Abstract
OBJECTIVES
To determine if body weight (BMI) is independently associated with cognitive function in postmenopausal women and the relationship between body fat distribution as estimated by waist-hip-ratio (WHR) and cognitive function.
DESIGN
Cross-sectional data analysis
SETTING
Baseline data from the Women's Health Initiative (WHI) hormone trials.
PARTICIPANTS
8745 postmenopausal women aged 65–79 years, free of clinical evidence of dementia and completed baseline evaluation in the Women's Health Initiative (WHI) hormone trials.
MEASUREMENTS
Participants completed a Modified Mini-Mental State Examination (3MSE), health and lifestyle questionnaires, and standardized measurements of height, weight, body circumferences and blood pressure. Statistical analysis of associations between 3MSE scores, BMI and WHR after controlling for known confounders.
RESULTS
With the exception of smoking and exercise, vascular disease risk factors, including hypertension, waist measurement, heart disease and diabetes, were significantly associated with 3MSE score and were included as co-variables in subsequent analyses. BMI was inversely related to 3MSE scores, for every 1 unit increase in BMI, 3MSE decrease 0.988 (p=.0001) after adjusting for age, education and vascular disease risk factors. BMI had the most pronounced association with poorer cognitive functioning scores among women with smaller waist measurements. Among women with the highest WHR, cognitive scores increased with BMI.
CONCLUSION
Increasing BMI is associated with poorer cognitive function in women with smaller WHR. Higher WHR, estimating central fat mass, is associated with higher cognitive function in this cross-sectional study. Further research is needed to clarify the mechanism for this association.
Keywords: obesity, cognition, dementia, waist-hip ratio, women
INTRODUCTION
Dementia is a major public health concern due to its rapidly increasing rate of occurrence in aging populations. Dementia currently affects 1–6% of the population over the age of 65, with the annual incidence rates doubling every 5 years between ages 75 and 89 years.1 It is estimated that by the year 2030, approximately 50 million, or 17–20% of the population, in the United States will be over the age of 65.2 Alzheimer disease (AD) is the most common form of dementia in the United States and currently affects approximately 4 million persons in the United States.3,4 Known risk factors for AD include family history and genetic predisposition and some studies have suggested associations between AD and vascular disorders such as atherosclerosis5, hypertension, coronary heart disease, and diabetes mellitus.6 Based on a recent meta-analysis of seven prospective studies, AD is 1.5 times more likely to develop in women than in men.12,13 Obesity and overweight increase risk of vascular disorders and may also increase risk of dementia. Furthermore, obesity is becoming a major public health issue.7–11 Obesity has been suggested as a risk factor for Alzheimer disease in older women14,15, and has been associated with poorer cognitive function in men15–17 but not in women.17–19 In fact, there is some evidence that obesity is protective against cognitive impairment in women possibly related to endogenous estrogens.20–22 This discrepancy between men and women in past studies may reflect sex-based differences in body weight and body fat distribution.
Early predictors of poor cognitive performance or cognitive decline may denote a population at increased risk for the development of dementia. Identifying populations at risk for dementia is becoming increasingly important as more information is available on possible preventative therapies to reduce or delay the incidence of dementia in the United States. In this study we explore the relationships between waist-to-hip ratios (WHR), BMI categories and cognition scores in a geographically diverse cohort of older, non-demented women. Since cognitive performance is predictive of subsequent dementia, the assessment of factors which may have a protective or detrimental effect on cognitive performance, in a cohort of normal older women is an important area for investigation.
METHODS
Our analyses utilized baseline data from Women's Health Initiative (WHI) hormone trials cohort. The WHI is a large, multifaceted study focused on common causes of morbidity and mortality among postmenopausal women aged 50–79 years. The WHI Estrogen plus Progestin (E+P) and Estrogen Alone (E-alone) randomized, placebo-controlled trials examined the risks and benefits of hormone therapies in women without and with hysterectomy, respectively. Detailed descriptions of eligibility criteria, recruitment methods, and study design have been published previously.23–25 Briefly, 40 clinical centers throughout the United States enrolled participants between 1993–1998. Eligible women were postmenopausal, had no medical condition associated with a predicted survival of less than 3 years, and were likely to be residing in the same geographic area for at least 3 years. Additional exclusion criteria included attention to safety issues and adherence and retention concerns. Women who were taking hormones, but who were interested in participating in the hormone trials, were required to stop their hormones for at least three months prior to joining the study and had to be willing to be randomized to either the hormone or placebo groups.
Participants completed health questionnaires assessing previous hormone use, history of cardiovascular disease, diabetes mellitus, stroke, lifestyle factors and demographic data. WHI hormone study participants who were 65 years and older also participated in baseline cognitive evaluation using the Modified Mini-Mental State Examination (3MSE), a 100-point validated test of global cognitive functioning.26 The 3MSE's 46 items contribute to a total score from 0–100, with a higher score reflecting better cognitive function. The test items measure temporal and spatial orientation, immediate and delayed recall, executive function, naming, verbal fluency, abstract reasoning, praxis, writing, and visuo-constructional abilities. The 3MSE has demonstrated good internal consistency and temporal reliability, sensitivity, and specificity for detecting cognitive impairment and dementia.27–29 Standardized 3MSE assessments were conducted by trained technicians in a quiet private area.
The standardized blood pressures were measured in the right arm after subjects had been seated quietly for at least 5 minutes. Hypertension was defined as a blood pressure equal to or greater than 140 mmHg systolic or 90 mmHg diastolic or if the participant was taking antihypertensive agents. Weight and height measurements were obtained using a calibrated beam balance scaled and a stadiometer anchored to the wall, with subjects wearing light street clothing and without shoes. Waist and hip measurements were obtained to the nearest 0.5 cm over non-binding undergarments at the level of the umbilicus and the fullest hip circumference. Body mass index (BMI) was calculated by dividing the weight in kilograms by the height in meters squared. Women were classified into BMI categories that correspond to standard World Health Organization-designated categories for underweight, desirable weight, overweight and obesity I, II, and III. In this classification, overweight is defined as BMI≥25.0 and obesity as BMI ≥ 30.0 according to World Health Organization criteria. All data were entered electronically into a centralized data base.
A majority of the study subjects were white (86.61%). Since ethnic minority women were not equally distributed among BMI categories, our analyses were restricted to white women. Means and distributions for age, 3MSE scores, education, waist circumference, and other variables of interest (disease and lifestyle) were stratified by BMI category. Means for variables of interest were also calculated after adjusting for age and education. Spearman Rank Correlation procedures were used to determine correlations between 3MSE score and BMI, waist circumference, age, education, systolic blood pressure, and smoking history. Odds ratios were calculated for atrial fibrillation, heart disease, hypertension, stroke, diabetes mellitus and previous hormone use. Multivariate logistic regression analyses were used to determine if increasing BMI category predicts 3MSE score when controlled for age, education hypertension, stroke, smoking, diabetes mellitus, and heart disease. Because the 3MSE scores were skewed to the right in this cognitively intact cohort, each score was subtracted from 100, which skewed the data to the left, permitting the use of a generalized linear model (GLM) with discrete values. The Poisson distribution with an over-dispersion parameter gamma was used because the transformed 3MSE scores had more variability than a pure Poisson process. Coefficients of the model are expressed in terms of the odds ratio and a 95% confidence interval. A log link function was used to model the transformed 3MSE scores. An ordinal logistic regression model was also used to validate the model independence of the results. This model (data not presented) lead to the same conclusions. Linear regression contrasts within the GLM were used for trend tests, such as age, education, waist-hip ratio, exercise and 3MSE. Tests for trends in the continuous covariates across BMI categories used a linear contrast within an analysis of variance. Tests for trends on binary variables, such as hypertension, heart disease, stroke, transient ischemic attack and diabetes were based on linear contrasts within logistic regression. SAS Version 9.1.3 was used for analyses.
RESULTS
A total of 8,745 postmenopausal women aged 65–79 completed 3MSE testing during baseline screening at 40 WHI clinical centers. Administration of the 3MSE was completed prior to randomization of women into the estrogen plus progestin or the estrogen alone studies. None of the women were taking hormone therapy at the time of screening. Baseline characteristics of the women are classified according to BMI category in Table 1. Because very few women (N= 70) had low body weight (BMI< 18.5), observations on women with very low relative weights were combined with observations on women in the normal weight category.
Table 1.
Means or Percents for Variable of interest by Obesity Categories (SE)
| Variables | Not Overweight BMI <24.9 | Overweight 25.0–29.9 | Obese I 30.0–34.9 | Obese II 35–39.9 | Obese III >40.0 |
|---|---|---|---|---|---|
| Number | 2263 | 2738 | 1659 | 645 | 256 |
|
| |||||
| 3MSE | 95.1 (0.1) | 95.0 (0.1) | 94.7 (0.1) + | 94.5 (0.2) + | 93.9 (0.3) + |
|
| |||||
| Age | 70.6 (0.1) | 70.3 (0.1) * | 69.9 (0.1) * | 69.4 (0.1) * | 68.7 (0.2) * |
|
| |||||
| Education | * | * | * | * | |
| <HS diploma | 4.9% | 5.5% | 7.4% | 9.0% | 6.6% |
| HS-Some college | 58.5% | 63.8% | 67.0% | 67.6% | 66.4% |
| >College grad | 36.6% | 30.7% | 25.6% | 23.4% | 27.0% |
|
| |||||
| Waist (cm) | 75.9 (0.2) | 87.1 (0.1) * | 97.6 (0.2) * | 106.7 (0.4) * | 113.4 (0.8) * |
|
| |||||
| Waist/Hip ratio | 0.79 (0.01) | 0.83 (0.01) * | 0.86 (0.01) * | 0.86 (0.01) * | 0.85 (0.01) * |
|
| |||||
| Exercise(mets) | 14.8 (0.3) | 11.9 (0.2) * | 8.9 (0.3) * | 7.2 (0.4) * | 6.4 (0.6) * |
|
| |||||
| Smoking (pack-years) | 24.5 (0.7) | 25.3 (0.7) | 27.1 (1.0) | 27.7 (1.7) | 28.6 (2.6) |
|
| |||||
| Hypertension | 35.7% | 44.8% * | 54.0% * | 60.8% * | 63.7% * |
|
| |||||
| Heart disease | 17.8% | 17.6% | 21.4% * | 24.3% * | 20.7% |
|
| |||||
| Diabetes | 2.6% | 5.3% * | 8.4% * | 12.4% * | 18.0% * |
P-value <0.05 was marked by * and P-value <0.1 was marked by +. Normal is the reference group.
Heart disease includes heart or circulation problems, cardiac arrest, heart failure, cardiac catheterization, heart bypass, atrial fibrillation
Table 1 presents the mean 3MSE scores, as well as other selected variables, for which previous associations with cognitive screening have been identified. As is apparent in the table, a large proportion of women (70.1%) were classified as overweight or obese. Overall, 3MSE scores decreased slightly with increasing BMI categories. Important co-variables were also associated with BMI. Higher educational attainment was seen among women in the normal and low BMI categories compared to women who were overweight or obese. Waist circumference and waist-to-hip circumference increased with BMI, as did hypertension and diabetes, while physical activity levels were inversely related to BMI. Current smoking was more common among women with low body weight. Overall, only a minority of women were current smokers (15.5%), although many had been smokers previously (47.2%).
Multiple logistic regression techniques were used to determine the independent relationships between each co-variable and 3MSE scores, after controlling for other confounders. The results of these analyses are presented in Table 2. Older women and those women who exercised less tended to have lower 3MSE scores, while women with larger waist circumferences and higher levels of education tended to have higher 3MSE scores. Women who reported having had a stroke, heart disease or diabetes or who were hypertensive had lower 3MSE scores. After adjusting for other variables, BMI was inversely associated with 3MSE score.
Table 2.
Multivariate Analyses of BMI, Activity and Waist Circumference as a predictor of Modified Mini-Mental State Examination (3MSE) Score
| Total 3MSE Score (Multiple Regression) | ||
|---|---|---|
| Odds Ratio(95%CI) | P-Value | |
| Body Mass Index (kg/m2) | 0.989 (0.985,0.995) | 0.0001 |
| Activity Level (METS/wk) | 0.999 (0.997,0.999) | 0.048 |
| Waist circumference (cm) | 1.002 (1.001,1.005) | 0.0140 |
| Age | 0.978 (0.973, 0.983) | 0.0001 |
| Education | 1.142 (1.132,1.153) | 0.0001 |
| Smoking | 0.972 (0.938, 1.007) | 0.1196 |
Control variables: Age, Education, Stroke, Hypertension, Diabetes Mellitus, Heart disease
Table 3 presents mean 3MSE scores by BMI category after adjusting for differences in 3MSE score due to age and educational attainment, both of which have important effects on 3MSE scores. Overall, a test for trend was significant (p< 0.001) indicating decreasing 3MSE scores are associated with increasing BMI, for every 1.0 unit increase in BMI, 3MSE decrease 0.988 (p<0.001) after adjusting for age, education and vascular disease risk factors.
Table 3.
Adjusted Means for Modified Mini-Mental State Examination (3MSE) Scores by Body Mass Index Category (SD)
| Not Overweight | Overweight | Obese I | Obese II | Obese III | |
|---|---|---|---|---|---|
| <=24.9 | 25.0–29.9 | 30.0–34.9 | 35–39.9 | >40.0 | |
| Age Adj. | 95.2 (0.09) | 94.9 (0.08) | 94.7 (0.10) | 94.5(0.17) | 93.8 (0.26) |
| Educ Adj. | 95.1 (0.09) | 95.0 (0.08) | 95.0 (0.10) | 95.0 (0.17) | 94.3 (0.26) |
| Age+Educ.Adj. | 95.2 (0.09) | 95.1 (0.08) | 95.0 (0.10) | 95.0 (0.17) | 94.1 (0.26) |
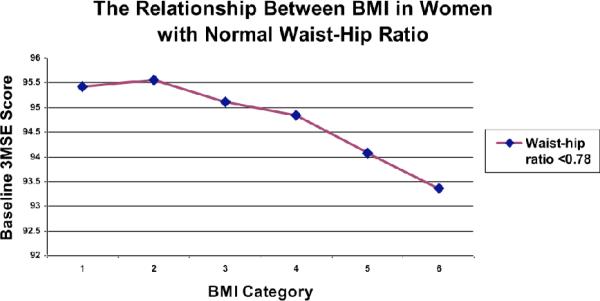
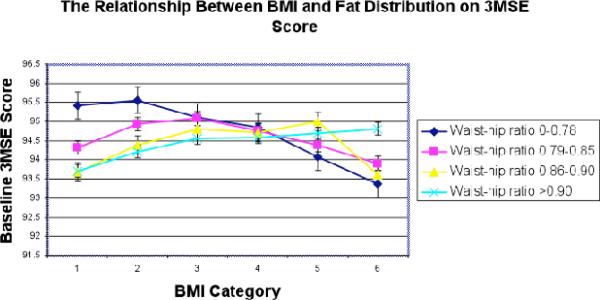
When we explored the relationships among 3MSE scores, waist-to-hip ratios and BMI categories, we found that they were complex (Figure 1). The 3MSE scores for women with low waist-to-hip ratios (Figure 2) decreased as BMI category increased (p<0.001). However, this association reverses for women with highest waist-to-hip ratios (Figure 2)._In the highest waist-to-hip quartile, 3MSE scores increased with increasing BMI categories (p<0.0102).
Figure 1.
The relationship between BMI and baseline 3MSE score.
Increasing BMI was inversely related to cognitive function scores, after adjusting for age, education, hypertension, heart disease, stroke, diabetes mellitus.
Figure 2.
The relationship between body fat distribution and BMI on cognitive scores
BMI category had the most pronounced association with poorer cognitive function scores among women with smaller waist measurements, suggesting that BMI is directly associated with cognitive function and not secondary to metabolic alterations associated with increased waist-tohip ratios. Women with the highest waist-hip ratios had higher cognitive scores with increasing BMI category suggesting that abdominal obesity may be associated with better cognitive functioning.
DISCUSSION
In our cross sectional analyses of associations of BMI categories and cognitive function in older women, we found a complex relationship that was modified by waist-to-hip ratio. Among women with a low waist-to-hip ratio, there was an inverse relationship between BMI category and 3MSE score after adjusting for demographic data and several weight-related comorbidities, that was not observed among women with higher waist-to-hip ratios.
Previous studies of associations between obesity and cognitive function have yielded mixed findings. Obesity, especially mid-life obesity, has been associated with increased risk of cognitive impairment and dementia in several studies.30–32 In gender-specific studies, obesity has been suggested as both protective and a risk factor for Alzheimer disease in women.14–22 It has been unclear to date if either of the suggested effects of obesity on cognition has been a direct or indirect effect either through increased endogenous estrogen levels improving cognition or the detrimental effects of vascular disease and inflammation associated with obesity causing increased risk of dementia. The present study suggests that body fat distribution is an important factor in obesity and its effects on cognitive function in older women.
Overall, obesity appears to be directly associated with cognitive function in postmenopausal older women. These data suggest that the detrimental effects of obesity on cognition may be mediated by factors in addition to vascular disease, hypertension, diabetes or inflammation, all of which have been associated with abdominal obesity. In our study, central adiposity was associated with higher cognitive screening scores on 3MSE at higher BMI categories and lower BMI categories were associated with lower 3MSE scores, even after adjusting for confounders. The associations cannot be explained by racial factors affecting the relationship, since the analyses was limited to white women. Several studies of exogenous estrogen therapy on dementia risk have not shown a protective effect.33–35 One possibility is that the production of endogenous estrogen by abdominal adipocytes may play a protective role for cognition.36–38
Limitations
This is a cross-sectional analysis of a longitudinal cohort of women and thus cause and effect cannot be concluded. The other major limitation of the cohort includes the inclusion of only white women were included in these analyses, and the fact that our dementia outcome likely combined participants with dementia of differing etiology. The cognitive data in this analysis is limited to the 3MSE, a measure of global cognitive functioning, which screens for but does not extensively assess domain specific cognitive functioning. Also, participants were healthy women who had relatively high levels of education and who were without dementia or cognitive impairment at baseline, thus participant-3MSE scores were clustered at a high level of cognitive functioning. None the less, we were able to detect an impact of BMI category on cognitive functioning.
CONCLUSION
BMI category and waist-hip ratio appear to be important factors in complex associations between obesity and cognitive function in older women. These findings suggest that central adiposity, estimated by higher waist-to-hip ratios, is somehow associated with higher cognitive function scores in older, postmenopausal women. These findings do not negate other research demonstrating that obesity is a major risk factor for cardiovascular disease and other chronic diseases, including those such as hypertension and diabetes which have been found to increase dementia risk.39 Qualitative as well as quantitative nutritional analyses to further differentiate relationships between diet, physical activity, BMI and cognitive functioning in older women are needed as well.
ACKNOWLEDGMENTS
Jennifer G Robinson, MD, MPH(JGR): Grants to Institution: Abbott, Aegerion, Bristol-Myers Squibb, Daiichi-Sankyo, Glaxo-Smith Kline, Hoffman La Roche, Merck, Merck Shering-Plough.
Funding Sources: This research was conducted while Diana Kerwin, MD was a T. Franklin Williams Scholar supported by the Foundation for Health in Aging, the American Geriatrics Society, Association of Subspecialty Professors and Atlantic Philanthropies, and a Dr. Judith Stitt Faculty Scholar supported by Wisconsin Women's Health Foundation.
The Women's Health Initiative is funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health, U.S. Department of Health and Human Services. Wyeth Pharmaceuticals provided the study drug and the placebo to the WHI trial.
Footnotes
SHORT LIST OF WHI INVESTIGATORS Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Barbara Alving, Jacques Rossouw, Linda Pottern
WHI Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Ruth E. Patterson, Anne McTiernan; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker, Pentti Rautaharju; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings; (University of Minnesota, Minneapolis, MN) John Himes; (University of Washington, Seattle, WA) Bruce Psaty
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Jennifer Hays; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Harbor-UCLA Research and Education Institute, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Evelyn Whitlock; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush-Presbyterian St. Luke's Medical Center, Chicago, IL) Henry Black; (Stanford Center for Research in Disease Prevention, Stanford University, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora Beth Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, Orange, CA) Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Howard Judd; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O'Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix
Conflict of Interest Disclosure: Diana R. Kerwin, MD(DRK): Honoraria, Speaker Forum, Consultant to Pfizer Pharmaceuticals, Forest Laboratories, Novartis Pharmaceuticals.
Sponsor's Role: Sponsor's had no role in the design, methods, recruitment, data collection analysis or preparation of this paper.
REFERENCES
- 1.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Leon M, editor. An Atlas of Alzheimer's Disease. Parthenon Press; New York: 1999. [Google Scholar]
- 3.U.S. General Accounting Office . Report no. AZDC06782. Health, Education and Human Services Division; Washington: 1998. Alzheimer's Disease: Estimates of Prevalence in the United States. Report to the Secretary of Health and Human Services; pp. 1–45. [Google Scholar]
- 4.Ernst RL, Hay JW. The US economic and social costs of Alzheimer disease revisited. Am J Public Health. 1994;84:1261–1264. doi: 10.2105/ajph.84.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 6.Skoog I, Kalaria RN, Breteler MM. Vascular factors and Alzheimer's disease. Alzheimer Dis Assoc Disord. 1999;12:S106–S114. doi: 10.1097/00002093-199912003-00016. [DOI] [PubMed] [Google Scholar]
- 7.Flegal KM, Carroll MD, Kuczmarski RJ, et al. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 8.Visscher TLS, Seidell JC, Menotti A. Underweight and overweight in relation to mortality among men aged 40–59 and 50–69 years: The Seven Countries Study. Am J Epidemiol. 2000;151:660–666. doi: 10.1093/oxfordjournals.aje.a010260. [DOI] [PubMed] [Google Scholar]
- 9.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 10.Stunkard AJ, Wadden TA, editors. Obesity: Theory and therapy. Second Edition Raven Press; New York: 1993. [Google Scholar]
- 11.National Institutes of Health . Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; Bethesda, Maryland: 1998. [Google Scholar]
- 12.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer disease in women. Am J Epidemiol. 1994;140:256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 13.Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: A meta-analysis. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- 14.Gustafson D, Rothenberg E, Blennow K, et al. An 18-Year follow-up of overweight and risk of Alzheimer disease. Arch Int Med. 2003;163:1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 15.Sorenson TI, Sonne-Holm S, Christensen U, et al. Reduced intellectual performance in extreme overweight. Hum Biol. 1982;54:765–775. [PubMed] [Google Scholar]
- 16.Kilander L, Myman H, Boberg M, et al. Cognitive function, vascular risk factors, and education. A cross sectional study based on a cohort of 70-year old men. J Intern Med. 1997;242:313–321. doi: 10.1046/j.1365-2796.1997.00196.x. [DOI] [PubMed] [Google Scholar]
- 17.Elias MF, Elias PK, Sullivan LM, et al. Lower cognitive function in the presence of obesity and hypertension: The Framingham Heart Study. Int J Obesity. 2006;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 18.Patel BN, Pang D, Stern Y, et al. Obesity enhances verbal memory in postmenopausal women with Down Syndrome. Neurobiol Aging. 2004;25:159–166. doi: 10.1016/s0197-4580(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 19.Berlinger WG, Potter JF. Low body mass index in demented outpatients. J Am Geriatr Soc. 1991;39:973–978. doi: 10.1111/j.1532-5415.1991.tb04043.x. [DOI] [PubMed] [Google Scholar]
- 20.Grundman M, Corey-Bloom J, Jernigan T, et al. Low body weight in Alzheimer's disease is associated with mesial temporal cortex atrophy. Neurol. 1996;46:1585–1592. doi: 10.1212/wnl.46.6.1585. [DOI] [PubMed] [Google Scholar]
- 21.Nourashemi F, Deshchamps V, Larrieu S, et al. Body mass index and incidence of dementia: The PAQUID Study. Neurol. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 22.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13(9 S):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 23.The Women's Health Initiative Study Group Design of the Women's Health Initiative Clinical Trial and Observational Study. Controlled Clinical Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 24.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative Recruitment Methods and Results. Ann Epidemiol. 2003;13(9 S):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 25.Teng EL, Chui HC. The 3MSE (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 26.Teng EL, Chui H, Gong A. Psychogeriatrics: Biomedical and Social Advances. Excerpta Medica; Tokyo, Japan: 1990. Comparisons between the Mini-Mental State Exam (MMSE) and its modified versión – the 3MS test; pp. 189–192. [Google Scholar]
- 27.McDowell I, Kristjansson B, Hill GB, et al. Community screening for dementia: The Mini-Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50:377–383. doi: 10.1016/s0895-4356(97)00060-7. [DOI] [PubMed] [Google Scholar]
- 28.Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:13–22. doi: 10.1159/000067109. [DOI] [PubMed] [Google Scholar]
- 29.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 30.Whitmer RA, Gunderson EP, Barrett-Connor E, et al. Obesity in middle age and future risk of dementia: A 27 years longitudinal population based study. BMJ. 2005;1330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molsa PK, Marttila RJ, Rinne UK. Epidemiology of dementia in a Finnish population. ACTA Neurol Scand. 1982;65:541–552. doi: 10.1111/j.1600-0404.1982.tb03109.x. [DOI] [PubMed] [Google Scholar]
- 32.Birge SJ. The role of estrogen in the treatment and prevention of dementia:introduction. Am J Med. 1997;103:1S–2S. doi: 10.1016/s0002-9343(97)00263-5. [DOI] [PubMed] [Google Scholar]
- 33.Judd HL, Judd GE, Lucas WE, et al. Endocrine function of the postmenopausal ovary: Concentrations of androgens in ovarian and peripheral vein blood. J Clin Endocrinol Metab. 1974;39:1020–1024. doi: 10.1210/jcem-39-6-1020. [DOI] [PubMed] [Google Scholar]
- 34.Women's Health Initiative Writing Group Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 35.Shumaker SA, Leagault C, Rapp SR, et al. Estrogen plus Progestin and the Incidence of Dementia and Mild Cognitive Impairment in Postmenopausal Women. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 36.Szymczak JMA, Thijssen JH, Blankenstein MA, et al. Concentration of sex steroids in adipose tissue after menopause. Steroids. 1998;63:319–321. doi: 10.1016/s0039-128x(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 37.Deslypere JP, Vermuelen A. Fat tissue: A steroid reservoir and site of steroid metabolism. J Clin Endocrinol Metab. 1985;61:564–570. doi: 10.1210/jcem-61-3-564. [DOI] [PubMed] [Google Scholar]
- 38.Kirschner MA, Samojilik E. Sex hormone metabolism in upper and lower body obesity. Int J Obes. 1991;Suppl 2:101–108. [PubMed] [Google Scholar]
- 39.Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late-life. Neurol. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]