Abstract
A dual navigator–gated, flow-sensitive alternating inversion recovery (FAIR) true fast imaging with steady precession (True-FISP) sequence has been developed for accurate quantification of renal perfusion. FAIR methods typically overestimate renal perfusion when respiratory motion causes the inversion slice to move away from the imaging slice, which then incorporates unlabeled spins from static tissue. In order to overcome this issue, the dual navigator scheme was introduced to track inversion and imaging slices, and thus to ensure the same position for both slices. Accuracy was further improved by a well-defined bolus length, which was achieved by a modification version of Q2TIPS [quantitative imaging of perfusion using a single subtraction (QUIPSS II) with interleaved thin-slice TI1 periodic saturation]: a series of saturation pulses was applied to both sides of the imaging slice at a certain time after the inversion. The dual navigator–gated technique was tested in eight volunteers. The measured renal cortex perfusion rates were between 191–378 ml/100g/min in the renal cortex with a mean of 376 ml/100g/min. The proposed technique may prove most beneficial for non-contrast based renal perfusion quantification in young children and patients who may have difficulty holding their breath for prolonged periods, or are sedated/anesthetized.
Keywords: arterial spin labeling (ASL), navigator gating, perfusion, renal blood flow (RBF), True-FISP
INTRODUCTION
The kidneys regulate acid–base balance and moderate blood pressure and fluid volume by filtering and excreting metabolic waste products. Many diseases of the kidney, such as renal artery stenosis, renal transplant nephropathy, chronic ischemic nephropathy, and drug nephropathy, are accompanied by changes in renal perfusion (1). Monitoring renal perfusion permits assessment of disease progression, prognosis, as well as patient management and therapy. In clinical routine, MR perfusion imaging is typically performed by using contrast agents, which may lead to the development of a debilitating condition known as nephrogenic systemic fibrosis (NSF) for patients with severely impaired renal function, endstage renal diseases, and those in acute renal failure (2). An alternative noninvasive technique is arterial spin labeling (ASL), which uses water spins in the blood as an endogenous tracer. ASL has been extensively used to assess tissue perfusion in the brain. In recent years, it has been increasingly used for other organs such as the kidneys (3–6).
Various imaging sequences, including half-Fourier single shot turbo spin echo (HASTE) (4), ultra fast low angle rapid acquisition with relaxation enhancement (UFLARE) (6), and true fast imaging with steady precession (True-FISP) (5,7–9), have been explored for assessing renal perfusion. With True-FISP, also termed steady-state free precession (SSFP), the SNR can be improved greatly, as both, transverse and longitudinal magnetization components achieve a steady state during image acquisition. This sequence, along with the flow-sensitive alternating inversion recovery (FAIR) labeling scheme (10), has proven suitable to quantify renal perfusion.
One of the challenges with current renal ASL techniques is that there are spatial variations in the transit delays of the labeled blood spins, which could affect the accuracy of quantifying perfusion. Quantitative imaging of perfusion using a single subtraction II (QUIPSS II) (11) was introduced to minimize this systematic error. In this scheme, a 15-lobe sinc saturation pulse is applied in the labeled region at time TI1 after the inversion pulse at the distal end of the labeled region. Later, QUIPSS II with thin-slice TI1 periodic saturation (Q2TIPS) (12) was proposed to improve the saturation slice profile by replacing the saturation pulse with a periodic train of thin-slice saturation pulses. Q2TIPS was then extended to two sets of saturation pulses applied to both the proximal and distal sides of the imaging slice to saturate the blood from both directions in the brain (13).
In contrast to the brain, the kidneys are affected by respiratory motion, which can severely reduce the accuracy of quantification for two reasons: first, during acquisition of the labeled image in FAIR, the imaging slice could move to the edge or even outside of the labeling (inversion) region. Some spins in the imaging slice cannot be fully inverted if they move near the edge of the labeled region because of the imperfect slice profile, and spins that move out of the labeling region cannot be inverted at all. Because the perfusion signal is extremely weak (only a small percentage of the equilibrium magnetization), even a small portion of unlabeled (uninverted) spins can cause large errors in quantifying perfusion. Second, because the renal perfusion rate is calculated from the difference of the control and labeling images in ASL, the mismatch between these two images caused by motion can generate considerable errors in estimating perfusion. Although in-plane motion could be rectified by image realigning in post-processing, it is impossible to correct out-of-plane motion. To eliminate these errors, multiple long breath-hold scans are usually required. Many individuals, including young children, may not be able to hold their breaths for prolonged periods. It is therefore desirable to avoid breath-hold scans by using a respiratory gating technique.
In this study, a dual navigator-gated technique that allows free breathing was implemented with a FAIR Q2TIPS True-FISP sequence to accurately quantify renal perfusion. The sequence incorporates an extended version of Q2TIPS to saturate blood flow and to reduce variations in the transit delay of labeled blood in the kidneys, thereby ensuring a bolus of well-defined length. More importantly, both the labeling and imaging slice are gated and shifted to the same reference position in order to eliminate the “non-labeling” artifact in perfusion imaging. The reduction of mismatch between the label and control images provides a more accurate measurement of renal perfusion rates.
MATERIALS AND METHODS
Pulse Sequence
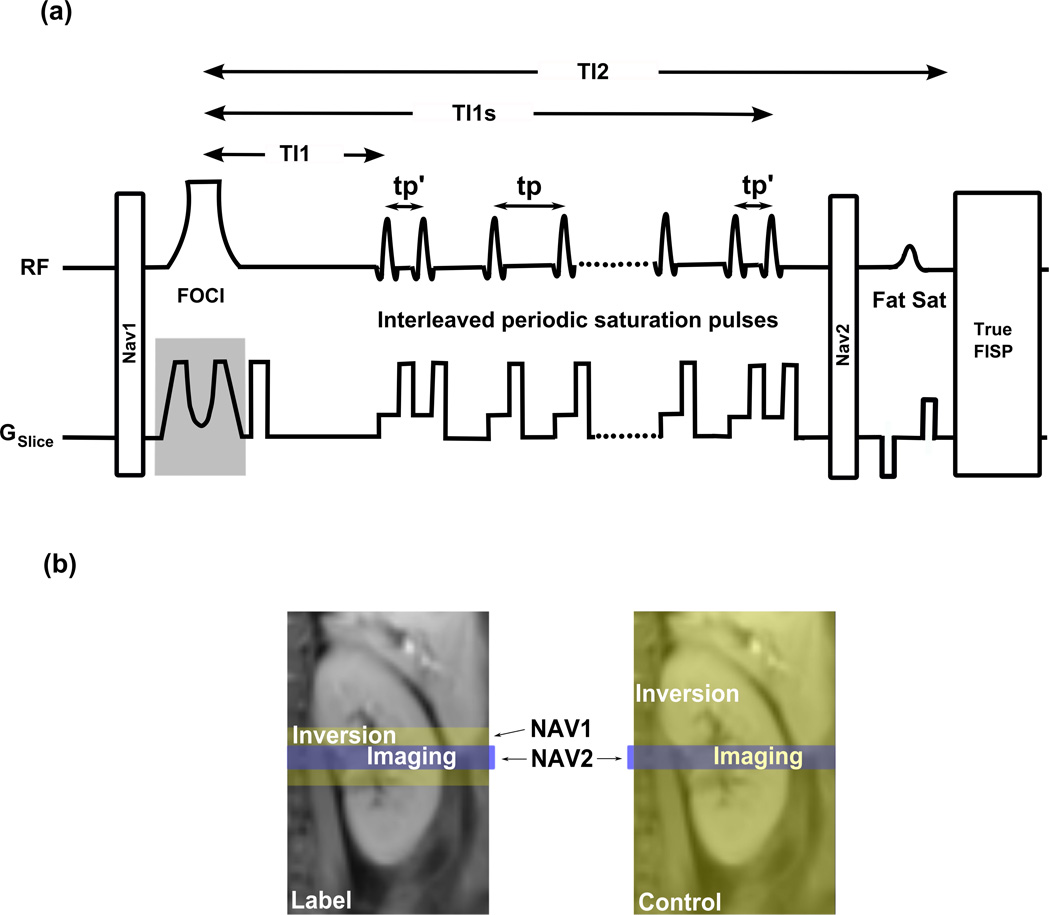
The dual navigator technique was combined with a FAIR perfusion preparation and a True-FISP image acquisition module (Figure 1). Following the first navigator, NAV1, a frequency offset corrected inversion (FOCI) pulse (14) was used to achieve a high inversion efficiency and slice profile. After a perfusion delay time of TI1, a series of interleaved periodic saturation pulses was applied from TI1 to TI1s. The second navigator, NAV2, was applied just before fat saturation preparation and True-FISP. The major sequence blocks are described as follows.
FIG. 1.
(a) Dual navigator-gated FAIR True-FISP sequence. Following the first navigator, NAV1, a FOCI inversion pulse is applied. After a perfusion delay time of TI1, a series of interleaved periodic saturation pulses is applied from TI1 to TI1s on both sides of the imaging slice. The second navigator, NAV2, is applied just before the fat saturation preparation and True-FISP. NAV1 gates the inversion (tagging) pulse while NAV2 gates the imaging sequence (True-FISP). The same reference position is used for both NAV1 and NAV2. The slice selective gradient for the FOCI pulse and NAV1 (in gray) are not applied during the control scans. tp′ is the separation between the first (and last) pair of saturation pulses, and tp is the interval between two successive saturation pulses. (b) Geometrical locations of imaging and inversion slices and their corresponding navigators for label and control scans. NAV2 is applied in both, label and control scans, to enforce label and control images to be acquired at the same location. NAV1 is only required for label scans to ensure inversion slices to be applied at the same position as the imaging slices. Because the inversion in control scans is non slice-selective, a navigator is not necessary. In our implementation, NAV1 was, however, applied with a very large acceptance window (±20 mm) to balance the MT effect as stated in the text.
Dual navigators
As discussed previously, in ASL scans, motion could cause the inclusion of unlabeled spins in the label image and a mismatch between control and label images. To eliminate these errors without breath-holding, the dual navigator technique was employed as shown in Figure 1. NAV1 monitored the inversion (labeling) slice while NAV2 gated for the imaging slice. It has been shown that gating and following is superior to gating only in achieving high image quality with the use of substantial wider windows and statistically significantly shorter acquisition times (15). Therefore, a gating and following (shifting) respiratory control (16) was used for both NAV1 and NAV2: The inversion and the imaging slices were prospectively shifted on-the-fly according to the diaphragm position detected by the navigators, NAV1 and NAV2, respectively. A tracking factor (ratio of diaphragm motion to kidney motion) of 0.67 was chosen based on previously reported investigations (17). In label scans, if a desired position was detected within the acceptance window by NAV1, the inversion was accepted and the inversion slice was shifted to a reference position that was obtained by the first successful NAV1. If the labeling passed with NAV1, NAV2 checked the imaging slice position with a new acceptance window that is η times that of NAV1 (η = 1.5 in this study). If the position fell in the acceptance window, the imaging slice was shifted to the reference position set by NAV1. In other words, label images were accepted only when both NAV1 and NAV2 succeeded within the acceptance windows. NAV1 and NAV2 were expected to ensure that the imaging and inverting slices were located at approximately the same position and the “non-labeling artifact” in the perfusion images could be significantly reduced. In control scans, since the inversion was nonselective, the imaging slice could not move out of the inversion slab (Fig. 1b). NAV1 was not required. Control images were accepted if NAV2 succeeded. Because no inversion was rejected by NAV1 in the control scans, the total number of control scans was approximately 50% less than that of the label scans when acquiring a full paired ASL data set. In our implementation, NAV1 was still applied, but with a large acceptance window (±20mm) so that all global inversions during control scans were accepted. Magnetization transfer effects caused by the navigators would therefore cancel out, since NAV1 and NAV2 were applied in both label and control scans.
A crossed-pair navigator was employed, comprising a 90° and a 180° slice-selective pulse. The intersecting region of the slices excited by the 90° and 180° pulse composed a navigator beam or column in the form of a spin echo, which was used to trace the motion of the diaphragm and liver (16). Since the two slices excited by the two radiofrequency pulses in the navigator appear as two dark bands in the images, they were carefully adjusted to avoid the dark bands from affecting quantification in the kidneys.
Interleaved Periodic Saturation Pulses
The extended Q2TIPS technique was applied in the FAIR labeling scheme, because the saturation pulses were required on both sides of the imaging slice (Figure 1). The periodic thin-slice saturation pulses were applied alternatively (time increment tp) on both sides of the imaging slice starting at the time point TI1 and ending at TI1s (TI1 stop time) to suppress arterial and venous inflow (12–13). The inter-pulse delay, tp′, of the first and last pair of saturation pulses was, different from tp, and minimized for the applied RF pulse length and gradient duration in order to provide almost identical TI1 times for both saturation slices. tp′ was 9 ms in this implementation. This ensured that all spins at both sides of the imaging slice were saturated from TI1 to TI1s, and a well-defined bolus of duration TI1 was established in this manner.
As the slice thickness of the periodic saturation pulses, dsat, is small, the interval between successive periodic saturation pulses on one side, 2tp, has to be appropriately chosen to saturate all spins flowing into the imaging slice at any possible velocity. 2tp has to be less than dsat/vmax, where vmax is the normal component of maximum renal blood flow velocity. In this study, the slice thickness of the periodic saturation pulses was 20 mm, and the interval between successive saturation pulses on one side was 20 ms. This ensured that the blood at velocities below vmax = dsat/(2tp) = 100 cm/s was saturated, which is well above the renal blood velocity of approximately 49 cm/s (18).
True-FISP Imaging Pulse Sequence
To reduce the oscillatory transition effects prone to True-FISP, which can severely degrade image quality, 14 Kaiser Bessel window ramp flip angle preparation RF pulses were employed (19). Also, in regions of high local magnetic field variations, images often suffer from characteristic bands of signal loss (banding artifacts). The artifacts are pronounced at high magnetic field strength B0. If these artifacts overlay the kidneys, renal perfusion cannot be appropriately quantified. Therefore, True-FISP pre-scans were iteratively performed before the ASL measurement and the resonant frequency was adjusted by offsets of 0–50 Hz to shift banding artifacts outside of the kidneys.
ΔM measured by EPI in the FAIR technique is proportional to the difference in the longitudinal magnetization (ΔMz) between the label and control images caused by labeled blood entering the imaging slice. However, because both transverse and longitudinal magnetization components achieve a steady state in the image acquisition, the dependence of ΔM on ΔMz in the FAIR True-FISP measurement is complicated by its dependence on the time course of magnetization (8). To minimize this effect, centric reordered phase encoding was applied to achieve reliable quantitative renal perfusion rate maps. Although the RF preparation lasts for about 50 ms, the perfusion signal persists due to the adaption of centric reordering encoding based on the experiments and simulations performed by Martirosian et al (8).
Imaging
All MR scans were performed with a 3T whole-body clinical MR scanner (Siemens Medical Solutions, Erlangen, Germany). A standard spine/torso and a body coil array were used for signal detection. Eight healthy volunteers (4 female, 4 male, 37 ± 14 years old) were recruited, and written informed consent was obtained from each participant before the exam.
Two sets of total 40 images (20 control/labeling pairs) were acquired using a single shot True-FISP sequence with FAIR labeling scheme. A 10.24 ms adiabatic FOCI “C-shape” pulse with a μ of 4.5, β of 1078, and bandwidth of 1544 Hz was used to achieve a sharp inversion slice profile (14). The imaging/inversion slice thickness was 8/22 mm. The gap between imaging and saturation slice was 10 mm to avoid interference due to respiratory motion. The FOCI pulse was tested in a 1.8% agarose gel phantom to verify the inversion slice profile. We found that the static tissue signal was fully suppressed in ΔM images. An axial/coronal slice through the kidneys was acquired with a FOV of 32–38 cm. Other parameters are as follows: TE 1.77 ms, acquisition bandwidth 650 Hz/pixel, flip angle 40°, matrix 128 × 123, TR 5 s, TI2 1.3 s, TI1 0.7 s, and TI1S 1.2 s. The TI1 time was optimized for the used labeling and imaging parameters (20). Because perfusion rates were calculated from the difference of the control and label images, a very stable longitudinal steady state had to be reached before the actual ASL measurement. Therefore, two dummy scans were performed to achieve the steady state prior to ASL data acquisition. Fat saturation preparation was applied just before the True-FISP imaging module to suppress the fat signal. The M0 image was acquired by turning off the inversion pulse.
The navigator beam was positioned at the dome of the right hemidiaphragm, and the end-expiratory position was chosen with an acceptance window of ±4 mm for NAV1 and ±6 mm for NAV2, resulting in acceptance rates of 40%–60%. As the result, the scan time for the dual navigator-gated scan was 6–9 min whereas the scan time for the ungated scan was 3.6 min.
Data Processing and Analysis
To compare the dual navigator scheme with single navigator (NAV1 only and NAV2 only) and ungated experiments, 1D navigator and image data were saved as raw signal intensities for the first group of three volunteers. The navigator signals and images were obtained by offline reconstruction from the raw data. The diaphragm position could be extracted from the navigator signal by exploiting the contrast change between liver and lung, in analogy to the algorithm used by the online reconstruction. If the diaphragm position is within the acceptance window as defined above, the position is accepted. Four different sets of images were created: 1) dual navigator image set: diaphragm position for NAV1 and NAV2 must lie within the acceptance window; 2) NAV1 only: NAV1 must lie within the acceptance window and there is an arbitrary position for NAV2; 3) NAV2 only: arbitrary position for NAV1 and NAV2 must lie within the acceptance window; 4) ungated acquisition: arbitrary positions for NAV1 and NAV2. For comparison of all four schemes, perfusion maps were calculated from each image set with the same number of pairs of label and control images.
For the second group of five volunteers, the subjects were scanned with ungated and dual navigator-gated Q2TIPS FAIR True-FISP sequences at the same slice position of the kidneys. Images were reconstructed online with the Image Calculation Environment (ICE) on our scanner. The images were sent to local computers for offline perfusion calculation.
A subtraction image was obtained by subtracting pairs of control and labeling images. Perfusion rates were computed on a pixel-by-pixel basis using the subtraction images, neglecting transit effects and exchange times of water molecules in the blood and tissue. If we assume that T1 values of the blood and tissue are similar in the kidney (8), the renal perfusion rate can be obtained by
| (1) |
where α is the labeling efficiency defined as the fraction of inversion of the arterial magnetization at the time of labeling (α = 0.95); ΔM is the magnetization difference at TI2; M0 represents the tissue equilibrium magnetization; λ is the tissue/blood partition coefficient (λ = 0.8 ml/g (21)). T1 of 1.15 s is assumed for the kidney cortex (22). The perfusion maps were calculated by using a modified version of the ASL data processing toolbox (23) developed in Matlab (MathWorks, Natick, MA). For each participant, the cortex regions of interest (ROIs) for both kidneys were drawn on the perfusion maps with reference to the labeled images.
RESULTS
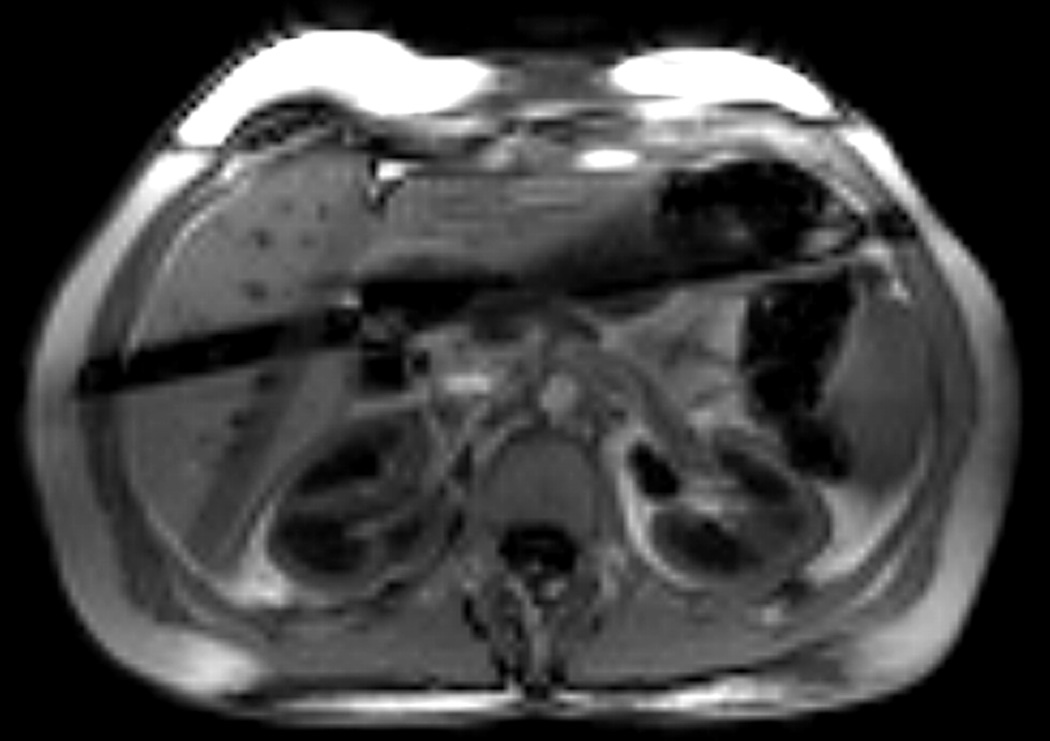
The True-FISP sequence allowed images of high quality and SNR to be acquired and reliable perfusion values to be assessed. Because the perfusion signal is very weak, there was no visible difference between the label and control images on the kidneys. Figure 2 shows a typical control image obtained by the dual navigator-gated Q2TIPS True-FISP sequence. The two dark bands on the liver were caused by the navigators, but they did not affect the estimation of renal perfusion as they were away from the kidneys.
FIG. 2.
A typical control image obtained with the dual navigator-gated Q2TIPS True-FISP sequence. Except for the two crossed dark bands caused by the navigator, good image quality and high SNR were achieved by the True-FISP imaging sequence.
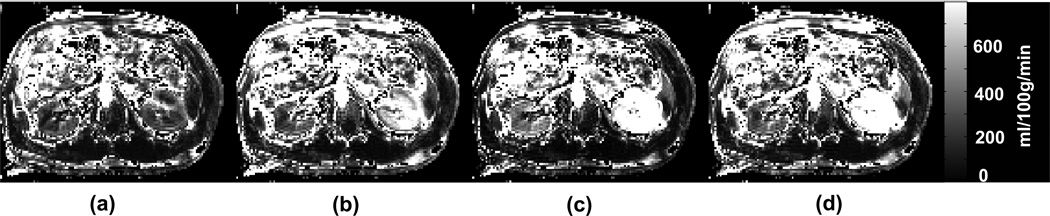
The perfusion maps obtained with the dual navigator gated, single navigator gated (NAV1 only and NAV2 only) and ungated methods are shown in Figure 3 (transversal orientation) and Figure 4 (coronal orientation) for a healthy volunteer. The depicted perfusion maps were not truncated. The kidney compartments, renal cortex and medulla, were clearly delineated in the dual navigator-gated image. The perfusion maps obtained by either single navigator technique or with ungated acquisition show an obvious overestimation of the perfusion values that are caused by the mismatch between inversion and imaging slices. This overestimation was clearly eliminated from the perfusion maps obtained with the dual navigator gated technique.
FIG. 3.
Axial renal perfusion images obtained by (a) dual navigator (both NAV1 and NAV2) gated, (b) NAV1 gated only, (c) NAV2 gated only, and (d) ungated acquisition for a healthy volunteer. Only for the dual navigator case, the kidney cortex is well delineated and the perfusion values are in agreement with previous investigations. Also, only for this case are the perfusion values in the same range for the right and the left kidney.
FIG. 4.
Coronal renal perfusion images obtained by (a) dual navigator (both NAV1 and NAV2) gated, (b) NAV1 gated only, (c) NAV2 gated only, and (d) ungated acquisition for a healthy volunteer. The dual navigator gated perfusion map shows less motion artifact, especially the left kidney is much better defined.
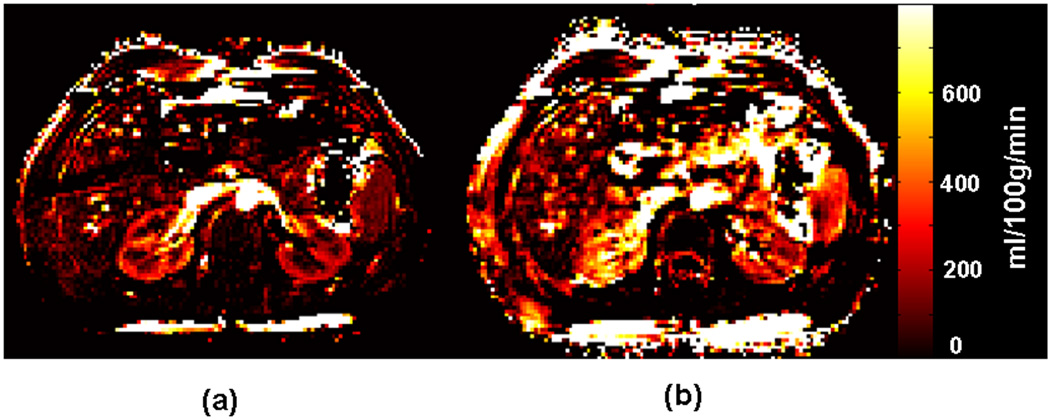
Figure 5 compares equally scaled, axial renal perfusion rate maps obtained by FAIR True-FISP with and without dual navigator gating in a healthy volunteer. The mean perfusion value of the cortex obtained by the dual navigator-gated Q2TIPS FAIR True-FISP method was 256 ml/100g/min, compared to 346 ml/100g/min without gating.
FIG. 5.
Axial perfusion rate maps obtained by FAIR True-FISP with (a) and without (b) the dual navigator-gated technique in the same scale. The perfusion values are not truncated. The kidneys are clearly delineated in the dual navigator-gated FAIR True-FISP perfusion image (a). The perfusion values in (b) (without navigator gating) are overestimated because of respiratory motion.
The mean kidney cortex perfusion values obtained by dual navigator gated, single navigator gated and ungated acquisition are summarized in Table 1 for the first group of three volunteers. Without the dual navigator gating, the overestimation of cortex perfusion values could be up to 250%, 392% and 343% for NAV1 only, NAV2 only and ungated acquisition, respectively.
Table 1.
Kidney cortex perfusion rates (ml/100g/min) obtained with dual navigator, NAV1 only, NAV2 only, and ungated acquisition in transversal(tra) and coronal(cor) orientations for the first group (three healthy volunteers).
| Volunteer | Dual nav | Nav1 only | Overestimation | Nav2 only | Overestimation | No nav | Overestimation |
|---|---|---|---|---|---|---|---|
| V1(tra) | 270 | 674 | 250% | 1057 | 392% | 924 | 343% |
| V2(tra) | 330 | 393 | 119% | 946 | 287% | 357 | 108% |
| V3(tra) | 358 | 531 | 148% | 901 | 251% | 876 | 244% |
| V3(cor) | 260 | 352 | 136% | 326 | 126% | 488 | 188% |
Table 2 provides a quantitative comparison of the perfusion rates of the renal cortices for the second group of five volunteers. With dual navigator gating, the measured perfusion rates ranged from 191 to 378 ml/100g/min, with a mean of 287 ml/100g/min. Without navigator gating, measured perfusion rates ranged from 332 to 1066 ml/100g/min, with a mean of 628 ml/100g/min, which are 1.2 to 3.3 times higher than those obtained by the dual navigator–gated scans because of the inclusion of uninverted spins in labeling images. For all eight volunteers, the mean cortex perfusion was 376 ml/100g/min with the dual navigator gated method.
Table 2.
Comparison of kidney cortex perfusion rates (ml/100g/min) in second group (five healthy volunteers) obtained by FAIR True-FISP with and without the dual navigator-gated technique.
| Volunteer | Left kidney | Right kidney | ||||
|---|---|---|---|---|---|---|
| dual nav | no nav | overestimation | dual nav | no nav | overestimation | |
| 4 | 242 | 730 | 302% | 234 | 780 | 333% |
| 5 | 377 | 587 | 156% | 329 | 484 | 147% |
| 6 | 348 | 965 | 277% | 378 | 1066 | 282% |
| 7 | 266 | 361 | 136% | 246 | 332 | 135% |
| 8 | 266 | 487 | 183% | 191 | 495 | 259% |
| mean (SD) | 300 (52) | 626 (208) | 209% (74%) | 276 (67) | 631 (261) | 229% (87%) |
The relative perfusion related signal change δM = ΔM /Mctrl and SNR = ΔM/σnoise in the cortex were calculated for the navigator gated study, where ΔM = (Mlbl − Mctrl), Mlbl and Mctrl are label and control image signal intensities, respectively, and σnoise is the standard deviation of noise in the subtraction image (8). δM = 15.8 ± 2.46% and SNR = 167 ± 30.7.
DISCUSSION
The newly proposed dual navigator-gated Q2TIPS FAIR True-FISP sequence has been developed and tested in eight healthy volunteers. True-FISP yields high imaging efficiency, which can reduce the total scan time and improve the reliability of the perfusion measurement. The proposed dual navigator-gated technique minimizes the overestimation of renal perfusion and thus facilitates a more accurate measurement.
The quantification of perfusion using the FAIR technique can be severely overestimated because of respiratory motion. Although the overestimation is prominent in axial orientation, it could be also a problem for other orientations as shown in Figure 4 and Table 1. Our data also shows that a single navigator scheme cannot eliminate the overestimation in the perfusion maps because the mismatch cannot be prevented by a single navigator. Maximum displacements along the superior/inferior, medial/lateral, and anterior/posterior directions due to respiratory motion are 15, 6.1, and 5.7 mm, respectively (17,24). If an axial slice is prescribed, the inversion slice could be shifted from the imaging slice by up to 15 mm. In the case of axial measurements, it may not be possible to quantify perfusion accurately with a FAIR experiment as the motion along superior/inferior direction during TI2 may cause imaging and labeling slice to disperse and a considerable amount of unlabeled spins to enter the imaging slice. Even if no motion is involved, to ensure that all spins in the imaging slice are fully inverted, the inversion slice has to be at least two times thicker than the imaging slice (8). If an axial slice scan is needed, an additional 30 mm should be added to the inversion slice to compensate for the superior/inferior motion. Even for a coronal or sagital scan about 12 additional mm may be required for a reliable measurement. Thicker inversion slices can result in substantial reduction in the perfusion signal (25) as well as inclusion of large supplying arteries, which may cause underestimation of perfusion values (6). They can also cause larger variations in transit times, which could also lead to underestimation of perfusion rates (8). In addition, errors in measurement of perfusion rates also arise in axial FAIR ASL scans if control and label image pairs are not acquired at the same position because of physiological motion.
Different approaches have been developed to overcome these problems. Breath-holding is usually an easy solution to artifacts caused by respiratory motion. However, combining the breath-holding method with ASL may not be very practical in clinical routine because dozens of long breath-holds are required to achieve a reliable perfusion map with a satisfactory SNR. As an alternative to breath-holding, synchronization of breathing with the measurement has been proposed (8,26). Here, study subjects are asked to adapt their breathing cycle to the repetition time of the scan by following the noise of the gradients to ensure appropriate inversion and imaging positions. However, the inversion and imaging slices still might not be in the same position. In addition, based on our experience, even healthy adults find it extremely difficult to follow this procedure over several minutes due to the noise from the many gradients (inversion, Q2TIPS, fat saturation, imaging gradients) which confused the volunteers. Background suppression and retrospectively image sorting based on respiratory positions were also introduced to renal ASL perfusion measurements (26), but these techniques are insufficient to completely address the mismatch problem. A preliminary study on continuous ASL (CASL) perfusion measurements using a single navigator was also reported (27). The proposed dual navigator technique gates both the inversion and imaging slices, and thereby effectively removes non-labeling artifacts from perfusion images.
Compared with typical breath-hold scans, navigator-gated imaging is usually more time consuming because data acquired outside of a preset acceptance window are rejected (16). Paradoxically, a breath-hold FAIR ASL exam does not save time, because dummy scans of approximately 10 s are required to achieve the longitudinal steady state in each breath-hold (6,9,28). As an example, for a typical breath-hold period of 20 s and a TR of 5 s, two sets of label and control images can be acquired of which only the second pair can be used for perfusion quantification because the first pair has not reached the steady state. To obtain 20 pairs of label and control images as acquired in this study, 20 long breath-holds are required. Accounting for breath-hold preparation, scan time, and recuperation periods, the total exam time of a multi breath-hold True-FISP ASL experiment could be 20 to 25 minutes, whereas the dual navigator approach can be completed within in 6–9 min and, mostly importantly, under free-breathing conditions.
In ASL, a systematic quantification error can be caused by spatial variations in the transit delay, during which labeled blood flows from the labeled region to the imaging slice. In the brain, this problem has been alleviated by using QUIPSS(11) and Q2TIPS(12). Because the blood flow pattern in the kidney is more complicated than that in the brain, this error could be even larger in the kidney. Therefore, we found that it is necessary to add a Q2TIPS module to ASL for a reliable quantification of renal perfusion. To the author’s best knowledge, this is the first time a Q2TIPS scheme has been successfully implemented in ASL to measure renal perfusions.
One of the challenges in quantifying renal blood flow using ASL is the contamination from macroscopic blood vessels, which present as bright spots or regions in perfusion weighted images. The contamination can be dephased by using bipolar crusher gradients in an EPI based ASL approach (29), but dephasing cannot be applied to the True-FISP ASL scheme because bipolar gradients prolong the repletion time. In previous studies, certain threshold values [500 ml/100g/min in ref. (6) and 600 ml/100g/min in ref. (8)] have been applied to truncate perfusion images, which may cause misrepresentation of perfusion values. The Q2TIPS saturation pulses used in this study can suppress this contamination effectively by introducing a delay time of TI2 − TI1 before image acquisition. The periodic pulses saturate the labeled blood flowing into the imaging slice, and the delay time allows the blood from the vessel to fully perfuse into the tissue. This eliminates the bright blood signal from macroscopic vessels, and no subjective threshold is needed to truncate the perfusion images.
As the interval, TI2, between the labeling and imaging events is 1.3 seconds, labeling and imaging usually occurs at different positions because of physiological motion. The dual navigator gating forces the inversion and imaging slice to be matched in two steps: First, both inversion and imaging only occurred within acceptance windows centered at the end-expiratory diaphragm position. Second, inversion and imaging slices were shifted to approximately the same reference position.
One respiratory cycle of 3 to 5 seconds may not be long enough to accommodate inversion and imaging. We set our acceptance windows to large values, i.e. ±4 mm for NAV1 and ±6 mm for NAV2, so that inversion and imaging could possibly be played out and accepted within one or two consecutive end-expiratory cycles. The incorporated navigators and imaging slice shifting algorithm will assure that inversion and imaging slices are matched across one or two respiratory cycles. TI2 is always constant, no matter whether the inversion and imaging occur in the same or different respiratory circles.
As opposed to dual navigator gating, a single navigator scheme could also be applied just before the inversion pulse (NAV1 only) or the TrueFISP imaging sequence (NAV2 only). A “NAV1 only” approach would lock the inversion slice at approximately the same location, but labeling and imaging slices may be mismatched because there is no gating for the imaging slice position. A “NAV2 only” approach would ensure that all the images are acquired at approximately the same position. However, because the inverting slice is not gated, inversion and imaging slices may not be located at the same position which leads to overestimation of renal perfusion values. Only a dual navigator method ensures that slices are labeled and imaged at the same position, thereby accurately measuring renal perfusion. This has been confirmed by comparing dual navigator gating technique with single navigator gating and ungating method in Figure 3, Figure 4 and Table 1.
As mentioned previously, the kidneys move in superior/inferior (SI), medial/lateral (ML), and anterior/posterior (AP) directions. The extensions of the ML and AP motions are 2 to 3 times smaller than the SI motion, and the motion patterns are correlated as they are mitigated by respiration. If the SI motion is traced and the imaging position is shifted correctly, ML and AP motion can be corrected (17,24). However, negligible residual motion may still be present, but presumably would not affect perfusion quantification.
The so-called T1 effect is another limitation in quantifying renal perfusion by ASL. After blood spins are labeled, they initially decay with the blood T1 and then begin to relax with the tissue T1 as entering the extravascular space and exchange with water in the tissue. Because it is difficult to determine the time of exchange of the labeled blood spins into the tissue, we used a single compartment model which assumes that labeled blood spins entering tissue voxels in the imaging slice relax with exactly the same T1 time as the surrounding tissue. In this study, the blood was assumed to exchange into the tissue immediately after the labeling, and a tissue T1 of 1150 ms was used in Eq.[1] (21)). In the case of slow exchange between blood and tissue water, the T1 value of blood would be used in Eq.[1]. Assuming a blood T1 of 1550 ms at 3T (30), the renal perfusion values would be exp(TI2/T1bood)/exp(TI2/T1cortex) = 75% that of those obtained by tissue T1.
Images acquired after application of crossed-pair navigators may contain two dark bands generated by the two RF pulses of the navigator. The dark bands can be shifted away from the area of interest by adjusting the position of the navigator pulses on respective localizer images. Prospective acquisition correction (PACE) (31–32) may be advantageous to avoid dark bands and could be incorporated in future dual navigator ASL methods.
The cortical perfusion values obtained with our dual navigator-gated technique are in good agreement with previous studies (5,8–9). We believe that our method allows easier and more reliable quantification of renal perfusion rates, even for axial slices. Although the dual navigator method has been implemented with a True-FISP sequence in this study, it can also be combined with other imaging sequences such as EPI. So far this technique has only been used to quantify renal perfusion, but it can easily be adapted to other abdominal organs such as the liver, pancreas and heart.
CONCLUSIONS
A free-breathing, dual navigator-gated FAIR True-FISP pulse sequence was developed for accurate measurement of renal perfusion. Enhancements to the sequence include dual navigators to gate both inversion and imaging slices, a series of periodical saturation pulses applied along both sides of the imaging slice, and a FOCI inversion pulse for a good slice profile. This technique enables the measurement of renal perfusion in free-breathing scans, thereby precluding the need for many long breath-held scans, avoiding possible misregistration, and improving patient tolerance. The method may prove most beneficial for young children and patients who may have difficulty to hold their breath over longer periods of time.
ACKNOWLEDGEMENTS
This work was supported by NHLBI 5 U54HL070590-06 (Scholar) and American Lebanese Syrian Associated Charities. We thank Dr. Vani J. Shanker at the Department of Scientific Editing, St. Jude Children’s Research Hospital for scientific editing.
Footnotes
This work was presented in part at the 16th Scientific Meeting of the International Society for Magnetic Resonance in Medicine in Toronto, Canada, 2008.
REFERENCES
- 1.Huang AJ, Lee VS, Rusinek H. MR imaging of renal function. Radiol Clin North Am. 2003;41(5):1001–1017. doi: 10.1016/s0033-8389(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 2.Newton BB, Jimenez SA. Mechanism of NSF: New evidence challenging the prevailing theory. J Magn Reson Imaging. 2009;30(6):1277–1283. doi: 10.1002/jmri.21980. [DOI] [PubMed] [Google Scholar]
- 3.Roberts DA, Detre JA, Bolinger L, Insko EK, Lenkinski RE, Pentecost MJ, Leigh JS., Jr Renal perfusion in humans: MR imaging with spin tagging of arterial water. Radiology. 1995;196(1):281–286. doi: 10.1148/radiology.196.1.7784582. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Siewert B, Bly BM, Warach S, Edelman RR. STAR-HASTE: Perfusion imaging without magnetic susceptibility artifact. Magn Reson Med. 1997;38(3):404–408. doi: 10.1002/mrm.1910380308. [DOI] [PubMed] [Google Scholar]
- 5.Boss A, Martirosian P, Graf H, Claussen CD, Schlemmer HP, Schick F. High resolution MR perfusion imaging of the kidneys at 3 Tesla without administration of contrast media. Fortschr Röntgenstr. 2005;177(12):1625–1630. doi: 10.1055/s-2005-858761. [DOI] [PubMed] [Google Scholar]
- 6.Karger N, Biederer J, Lusse S, Grimm J, Steffens JC, Heller M, Gluer CC. Quantitation of renal perfusion using arterial spin labeling with FAIR-UFLARE. Magn Reson Imaging. 2000;18(6):641–647. doi: 10.1016/s0730-725x(00)00155-7. [DOI] [PubMed] [Google Scholar]
- 7.Oppelt A, Gaumann R, Barfuss H, Fischer H, Hartl W, Schajor W. FISP: eine neue schnelle Pulssequenz für die Kernspintomographie. Electromedica. 1986;54:15–18. [Google Scholar]
- 8.Martirosian P, Klose U, Mader I, Schick F. FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med. 2004;51(2):353–361. doi: 10.1002/mrm.10709. [DOI] [PubMed] [Google Scholar]
- 9.Fenchel M, Martirosian P, Langanke J, Giersch J, Miller S, Stauder NI, Kramer U, Claussen CD, Schick F. Perfusion MR imaging with FAIR True FISP spin labeling in patients with and without renal artery stenosis: Initial experience. Radiology. 2006;238(3):1013–1021. doi: 10.1148/radiol.2382041623. [DOI] [PubMed] [Google Scholar]
- 10.Kim S-G. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: Application to functional mapping. Magn Reson Med. 1995;34(3):293–301. doi: 10.1002/mrm.1910340303. [DOI] [PubMed] [Google Scholar]
- 11.Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39(5):702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- 12.Luh W-M, Wong EC, Bandettini PA, Hyde JS. QUIPSS II with thin-slice TI1 periodic saturation: A method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med. 1999;41(6):1246–1254. doi: 10.1002/(sici)1522-2594(199906)41:6<1246::aid-mrm22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Günther M, Oshio K, Feinberg DA. Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med. 2005;54(2):491–498. doi: 10.1002/mrm.20580. [DOI] [PubMed] [Google Scholar]
- 14.Payne GS, Leach MO. Implementation and evaluation of frequency offset corrected inversion (FOCI) pulses on a clinical MR system. Magn Reson Med. 1997;38(5):828–833. doi: 10.1002/mrm.1910380520. [DOI] [PubMed] [Google Scholar]
- 15.Danias PG, McConnell MV, Khasgiwala VC, Chuang ML, Edelman RR, Manning WJ. Prospective navigator correction of image position for coronary MR angiography. Radiology. 1997;203(3):733–736. doi: 10.1148/radiology.203.3.9169696. [DOI] [PubMed] [Google Scholar]
- 16.Ehman R, Felmlee J. Adaptive technique for high-definition MR imaging of moving structures. Radiology. 1989;173(1):255–263. doi: 10.1148/radiology.173.1.2781017. [DOI] [PubMed] [Google Scholar]
- 17.Tipirneni A, Song R, Loeffler RB, Hillenbrand CM. Evaluation of the relationship between respiratory hepatic and renal motion using real-time MRI. Proceedings of the 17th Annual meeting of ISMRM; Honolulu, USA. 2009. p. 2071. [Google Scholar]
- 18.Momen A, Leuenberger UA, Handly B, Sinoway LI. Effect of aging on renal blood flow velocity during static exercise. Am J Physiol Heart Circ Physiol. 2004;287(2):H735–H740. doi: 10.1152/ajpheart.00959.2003. [DOI] [PubMed] [Google Scholar]
- 19.Le Roux P. Simplified model and stabilization of SSFP sequences. J Magn Reson. 2003;163(1):23–37. doi: 10.1016/s1090-7807(03)00115-0. [DOI] [PubMed] [Google Scholar]
- 20.Song R, Loeffler R, Hillenbrand C. QUIPSS II with interleaved thin-slice TI1 periodic saturation for FAIR sequence. Proceedings of the 16th Annual meeting of ISMRM; Toronto, Canada. 2008. p. 3683. [Google Scholar]
- 21.Kundel HL, Schlakman B, Joseph PM. Water content and NMR relaxation time gradients in the rabbit kidney. Invest Radiol. 1986;21(1):12–17. doi: 10.1097/00004424-198601000-00002. [DOI] [PubMed] [Google Scholar]
- 22.de Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: Preliminary results. Radiology. 2004;230(3):652–659. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, Detre JA. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn Reson Imaging. 2008;26(2):261–269. doi: 10.1016/j.mri.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandner ED, Wu A, Chen H, Heron D, Kalnicki S, Komanduri K, Gerszten K, Burton S, Ahmed I, Shou Z. Abdominal organ motion measured using 4D CT. International Journal of Radiation Oncology Biology Physics. 2006;65(2):554–560. doi: 10.1016/j.ijrobp.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 25.Holm DA, Sidaros K. Slice profile optimization in arterial spin labeling using presaturation and optimized RF pulses. Magn Reson Imaging. 2006;24(9):1229–1240. doi: 10.1016/j.mri.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Robson PM, Madhuranthakam AJ, Dai W, Pedrosa I, Rofsky NM, Alsop DC. Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling. Magn Reson Med. 2009;61(6):1374–1387. doi: 10.1002/mrm.21960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gach HM, Nguyen T, Wang Y. CASL perfusion MRI of the kidney using real-time tracking, free breathing navigator. Proceedings of the 14th Annual meeting of ISMRM; Seattle, USA. 2006. p. 2990. [Google Scholar]
- 28.De Bazelaire C, Rofsky NM, Duhamel G, Michaelson MD, George D, Alsop DC. Arterial spin labeling blood flow magnetic resonance imaging for the characterization of metastatic renal cell carcinoma(1) Acad Radiol. 2005;12(3):347–357. doi: 10.1016/j.acra.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Ye FQ, Mattay VS, Jezzard P, Frank JA, Weinberger DR, McLaughlin AC. Correction for vascular artifacts in cerebral blood flow values measured by using arterial spin tagging techniques. Magn Reson Med. 1997;37(2):226–235. doi: 10.1002/mrm.1910370215. [DOI] [PubMed] [Google Scholar]
- 30.Noeske R, Seifert F, Rhein KH, Rinneberg H. Human cardiac imaging at 3 T using phased array coils. Magn Reson Med. 2000;44(6):978–982. doi: 10.1002/1522-2594(200012)44:6<978::aid-mrm22>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Klessen C, Asbach P, Kroencke TJ, Fischer T, Warmuth C, Stemmer A, Hamm B, Taupitz M. Magnetic resonance imaging of the upper abdomen using a free-breathing T2-weighted turbo spin echo sequence with navigator triggered prospective acquisition correction. J Magn Reson Imaging. 2005;21(5):576–582. doi: 10.1002/jmri.20293. [DOI] [PubMed] [Google Scholar]
- 32.Asbach P, Klessen C, Kroencke TJ, Kluner C, Stemmer A, Hamm B, Taupitz M. Magnetic resonance cholangiopancreatography using a free-breathing T2-weighted turbo spin-echo sequence with navigator-triggered prospective acquisition correction. Magn Reson Imaging. 2005;23(9):939–945. doi: 10.1016/j.mri.2005.07.002. [DOI] [PubMed] [Google Scholar]