed in axonal profiles (axons and axon terminals) of both WT (Fig. 2A) and KO (Fig. 2B). Quantification confirmed that, out of all μ-OR-labeled neuronal (dendritic and axonal) profiles in the BSTal of WT mice, 79% (893/1131) were dendritic and 21% (238/1131) were axonal. Similarly, in CRF-1 receptor KO mice, 74% (809/1088) of all μ-OR-labeled neuronal profiles in the BSTal were dendritic and 26% (279/1088) were axonal. Cluster analysis revealed μ-OR labeling in three major size groups of dendrites- small (0.70-1.17 μm), medium (1.20-1.40 μm), and large (2.0-3.8 μm). The μ-OR labeling was observed in 401 small, 294 medium, and 104 large dendrites within the BSTal of WT mice. Similar patterns were observed in the BSTal of CRF-1 receptor KO mice where there were 319 small, 331 medium, and 90 large dendrites.
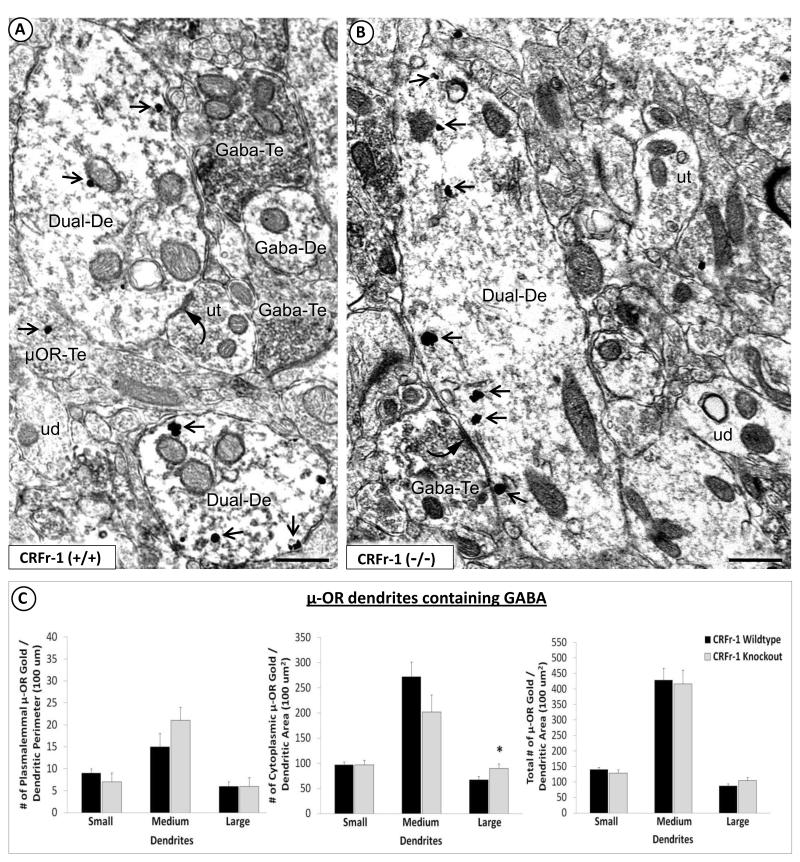
Fig. 2.
Qualitative and quantitative evidence for enhanced cytoplasmic μ-OR immunolabeling in large GABA-containing dendrites of the BSTal in CRF-1 receptor KO compared with WT mice. Electron micrographs show μ-OR immunogold particles in dendrites that contain GABA immunoperoxidase in the BSTal of CRF-1 receptor WT (A) and KO mice (B). Shown in (A) are GABA immunoperoxidase-labeled dendrites with (Dual-De) or without (GABA-De) μ-OR immunogold particles (arrow) within the cytoplasm and contacting the plasma membrane. Dendrites co-labeled for the μ-OR and GABA (Dual-De) in (A) are apposed to, or receive symmetric, inhibitory-type synapses (curved arrow) from, axon terminals that are unlabeled (ut) or that contain μ-OR immunogold labeling (μOR-Te). Also seen in the neuropil in (A) is a dendrite that contains GABA peroxidase (GABA-De) but lacks any detectable μ-OR immunogold labeling. The dual-labeled dendrite in (B) receives a symmetric synapse (curved arrow) from an axon terminal that contain GABA peroxidase (GABA-Te). In (A) and (B) are unlabeled dendrites that are without either immunoperoxidase or immunogold labeling (ud). Scale bars = 500 nm. Bar graphs in (C) show plasmalemmal (number/perimeter length), cytoplasmic (number/area) and total (numer/area) density of μ-OR immunogold particles in GABA-immunoreactive dendrites of varying sizes (small, medium, and large) in 17,893 um2 of the BSTal in CRF-1 receptor KO and WT mice (n = 4 mice per group). The asterisk (*) indicates that, as compared with WT, the CRF-1 receptor KO group has a significantly greater cytoplasmic μ-OR immunogold particle density. * p < 0.05. Error bars indicate SEM.
The μ-OR immunogold particles were commonly distributed within the cytoplasm and along the plasma membrane of dendritic profiles that also contained GABA immunoperoxidase in both WT (Fig. 2A) and CRF-1 receptor KO mice (Fig. 2B). Unlike the predominantly dendritic location of the μ-OR, the distribution of GABA immunoreactivity was almost equally prevalent in dendritic (53%; 980/1859) and axonal (47%; 879/1859) profiles of WT mice as well as in dendritic (50%; 702/1408) and axonal (50%; 706/1408) profiles of KO mice. A subset of neurons that were immunogold-labeled for the μ-OR also contained GABA immunoperoxidase reaction product. Forty percent (354/893) of dendritic, and 33% (78/238) of axonal profiles containing μ-OR immunogold particles were immunoperoxidase-labeled for GABA in WT mice. In CRF-1 receptor KO mice, 26% (210/809) of dendritic, and 22% (60/279) of axonal profiles contained both μ-OR immunogold and GABA immunoperoxidase labeling.
Synaptic associations of μ-OR-labeled neurons
The types of synaptic associations between axon terminals and μ-OR-labeled neurons in the BSTal of WT mice were similar to those observed in CRF-1 receptor KO mice. Out of all synaptic contacts seen on μ-OR-labeled dendrites with or without GABA immunoreactivity in the BSTal of WT mice (n= 322), the most common were from unlabeled axon terminals (272/322). These contacts were characterized by asymmetric, excitatory-type (180/322) or less often, symmetric, inhibitory-type (92/322) synapses (Fig. 2A, 3A). Interestingly, the μ-OR immunogold particles were preferentially located near asymmetric, excitatory-type synapses (Fig. 3A). The μ-OR-labeled dendrites also received inhibitory-type synapses (43/322) from, or were apposed (n=97) to, GABA-immunoreactive axon terminals (Fig. 2A). There were rarely recognizable affiliations of dendritic μ-OR immunogold particles with symmetric synapses.
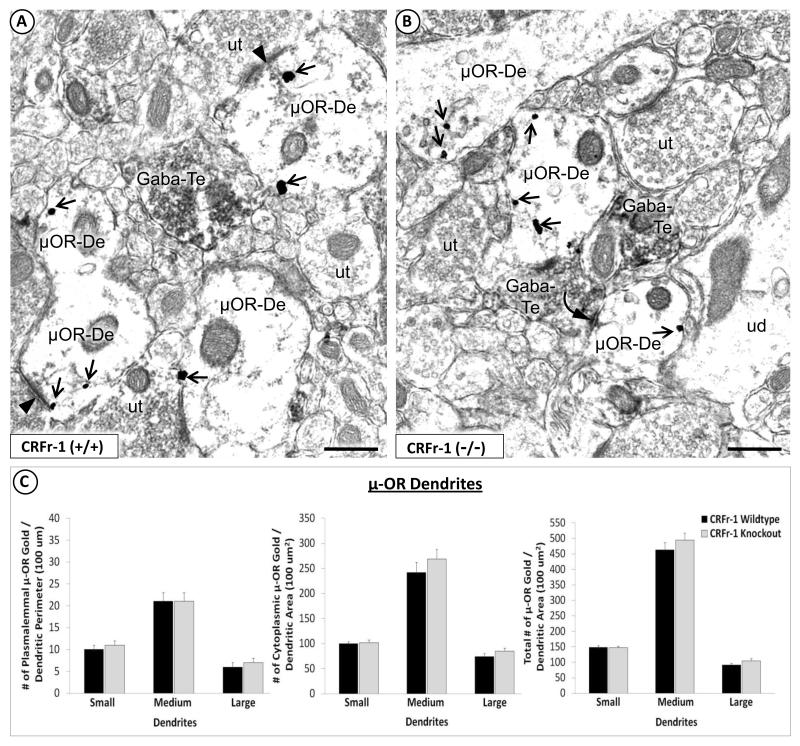
Fig. 3.
Absence of a global effect of CRF-1 receptor deletion in the total population of μ-OR-containing dendrites. Electron micrographs show the similarity of μ-OR immunogold distribution in dendrites that lack detectable GABA peroxidase in the BSTal of CRF-1 receptor WT (A) or KO mice (B). In (A), μ-OR immunogold particles (arrow) are distributed in the cytoplasm, on the plasma membrane and near asymmetric, excitatory-type synapses (arrowhead) in dendrites (μOR-De). The μ-OR-labeled dendrites in (A) receive asymmetric, excitatory-type synapses (arrowhead) from unlabeled axon terminals (ut). μ-OR-labeled dendrites also receive symmetric, inhibitory-type synapses (curved arrow) from axon terminals that contain GABA peroxidase (GABA-Te), as seen in (B). ud = unlabeled dendrite. Scale bars = 500 nm. Bar graphs in (C) show a lack of significant differences between CRF-1 receptor KO and WT mice in the plasmalemmal (number/perimeter length), cytoplasmic (number/area) or total (number/area) density of μ-OR immunogold particles in the total population of BSTal dendrites whether or not they contain GABA immunoperoxidase (n = 4 mice per group). Error bars indicate SEM.
Preferential enhancement of the cytoplasmic density of μ-OR immunogold in GABA-containing dendrites of the BSTal in CRF-1 KO
Quantitative comparisons of μ-OR immunogold labeling between KO and WT groups were first carried out for only those μ-OR-labeled dendritic profiles with detectable GABA peroxidase (Fig. 2A, 2B), as described below. Compared with WT mice, CRF-1 receptor KO mice showed similar plasmalemmal (Fig. 2C), but significantly more cytoplasmic μ-OR immunogold particles per unit cross-sectional area of large GABA-immunoreactive dendrites (F(1,87) = 4.1; p < 0.05); (Fig. 2C). In contrast with large dendrites, there was an apparent decrease in the cytoplasmic density of μ-OR immunogold in medium dendrites of CRF-1 receptor KO mice (Fig. 2C). However, neither medium nor small dendrites significantly differed in dendritic μ-OR immunogold in CRF-1 receptor KO compared with WT mice. In GABA-immunoreactive dendrites regardless of size, the percentage of μ-OR immunogold particles that were associated with synapses was significantly less in CRF-1 receptor KO (1.4% ± 0.01) compared with WT (6.2% ± 0.01) mice (F(1,510) = 6.3; p < 0.01). There were no significant between-group differences in the cross-sectional area or perimeter of dendrites in the BSTal.
When quantitative comparisons of μ-OR immunogold labeling between KO and WT groups were carried out for the total population of μ-OR-labeled dendritic profiles (with and without detectable GABA peroxidase; Fig. 3A, 3B), KO and WT mice did not significantly differ in the plasmalemmal, cytoplasmic or total number of μ-OR immunogold particles for any dendritic size class (Fig. 3C). However, the percentage of synapse-associated μ-OR immunogold was significantly less in dendrites of CRF-1 receptor KO (3.4% ± 0.01) compared with WT (6.1% ± 0.01) mice (F(1,1537) = 7.7; p < 0.01). This difference was also observed specifically in small dendrites of CRF-1 receptor KO (3.2% ± 0.01) compared with WT (6.7% ± 0.01) mice (F(1,718) = 9.0; p < 0.01). No significant between-group differences were seen for the number of μ-OR-expressing dendrites in the BSTal.
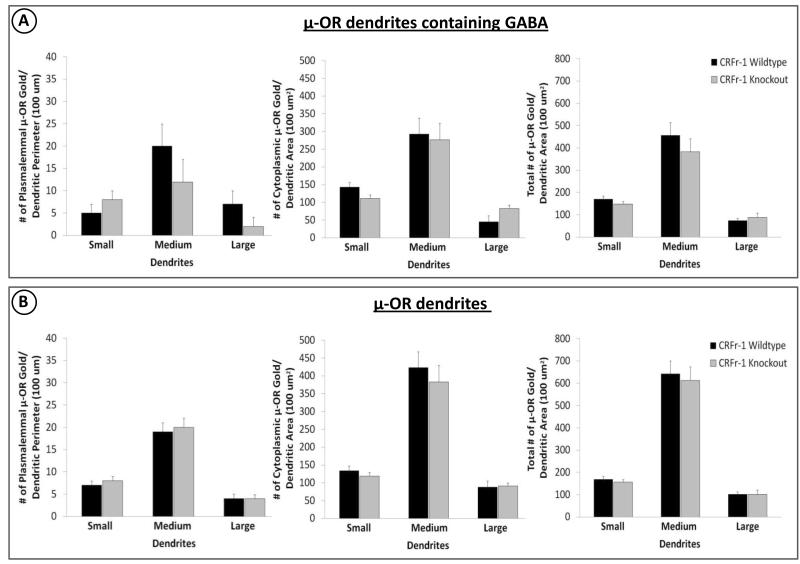
As a comparison for the BSTal, quantitative analysis of μ-OR immunogold labeling between WT and KO groups was carried out in the dorsal striatum. Quantitative analyses were conducted for μ-OR-labeled dendritic profiles with detectable GABA peroxidase (Fig. 4A) separately from the total μ-OR-labeled dendritic population (Fig. 4B). For either population of dendritic profiles, no significant between-group differences were observed for small (0.70-1.18 μm), medium (1.2-1.3 μm) or large (2.0-5.0 μm) dendrites (Fig. 4A, 4B).
Fig. 4.
Quantitative analyses of μ-OR immunogold labeling in dendrites of varying sizes (small, medium, large) are shown for the dorsal striatum, used as a region of comparison for the BSTal. Bar graphs show plasmalemmal (number/perimeter length), cytoplasmic (number/area) and total (number/area) density of μ-OR immunogold particles in GABA-immunoreactive dendrites (A) or in all dendrites with and without GABA immunoreactivity (B) in CRF-1 receptor KO and WT mice (n = 4 mice per goup). Error bars indicate SEM.
Reduction in numbers of GABA-immunoreactive dendritic and axonal profiles in BSTal and striatum of CRF-1 KO mice
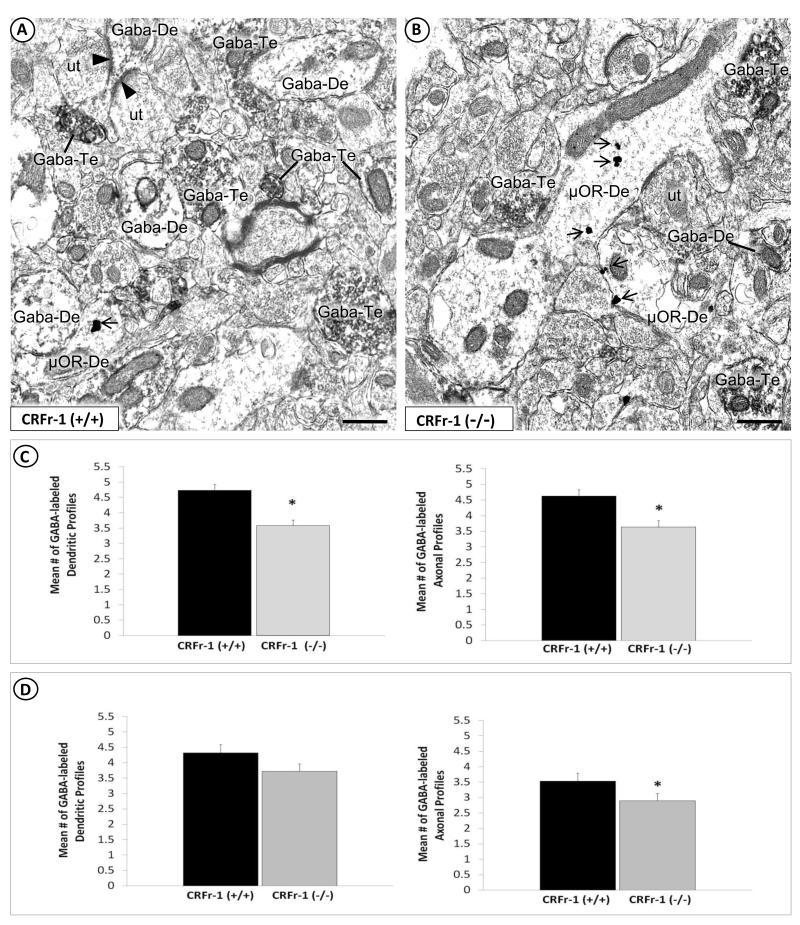
In the BSTal of both CRF-1 receptor KO and WT mice, numerous dendritic and axonal profiles containing GABA immunoperoxidase were apposed or formed symmetric synaptic contacts with μ-OR-labeled neurons (Fig. 5A, 5B). Quantitative analysis of GABA immunoperoxidase-labeled neuronal profiles in the BSTal showed significant between-group differences in the abundance of labeled profiles. Significantly fewer GABA immunoperoxidase-labeled (F(1,399) = 19.5; p < 0.01), and significantly more unlabeled (F(1,350) = 40.5; p < 0.01) dendritic profiles were observed in the BSTal of CRF-1 receptor KO compared with WT mice (Fig. 5C). In the dorsal striatum, there was a trend towards fewer GABA-immunoreactive dendritic profiles in CRF-1 receptor KO compared to WT mice (F(1,238) = 2.9; p = 0.09) (Fig. 5D). In addition, there were significantly fewer GABA immunoreactive axonal profiles in both the BSTal (F(1,382) = 11.9; p < 0.01) and striatum (F(1,203) = 5.0; p < 0.05) of the CRF-1 receptor KO compared with the WT mice (Fig. 5C, 5D).
Fig. 5.
Region-specific differences between CRF-1 receptor KO and WT in the prevalence of GABA-labeled dendrites and axon terminals in the BSTal, but only axon terminals in the dorsal striatum. Electron micrographs show GABA immunoperoxidase-labeled dendrites (GABA-De) and axon terminals (GABA-Te) of CRF-1 receptor WT (A) and KO (B) mice. A GABA-immunoreactive dendrite in (A) receives two asymmetric, excitatory-type synapses (arrowhead) from unlabeled axon terminals (ut). GABA peroxidase-containing dendrites and axon terminals in (A) and (B) are seen in the neuropil with dendrites (μOR-De) that contain μ-OR immunogold particles (arrow). Note that many more GABA-labeled dendrites as well as axon terminals are seen in the BSTal of WT (A) compared with CRF-1 receptor KO (B). Scale bars = 500 nm. Bar graphs in (C) show a significant reduction in the mean number of GABA immunoperoxidase-labeled dendrites and axon terminals per neuropil area in the BSTal of CRF-1 receptor KO and WT mice. In the striatum (D), there is also a significant reduction in GABA-labeled axon terminals, but no significant difference in dendrites of CRF-1 receptor KO compared with WT mice (n = 4 mice per group). * p < 0.05. Error bars indicate SEM.
Discussion
Our results provide the first ultrastructural evidence that, as compared to WT mice, those deficient in the CRF-1 receptor have a greater cytoplasmic density of μ-OR immunogold in large dendrites of GABAergic neurons in the BSTal. We also show a concomitant reduction in synapse-associated μ-OR immunogold in small dendrites of CRF-1 receptor KO compared with WT mice. These changes in dendritic μ-OR immunogold labeling are specific to the BSTal and not observed in the dorsal striatum of CRF-1 receptor KO mice. The results suggest that the availability of μ-ORs in GABAergic dendrites of the BSTal is dictated in part by expression of the CRF-1 receptor. In addition, however, we observed fewer GABA-immunoreactive dendritic and axonal profiles in both the BSTal and dorsal striatum of CRF-1 receptor KO compared to WT mice. Together, these findings have important implications for understanding the capacity of CRF-1 receptor agonists to modulate the response to opiates active at the μ-OR, and to influence GABAergic expression in limbic and striatal neurons.
Methodological Considerations
In both the rat and mouse brain, GABA immunoreactivity is localized in somatodendritic and axonal profiles within the BSTal (Sun & Cassell, 1993; Bali et al, 2005) and striatum (Delle Donne et al, 1987; Pickel & Heras, 1996; Chen et al, 2003). However, the detection of GABA immunoreactivity is often limited to larger dendrites and axon terminals, and less commonly seen in smaller dendrites and dendritic spines (Pickel & Heras, 1996; Chen et al, 2003). Thus, in the present quantitative analyses, co-localization of the μ-OR and GABA may have been particularly underestimated in smaller dendrites. We cannot rule out the possibility that dendrites categorized as unlabeled (i.e., without GABA peroxidase) could arise from GABAergic cell bodies having undetectable dendritic labeling. This limitation, however, is equally applicable to CRF-1 receptor KO and WT mice and is not likely to significantly affect the interpretation of the results in the present study.
Enhancement of cytoplasmic μ-OR density in GABA dendrites of the BSTal in CRF-1 KO
In the BSTal of CRF-1 receptor KO mice, the observed significant increase in cytoplasmic μ-OR density in large GABAergic dendrites without change in plasmalemmal μ-OR suggests that this increase is not related to internalization of surface/synaptic receptors. In contrast, however, the increase in μ-OR immunogold density in large dendrites may be attributed in part to a CRFr1-dependent shift in the locations of the cytoplasmic reserve of μ-ORs from medium to large dendrites of GABA-containing neurons in the BSTal. This is suggested by the decrease in cytoplasmic μ-ORs in medium GABAergic dendrites of CRF-1 receptor KO mice. Furthermore, the proportion of μ-ORs associated with synapses in small BSTal dendrites was significantly less in CRF-1 receptor KO compared with WT mice. Therefore, the increase in cytoplasmic μ-OR immunogold in large dendrites of CRF-1 receptor KO mice may also be attributed to CRF-1 receptor dependent trafficking of μ-ORs from synaptic locations in distal dendritic spines to the cytoplasm of proximal dendrites.
The enhanced cytoplasmic density of μ-ORs in CRF-1 receptor KO mice may preferentially occur in those BSTal GABAergic neurons in which there is lost expression of CRF-1 receptors. This is suggested by the frequent co-expression of μ-ORs and CRF-1 receptors in BSTal dendrites of WT mice (Jaferi & Pickel, 2009). In addition, we have observed, in WT mice, that both μ-ORs and CRF-1 receptors within the extended amygdala are frequently associated with excitatory-type synapses (Jaferi & Pickel, 2009; Treweek et al, 2009). It is conceivable that the enhanced accumulation of μ-ORs in the cytoplasm of large dendrites is a reflection that these receptors are no longer necessary at excitatory-type synapses devoid of CRF-1 receptors. This would be somewhat analogous to observed CRF-1 receptor trafficking in BSTal dendrites following chronic morphine-induced activation of the μ-OR (Jaferi et al, 2009).
Unlike the BSTal, our examination of the dorsal striatum revealed a lack of significant changes in dendritic μ-OR immunogold densities in GABAergic dendrites of CRF-1 receptor KO compared to WT mice. The BSTal and dorsal striatum differ in their anatomical connections and specific contributions to drug addiction. The dorsal striatum is implicated in the formation of habits, including those that that are drug-motivated (Everitt & Robbins, 2005). Habits and motor functions are strongly dependent on striatal inputs from the substantia nigra, somatosensory cortex and the motor, premotor, supplementary and cingulate motor cortical areas (Nakano et al, 2000). In contrast, the BSTal, involved in mediating the interaction between stress and drug reward (Shaham et al, 2000; Koob, 2003), is innervated by the insular and prefrontal cortices (Yasui et al, 1991; Radley et al, 2009), the central amygdala (Dong et al, 2001) and the reward-associated ventral tegmental area (Hasue & Shammah-Lagnado, 2002) as well as brainstem regions conveying visceral sensory information through the nucleus of the solitary tract and ventrolateral medulla (Gaykema et al, 2007). The BSTal is also among a select group of regions that receives dense, likely glutamatergic, afferent input from the paraventricular thalamus (Frassoni et al, 1997; Li & Kirouac, 2008), a critical regulator of neuroendocrine responses to repeated stress (Jaferi & Bhatnagar, 2006). Neurons in many of the regions providing input to the BSTal normally express CRF-1 receptors (Justice et al, 2008; Treweek et al, 2009). Thus, the observed increase in μ-OR density in large BSTal dendrites of CRF-1 receptor KO mice may be due not only to changes occurring in single BSTal neurons but also to CRF-1 receptor-containing neurons in brain regions that project extensively to the BSTal and minimally innervate the dorsal striatum.
Receptor changes in genetic KO models are often attributed to compensatory adaptations resulting from absence of the gene during development (Clifton et al, 2003; Wittmann et al, 2005; Kralic et al, 2006). However, evidence showing that acute intracerebroventricular administration of an anti-CRF IgG significantly increases brain μ-OR density in adult mice suggests continued plasticity into adulthood (Rothman et al, 2002). CRF-1 receptor-mediated regulation of μ-OR availability in BSTal neurons may arise not just as a compensatory adaptation in response to CRF-1 receptor deletion, but may also normally exist in association with short-term changes in CRF-1 receptor activation in the adult brain.
The CRF-1 receptor KO and WT mice showed no significant differences in μ-OR density in non-GABA-immunoreactive dendrites of either the BSTal or dorsal striatum. Large, neurochemically-identified populations of neurons other than GABAergic neurons have also been localized in the BSTal and striatum. Neurons rich in dopamine (Pickel et al, 1981; Phelix et al, 1992), and opioids such as dynorphin and enkephalin are prevalent in each of these regions (Pickel et al, 1980; Yakovleva et al, 2006; Poulin et al, 2009). In addition, the BSTal has many CRF-containing neurons (Phelix & Paull, 1990; Jaferi et al, 2009). In the present study, μ-OR-labeled dendrites without GABA-immunoreactivity may represent any of these neuronal populations and also likely include those of GABAergic neurons with non-detectable GABA levels.
Decreased numbers of GABA neurons in BSTal and dorsal striatum of CRF-1 KO
Fewer GABA-immunoreactive dendrites, and more unlabeled dendrites, were observed in the BSTal of CRF-1 receptor KO compared with WT mice. Furthermore, in both the BSTal and striatum of CRF-1 receptor KO mice, fewer GABA-immunoreactive axon terminals were observed compared to WT mice. Mice deficient in the CRF-1 receptor gene are known to display morphological and functional alterations in developing brain regions including significantly increased dendritic branching in the hippocampus (Chen et al, 2004), attenuated synaptic potentiation in inhibitory hippocampal neurons (Schierloh et al, 2007), and a lost capacity of CRF to enhance GABAA inhibitory postsynaptic currents in central amygdala neurons (Nie et al, 2004). The results of the present study suggest that a permanent absence of CRF-1 receptors also induces compensatory changes in the BSTal and dorsal striatum resulting in downregulation of GABAergic expression.
Implications
In the present study, the enhanced cytoplasmic reserve of μ-ORs in large dendrites, and the decreased density of synapse-associated μ-ORs in small dendrites, of GABAergic neurons in the BSTal of CRF-1 receptor-deficient mice may profoundly affect the capacity of these neurons to respond to repeated opiate administration. Behavioral studies have revealed a compelling role for CRF, primarily via CRF-1 receptor activation, in modulation of the response to opiates (Heinrichs et al, 1995; Shaham et al, 1997; Shaham et al, 1998; Iredale et al, 2000; Lu et al, 2000; McNally & Akil, 2002; Contarino & Papaleo, 2005; Stinus et al, 2005; Wang et al, 2006). For example, genetic deletion or pharmacological blockade of the CRF-1 receptor attenuates stress-induced relapse to opiate seeking (Shaham et al, 1998, Wang et al, 2006) as well as the somatic and affective symptoms of opiate withdrawal (Iredale et al, 2000; Lu et al, 2000; Contarino & Papaleo, 2005; Stinus et al, 2005). More direct region-specific evidence demonstrates that blockade of CRF-1 receptors within the BNST attenuates stress-induced reinstatement of morphine conditioned place preference (Wang et al, 2006).
A significant decrease in GABA expression was observed in neurons of both the BSTal and dorsal striatum of CRF-1 receptor KO mice. This observation is consistent with evidence showing that CRF enhances GABAergic synaptic transmission by activation of the CRF-1 receptor in several different brain regions including the BNST (Nie et al, 2004; Kash & Winder, 2006; Schierloh et al, 2007; Bagosi et al, 2008; Kirby et al, 2008). GABAergic neurons in the BNST also regulate anxiety-like behavior (Sajdyk et al, 2008) and hypothalamic-pituitary-adrenal responses to acute emotional stressors (Radley et al, 2009). Therefore, a reduction or loss of CRF-1 receptors available for activation in the BSTal and associated brain regions may have a major impact on GABAergic neurotransmission in BSTal neurons and in their outputs controlling behavioral and neuroendocrine responses to stress. Given the involvement of the BNST in stress-induced opiate relapse (Wang et al, 2006), and of the striatum in the compulsive nature of addiction (Everitt & Robbins, 2005), a marked decrease in GABAergic inhibitory tone in both the BSTal and dorsal striatum has important implications for the maintenance of opiate seeking during addiction.
Acknowledgements
This work was supported by NIDA grants (DA00727416) to AJ, and DA04600 to VMP, whose research support was also provided by the center grant DA005130 under the direction of Dr. MJ Kreek (Rockefeller University).
References
- Aoki C, Pickel VM. Neuropeptide Y in the cerebral cortex and the caudate-putamen nuclei: ultrastructural basis for interactions with GABAergic and non-GABAergic neurons. J Neurosci. 1989;9(12):4333–54. doi: 10.1523/JNEUROSCI.09-12-04333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27(1):1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Bagosi Z, Jászberényi M, Szabó G, Telegdy G. The effects of CRF and the urocortins on [3H]GABA release from the rat amygdala--an in vitro superfusion study. Brain Res Bull. 2008;75(1):15–7. doi: 10.1016/j.brainresbull.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Bali B, Erdélyi F, Szabó G, Kovács KJ. Visualization of stress-responsive inhibitory circuits in the GAD65-eGFP transgenic mice. Neurosci Lett. 2005;380(1-2):60–5. doi: 10.1016/j.neulet.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559(1):44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- Cabral A, Ruggiero RN, Nobre MJ, Brandão ML, Castilho VM. GABA and opioid mechanisms of the central amygdala underlie the withdrawal-potentiated startle from acute morphine. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):334–44. doi: 10.1016/j.pnpbp.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33(2-3):113–27. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemicon [11/26/08];Guinea-pig anti-μ opioid receptor-1 polyclonal antibody AB1774 Product Information. 2005 Downloaded from http://www.chemicon.com/webfiles/PDF/AB1774.pdf.
- Chen LW, Cao R, Liu HL, Ju G, Chan YS. The striatal GABA-ergic neurons expressing substance P receptors in the basal ganglia of mice. Neuroscience. 2003;119(4):919–25. doi: 10.1016/s0306-4522(03)00223-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bender RA, Brunson KL, Pomper JK, Grigoriadis DE, Wurst W, Baram TZ. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101(44):15782–7. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton PG, Lee MD, Somerville EM, Kennett GA, Dourish CT. 5-HT1B receptor knockout mice show a compensatory reduction in 5-HT2C receptor function. Eur J Neurosci. 2003;17(1):185–90. doi: 10.1046/j.1460-9568.2003.02437.x. [DOI] [PubMed] [Google Scholar]
- Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci U S A. 2005;102(51):18649–54. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Kieffer BL, Befort K. Mu opioid receptor: a gateway to drug addiction. Curr Opin Neurobiol. 2004;14(3):370–8. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Delle Donne KT, Sesack SR, Pickel VM. Ultrastructural immunocytochemical localization of the dopamine D2 receptor within GABAergic neurons of the rat striatum. Brain Res. 1997;746(1-2):239–55. doi: 10.1016/s0006-8993(96)01226-7. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38(1-2):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12(2):119–36. doi: 10.1002/hipo.1107. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128(4):408–12. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8(11):1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Apfelbach R. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J Neurosci. 2003;23(1):23–8. doi: 10.1523/JNEUROSCI.23-01-00023.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan TF, Chen SR, Pan HL. Effect of the mu-opioid on excitatory and inhibitory synaptic inputs to periaquaductal gray-projecting neurons in the amygdala. J Pharmacol Exp Ther. 2005;312:441–448. doi: 10.1124/jpet.104.074633. [DOI] [PubMed] [Google Scholar]
- Frassoni C, Spreafico R, Bentivoglio M. Glutamate, aspartate and co-localization with calbindin in the medial thalamus. An immunohistochemical study in the rat. Exp Brain Res. 1997;115(1):95–104. doi: 10.1007/pl00005689. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Chen CC, Goehler LE. Organization of immune-responsive medullary projections to the bed nucleus of the stria terminalis, central amygdala, and paraventricular nucleus of the hypothalamus: evidence for parallel viscerosensory pathways in the rat brain. Brain Res. 2007;1130(1):130–45. doi: 10.1016/j.brainres.2006.10.084. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Kruzich PJ, Colago EE, Kreek MJ, Pickel VM. Increased AMPA GluR1 receptor subunit labeling on the plasma membrane of dendrites in the basolateral amygdala of rats self-administering morphine. Synapse. 2005;58(1):1–12. doi: 10.1002/syn.20176. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Progress in Neurobiology. 2008;86:141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Morsen CP, Wolfling K, Flor H. The relationship of stress, coping, effect expectancies and craving. Eur Addict Res. 2007;13(1):31–8. doi: 10.1159/000095813. [DOI] [PubMed] [Google Scholar]
- Hara Y, Pickel VM. Dendritic distributions of dopamine D1 receptors in the rat nucleus accumbens are synergistically affected by startle-evoking auditory stimulation and apomorphine. Neuroscience. 2007;146(4):1593–605. doi: 10.1016/j.neuroscience.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6(1):74–80. [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative Cytoarchitectonic Atlas of the C57BL/6 and 129/Sv Mouse Brains. Elsevier Science; Amsterdam: 2000. [Google Scholar]
- Iredale PA, Alvaro JD, Lee Y, Terwilliger R, Chen YL, Duman RS. Role of corticotropin-releasing factor receptor-1 in opiate withdrawal. J Neurochem. 2000;74(1):199–208. doi: 10.1046/j.1471-4159.2000.0740199.x. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147(10):4917–30. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Pickel VM. Mu-opioid and corticotropin-releasing factor receptors show largely postsynaptic co-expression, and separate presynaptic distributions, in the mouse central amygdala and bed nucleus of the stria terminalis. Neuroscience. 2009;159(2):526–539. doi: 10.1016/j.neuroscience.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaferi A, Lane DA, Pickel VM. Subcellular plasticity of the corticotropin-releasing factor receptor in dendrites of the mouse bed nucleus of the stria terminalis following chronic opiate exposure. Neuroscience. 2009;163(1):143–54. doi: 10.1016/j.neuroscience.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W. Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol. 2008;511(4):479–96. doi: 10.1002/cne.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyuzhny AE, Wessendorf MW. Relationship of mu- and delta-opioid receptors to GABAergic neurons in the central nervous system, including antinociceptive brainstem circuits. J Comp Neurol. 1998;392(4):528–47. [PubMed] [Google Scholar]
- Kash TL, Nobis WP, Matthews RT, Winder DG. Dopamine enhances fast excitatory synaptic transmission in the extended amygdala by a CRF-R1-dependent process. J Neurosci. 2008;28(51):13856–65. doi: 10.1523/JNEUROSCI.4715-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20(1):19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28(48):12927–37. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13(6):442–52. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rounsaville BJ, Kleber HD. A 2.5-year follow-up of depression, life crises, and treatment effects on abstinence among opioid addicts. Arch Gen Psychiatry. 1986;43(8):733–8. doi: 10.1001/archpsyc.1986.01800080019003. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Sidler C, Parpan F, Homanics GE, Morrow AL, Fritschy JM. Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of gamma-aminobutyric acid type A receptor alpha1 subunit knockout mice. J Comp Neurol. 2006;495(4):408–21. doi: 10.1002/cne.20866. [DOI] [PubMed] [Google Scholar]
- Krueger DW. Stressful life events and the return to heroin use. J Human Stress. 1981;7(2):3–8. doi: 10.1080/0097840X.1981.9936820. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Han VK, Henderson P, Verdoorn T, Towle AC. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neuroscience. 1986;19(2):465–93. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506(2):263–87. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- Lu L, Liu D, Ceng X, Ma L. Differential roles of corticotropin-releasing factor receptor subtypes 1 and 2 in opiate withdrawal and in relapse to opiate dependence. Eur J Neurosci. 2000;12(12):4398–404. [PubMed] [Google Scholar]
- Lane DA, Lessard AA, Chan J, Colago EE, Zhou Y, Schlussman SD, Kreek MJ, Pickel VM. Region-specific changes in the subcellular distribution of AMPA receptor GluR1 subunit in the rat ventral tegmental area after acute or chronic morphine administration. J Neurosci. 2008;28(39):9670–81. doi: 10.1523/JNEUROSCI.2151-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer P, Erdtmann-Vourliotis M, Riechert U, Ammon S, Höllt V. Mild stress sensitizes the brain’s response to morphine. Brain Res Mol Brain Res. 2002;104(2):143–7. doi: 10.1016/s0169-328x(02)00330-3. [DOI] [PubMed] [Google Scholar]
- McNally GP, Akil H. Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience. 2002;112(3):605–17. doi: 10.1016/s0306-4522(02)00105-7. [DOI] [PubMed] [Google Scholar]
- Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247(Suppl 5):V1–15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR. Ethanol Augments GABAergic Transmission in the Central Amygdala via CRF1 Receptors. Science. 2004;303(5663):1512–1514. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- Oertel WH, Riethmüller G, Mugnaini E, Schmechel DE, Weindl A, Gramsch C, Herz A. Opioid peptide-like immunoreactivity localized in GABAErgic neurons of rat neostriatum and central amygdaloid nucleus. Life Sci. 1983;33(Suppl 1):73–6. doi: 10.1016/0024-3205(83)90447-2. [DOI] [PubMed] [Google Scholar]
- Ottersen OP, Storm-Mathisen J. Glutamate- and GABA-containing neurons in the mouse and rat brain, as demonstrated with a new immunocytochemical technique. J Comp Neurol. 1984;229(3):374–92. doi: 10.1002/cne.902290308. [DOI] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System. Oxford University Press; New York: 1991. [Google Scholar]
- Phelix CF, Paull WK. Demonstration of distinct corticotropin-releasing factor-containing neuron populations in the bed nucleus of the stria terminalis. A light and electron microscopic immunocytochemical study in the rat. Histochemistry. 1990;94(4):345–364. doi: 10.1007/BF00266441. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992;28(6):949–65. doi: 10.1016/0361-9230(92)90218-m. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19(2):67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Sumal KK, Beckley SC, Miller RJ, Reis DJ. Immunocytochemical localization of enkephalin in the neostriatum of rat brain: a light and electron microscopic study. J Comp Neurol. 1980;189(4):721–40. doi: 10.1002/cne.901890408. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Beckley SC, Joh TH, Reis DJ. Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res. 1981;225(2):373–85. doi: 10.1016/0006-8993(81)90843-x. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Heras A. Ultrastructural localization of calbindin-D28k and GABA in the matrix compartment of the rat caudate-putamen nuclei. Neuroscience. 1996;71(1):167–78. doi: 10.1016/0306-4522(95)00441-6. [DOI] [PubMed] [Google Scholar]
- Poulin JF, Arbour D, Laforest S, Drolet G. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.06.021. (in press) [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Gehlert DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci. 2003;985:263–72. doi: 10.1111/j.1749-6632.2003.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–40. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan AK, Sribanditmongkol P. Effect of morphine-induced catalepsy, lethality, and analgesia by a benzodiazepine receptor agonist midazolam in the rat. Pharmacol Biochem Behav. 1994;48(2):357–61. doi: 10.1016/0091-3057(94)90538-x. [DOI] [PubMed] [Google Scholar]
- Reading PJ, Dunnett SB, Robbins TW. Dissociable roles of the ventral, medial and lateral striatum on the acquisition and performance of a complex visual stimulus-response habit. Behav. Brain Res. 1991;45:147–161. doi: 10.1016/s0166-4328(05)80080-4. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–12. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. Journal of Neuroscience. 2001;21(3):823–33. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Vu N, Xu H, Baumann MH, Lu YF. Endogenous corticotropin releasing factor regulates adrenergic and opioid receptors. Peptides. 2002;23(12):2177–80. doi: 10.1016/s0196-9781(02)00245-0. [DOI] [PubMed] [Google Scholar]
- Sajdyk T, Johnson P, Fitz S, Shekhar A. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J Psychopharmacol. 2008;22(6):633–41. doi: 10.1177/0269881107082902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52(4):701–6. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Fan LW, Tien LT, Ma T, Loh HH, Ho IK. The interaction of morphine and gamma-aminobutyric acid (GABA)ergic systems in anxiolytic behavior: using mu-opioid receptor knockout mice. Brain Res Bull. 2002;57(5):689–94. doi: 10.1016/s0361-9230(01)00785-7. [DOI] [PubMed] [Google Scholar]
- Schierloh A, Deussing J, Wurst W, Zieglgänsberger W, Rammes G. Corticotropin-releasing factor (CRF) receptor type 1-dependent modulation of synaptic plasticity. Neurosci Lett. 2007;416(1):82–6. doi: 10.1016/j.neulet.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119(3):334–41. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17(7):2605–14. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137(2):184–90. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33(1):13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Stobbs SH, Colago EE, Lee RS, Koob GF, Gallegos RA, Henriksen SJ. Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exp Neurol. 2006;202(1):139–51. doi: 10.1016/j.expneurol.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Stinus L, Cador M, Zorrilla EP, Koob GF. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005;30(1):90–8. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330(3):381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Moriwaki A, Wang JB, Uhl GR, Pickel VM. mu-Opioid receptors are localized to extrasynaptic plasma membranes of GABAergic neurons and their targets in the rat nucleus accumbens. J Neurosci. 1997;17(7):2585–94. doi: 10.1523/JNEUROSCI.17-07-02585.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejwani GA, Rattan AK, Sribanditmongkol P, Sheu MJ, Zuniga J, McDonald JS. Inhibition of morphine-induced tolerance and dependence by a benzodiazepine receptor agonist midazolam in the rat. Anesth Analg. 1993;76(5):1052–60. doi: 10.1213/00000539-199305000-00025. [DOI] [PubMed] [Google Scholar]
- Treweek J, Jaferi A, Colago E, Zhou P, Pickel VM. Electron microscopic localization of corticotropin releasing factor (CRF) and CRF receptor in rat and mouse central nucleus of the amygdala. Journal of Comparative Neurology. 2009;512(3):323–35. doi: 10.1002/cne.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Stoeckel M, Lasbennes F, Freund-Mercier MJ. c-Fos and peptide immunoreactivities in the central extended amygdala of morphine-dependent rats after naloxone-precipitated withdrawal. Eur J Neurosci. 2003;18(5):1295–305. doi: 10.1046/j.1460-9568.2003.02837.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213(1-2):29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of mu-opioid receptors in dendritic targets of dopaminergic terminals in the rat caudate-putamen nucleus. Neuroscience. 1997;81(3):757–71. doi: 10.1016/s0306-4522(97)00253-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl. 2006;185(1):19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- White NM. Some highlights of research on the effects of caudate nucleus lesions over the past 200 years. Behav Brain Res. 2009;199(1):3–23. doi: 10.1016/j.bbr.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Wittmann W, Loacker S, Kapeller I, Herzog H, Schwarzer C. Y1-receptors regulate the expression of Y2-receptors in distinct mouse forebrain areas. Neuroscience. 2005;136(1):241–50. doi: 10.1016/j.neuroscience.2005.07.047. [DOI] [PubMed] [Google Scholar]
- Yakovleva T, Bazov I, Cebers G, Marinova Z, Hara Y, Ahmed A, Vlaskovska M, Johansson B, Hochgeschwender U, Singh IN, Bruce-Keller AJ, Hurd YL, Kaneko T, Terenius L, Ekström TJ, Hauser KF, Pickel VM, Bakalkin G. Prodynorphin storage and processing in axon terminals and dendrites. FASEB J. 2006;20(12):2124–6. doi: 10.1096/fj.06-6174fje. [DOI] [PubMed] [Google Scholar]
- Yasui Y, Breder CD, Saper CB, Cechetto DFA. Autonomic responses and efferent pathways from the insular cortex in the rat. J Comp Neurol. 1991;303:355–374. doi: 10.1002/cne.903030303. [DOI] [PubMed] [Google Scholar]