Abstract
Disseminated peritoneal leiomyomatosis (DPL) is a rare condition characterized by scattered smooth muscle nodules over the peritoneal surfaces. The pathogenesis of DPL remains unclear. Herein we report a case of DPL occurring seven years after laparoscopic supracervical hysterectomy with morcellation for uterine leiomyomata (UL). We analyzed both the original UL and the subsequent DPL by molecular cytogenetics to assess the role of chromosomal abnormalities in DPL pathobiology. Interestingly, all of the chromosomal aberrations detected in this case of DPL, including r(1)(p34.3q41), del(3)(q23q26.33) and t(12;14)(q14.3;q24.1), are characteristic chromosomal abnormalities detected in UL. FISH analysis of the initial UL confirmed an interstitial deletion spanning at least 3q24 and 3q25.1, suggesting that functional alteration of a potential gene in this chromosomal region may play a role in DPL development from UL. With the increasing rate of hysterectomy through laparoscopic approach to UL, the unique complications of laparoscopy with morcellation, especially seeding and proliferation of tumor cells over abdominal organs and peritoneum, are becoming more significant and may necessitate review of current surgical protocols to prevent future seeding of the pelvic region with tumor particles.
INTRODUCTION
Smooth muscle tumors of the uterus have a spectrum from benign leiomyoma to malignant leiomyosarcoma including a variety of tumors with unusual growth patterns. Benign uterine smooth muscle tumors, known as uterine leiomyomata (UL) or fibroids, are the most common tumors in women of reproductive age. Prevalence estimates of these tumors are as high as 77%, and 25% of reproductive age women have clinically apparent UL (Buttram and Reiter, 1981; Cramer and Patel, 1990). Consequently, UL are the primary indication for hysterectomy in the US (Lepine et al., 1997). In contrast to UL, malignant leiomyosarcoma is much less frequent, has an aggressive clinical behavior and can be distinguished from UL by the presence of coagulative tumor necrosis, severe nuclear or cytological atypia, elevated mitotic activity, and complex cytogenetic rearrangements (Fletcher et al., 1990; Sreekantaiah et al., 1993; Bell et al., 1994). Smooth muscle tumors with histological features intermediate to benign UL and malignant leiomyosarcoma have an uncertain malignant potential (Bell et al., 1994; Peters et al., 1994).
The group of smooth muscle tumors resembling UL at both gross and microscopic levels but present in atypical locations with unusual growth patterns includes intravenous leiomyomatosis (IVL), benign metastasizing leiomyoma, and disseminated peritoneal leiomyomatosis (DPL). IVL is a smooth muscle proliferation in the uterine veins, vena cava and right atrium of the heart with or without a concurrent UL (Mulvany et al., 1994; Nakayama et al., 1994). Benign metastasizing leiomyoma manifests as smooth muscle nodules or masses usually found in the lungs of women with a history of UL (Abramson et al., 2001).
DPL is a rare condition characterized by discrete smooth muscle nodules scattered throughout the peritoneum of the abdomen and pelvis (Aruh et al., 1993; Hardman and Majmudar, 1996). Despite its benign histological features, DPL can mimic peritoneal carcinomatosis macroscopically. Women of reproductive age are most commonly affected and rare cases affecting men are reported among approximately 100 documented DPL cases (Lausen et al., 1990; Bekkers et al., 1999; Yamaguchi et al., 2003). The actual incidence of DPL might be underestimated, considering its asymptomatic nature (Rajab et al., 2000). DPL is usually associated with pregnancy, oral contraceptive use or hormone replacement therapy, and estrogen secreting tumors of the ovary (Aterman et al., 1977; Bekkers et al., 1999; Drake et al., 2001). Most DPL cases are clinically benign and some may regress partially or completely, especially after pregnancy(Lausen et al., 1990; Hales et al., 1992). Alternatively they can progress, recur or undergo malignant transformation (Sharma et al., 2004).
Although the etiology of DPL remains controversial, the most widely known (but likely incorrect) hypothesis is the smooth muscle metaplasia of subperitoneal mesenchymal cells (Drake et al., 2001). Determining the underlying molecular mechanisms of DPL should inform an understanding of the causal associations of DPL as well as the malignant and metastatic potential of uterine smooth muscle tumors.
In the current study, we present a female patient with DPL seven years subsequent to laparoscopic supracervical hysterectomy with morcellation for UL. Molecular cytogenetic analyses were pursued to assess the role of chromosomal rearrangements in DPL pathobiology and revealed an association between the chromosomal alterations of the DPL and UL occurring consecutively in the same patient.
MATERIALS AND METHODS
Patient History and Pathological Evaluation
The patient is a 48 year old nulligravid caucasian female presenting for evaluation and management of an asymptomatic solid pelvic mass identified upon pelvic examination and confirmed by ultrasound. Seven years prior to presentation she underwent a laparoscopic supracervical hysterectomy with morcellation. The patient had menorrhagia and pelvic pressure prior to this surgery and the uterus was irregularly enlarged to the size of a 15 week pregnancy by clinical examination. At the time of the initial procedure, the surgeon noted that the uterus was “removed in small pieces.”. Final pathology revealed uterine leiomyoma (Fig. 1). The patient continued to menstruate postoperatively suggesting incomplete removal of the uterus.
Fig. 1.
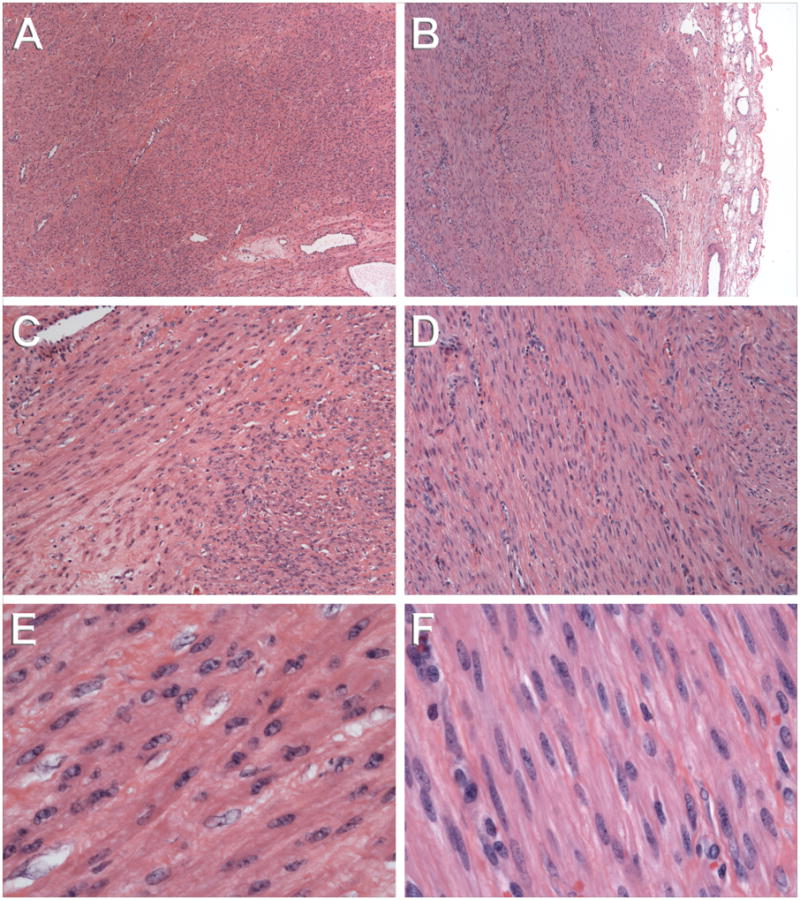
Histology of the morcellated uterine leiomyoma (A, C, E) and a 6.5 cm implant of disseminated peritoneal leiomyomatosis (B, C, F) involving the sigmoid serosa. At low (4x objective) magnification, both the original uterine leiomyoma (A) and subsequent peritoneal leiomyoma (B) show slightly hypercellular, densely packed smooth muscle cells and thick-walled blood vessels. Of note, the sigmoid tumor is a well circumscribed nodule covered by a thin rim of mesenteric fibroadipose tissue (right edge in panel B). At intermediate (10x objective) magnification, the characteristic fascicular arrangement of smooth muscle cells in the uterine (C) and peritoneal (D) tumors is seen. Slightly more collagenous extracellular matrix material (hyaline) and intercellular edema (hydropic change) were present in the uterine tumor (C). At high (40x objective) magnification, the uterine (E) and peritoneal (F) tumors are histologically indistinguishable, both being composed of uniform, spindled smooth muscle cells without atypia or geographic tumor necrosis, and with abundant eosinophilic cytoplasm and a very low mitotic index (viz., <1 mitotic figure per 10 high power fields). Scattered mast cells were noted in the peritoneal tumors (not shown), but mast cells are a common companion to smooth muscle cells in benign uterine leiomyomata.
Based upon the preoperative imaging studies, an informed consent was obtained for re-exploration. An uncomplicated exploratory laparotomy, bilateral salpingo-ophorectomy, trachelectomy and small bowel resection with resection of peritoneal tumor masses was performed. Findings included multiple discrete tumor masses throughout the abdomen and pelvis (Fig. 2). A total of 16 masses ranging in size from 0.6 to 9.0 cm were identified and resected. Microscopic examination of the tumor specimens revealed smooth muscle tumors consistent with DPL (Fig. 1).
Fig. 2.
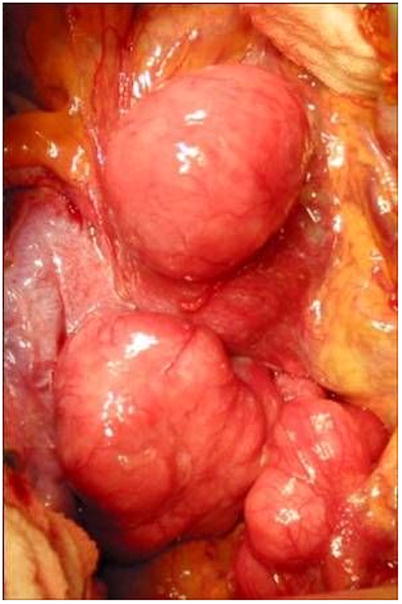
Intra-operative appearance of DPL nodules scattered over the peritoneum.
Tissue Samples
Tissue samples from the DPL surgery were obtained under a Partners HealthCare IRB approved discarded tissue protocol. GTG-banded karyotyping (Rein et al., 1991) of five geographically separate DPL masses and array comparative genomic hybridization analysis (aCGH) of one mass were performed.
Formalin-fixed, paraffin embedded tissue blocks from the UL surgery were obtained from archives of the Division of Women’s and Perinatal Pathology at Brigham and Women's Hospital. Based on the karyotype of the DPL, FISH (described below) was performed on interphase nuclei isolated from 50 micron sections of the archived paraffin blocks (Weremowicz and Schofield, 2007).
Array Comparative Genomic Hybridization (aCGH) Analysis
DNA isolation and aCGH were performed as previously described (Hodge et al., 2009a). The assay compared the tumor and the normal colon tissue DNA obtained from the DPL surgery, using an oligo-based 1M array platform (Agilent, Inc., Santa Clara, CA) containing 974,016 probes with a median effective resolution of 6.3 kb using a three consecutive probe cutoff. Probe intensity information was obtained using the Feature Extraction Software (Agilent), and subsequently feature extraction files were imported to the DNA Analytics Software (Agilent). A genomic imbalance was noted when three or more adjacent oligos detected at least a single copy number change, as compared to the control DNA. Regions of copy number variation are reported as distance in base pairs from the terminus of the p arm of the respective chromosome.
Fluorescence In Situ Hybridization
FISH was performed on interphase nuclei isolated from the paraffin blocks of the UL using bacterial artificial chromosome (BAC) and TelVision™ (Abbott Laboratories, Abbott Park, IL) probes. Tissues embedded in paraffin were confirmed to be UL by hematoxylin and eosin staining of 5 micron sections prior to interphase nuclei extraction.
BAC clones were selected for FISH probes using the University of California Santa Cruz Genome Browser (http://genome.ucsc.edu) (February 2009 assembly). BAC DNAs were isolated following a standard protocol consisting of alkaline lysis, neutralization, and ethanol precipitation (QIAGEN, Valencia, CA). DNAs were labeled with SpectrumGreen or SpectrumRed-dUTP using the Abbott Molecular Nick Translation Kit™ (Abbott). Probes were validated on 100 nuclei and three metaphase spreads prepared from peripheral blood lymphocytes from karyotypically normal individuals. False positive cutoff values were established by doubling the false positive rate deduced from the probe validation.
Extraction of interphase nuclei from 50 micron sections of formalin-fixed, paraffin-embedded tumor tissue from the UL surgery and FISH on these nuclei were performed as previously described (Kuchinka et al., 1995). Interphase nuclei were counterstained with DAPI and hybridization was analyzed with a Zeiss Axioskop epifluorescence microscope (Thornwood, NY) and Applied Imaging CytoVision Software (Santa Clara, CA). A minimum of 50 nuclei were scored per probe.
RESULTS
Molecular Cytogenetic Findings of the DPL
Fourteen metaphase cells from five geographically separate DPL masses were analyzed by GTG-banding. Each tumor revealed the identical abnormal karyotype reported as 46,XX,r(1)(p34.3q41),del(3)(q23q26.33),del(9)(q2?2),t(12;14)(q14.3;q24.1) (Fig. 3) except for one tumor that was a mosaic with 46,XX cells.
Fig. 3.
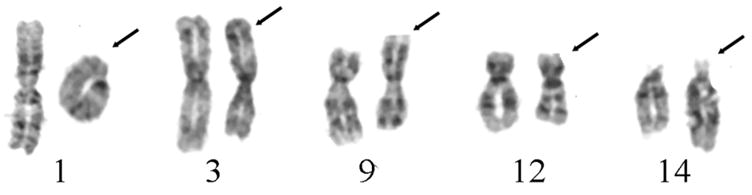
Partial karyotypes with GTG-banded chromosomes with structural aberrations observed in metaphase cells of the DPL: r(1)(p34.3q41), del(3)(q23q26.33), del(9)(q2?2) and t(12;14)(q14.3;q24.1) from left to right, with arrows designating abnormal chromosomes.
Locations of the chromosomal deletions observed by GTG-banding were confirmed and refined by aCGH with the exception of the del(9)(q2?2). The assay compared DNA extracted from a DPL mass with DNA prepared from the patient’s normal colon tissue to avoid the confounding effect of constitutional copy number variants (CNVs). Seven one-copy genomic imbalances were detected and three of them confirmed the GTG-banded karyotype including a 35.4 Mb deletion of 1p36.33-p34.3 (742,580-36,126,342 bp), a 31.5 Mb deletion of 1q41-q44 (215,660,437-247,186,573 bp) (Table 1), and a 38 Mb deletion of 3q23-q26.33 (142,935,925-181,010,217 bp) (Table 1). Four additional one-copy number aberrations, not visible by GTG-banding, were detected including two deletions of chromosome 2 at 2p24.2-p24.1 and 2q32.3 (18,914,304-19,608,288 bp; 193,379,909-194,123,897 bp) with sizes of 694 kb and 744 kb respectively, a 22.5 kb gain at 11p15.5 (2,020,604-2,043,167 bp), and a 65.5 kb deletion of 14q11.2 (23,115,930-23,181,491 bp) (Table 1). Genes located in these altered chromosomal regions are indicated in supplementary data.
Table 1.
Locations of the chromosomal alterations detected by aCGH
| Chromosome Location | Alteration |
|---|---|
| 1p36.33-p34.3 (742,580-36,126,342 bp) | Deletion |
| 1q41-q44 (215,660,437-247,186,573 bp) | Deletion |
| 2p24.2-p24.1 (18,914,304-19,608,288 bp) | Deletion |
| 2q32.3 (193,379,909-194,123,897 bp) | Deletion |
| 3q23-q26.33 (142,935,925-181,010,217 bp) | Deletion |
| 11p15.5 (2,020,604-2,043,167 bp) | Gain |
| 14q11.2 (23,115,930-23,181,491 bp) | Deletion |
Molecular Cytogenetic Findings of the UL
Based on the GTG-banded karyotype of the DPL, FISH experiments were designed and performed on interphase nuclei extracted from 50 micron paraffin sections of the UL from the surgery in 2002. To ascertain whether the UL had similar chromosomal abnormalities with the DPL, three probe sets were prepared.
Presence of the r(1)(p34.3q41) was assessed by hybridization of BAC clone RP11-146K22 located at 1q43 (within putative deletion) and BAC clone RP11-717K8 at 1p13.2 (control probe for chromosome 1 copy number). The hybridization pattern of this probe set was interpreted to be normal without evidence for the deletion being assessed on chromosome 1.
The del(3)(q23q26.33) was evaluated by hybridization of BAC clone RP11-959L4 located at 3q24-q25.1 (within putative deletion) and the TelVysion™ 3p SpectrumGreen probe (Abbott, control probe within 300 kb end of the short arm of chromosome 3). Approximately 27% of interphase nuclei were interpreted to harbor a 3q deletion (false positive cutoff of 10%).
The t(12;14)(q14.3;q24.1) was screened for using a fusion signal of BAC clones RP11-366L20 located at 12q14.3 and RP11-195L9 at 14q24.1. The hybridization pattern of this probe set was interpreted to be normal without evidence for a t(12;14).
DISCUSSION
Potential Relationship between UL and DPL Pathogenesis Based on Molecular Cytogenetic Findings
DPL is a rare entity with multiple small, histologically benign smooth muscle nodules on superficial peritoneal surfaces of the abdominal and pelvic cavities (Aruh et al., 1993; Hardman and Majmudar, 1996). Despite its unusual location and growth pattern, the macroscopic and histological findings of DPL are quite similar to UL (Sutherland et al., 1980). Also, both UL and DPL are usually seen in women of reproductive age, associated with increased estrogen and progesterone levels such as pregnancy or prolonged oral contraceptive use (Cramer and Patel, 1990; Bekkers et al., 1999; Drake et al., 2001; Wise et al., 2004). First degree relatives of women diagnosed with fibroids have a 2.5-fold increased risk for developing UL (Vikhlyaeva et al., 1995; Schwartz et al., 2000), and a familial occurrence of DPL, albeit rare, has been reported (Halama et al., 2005).
Cytogenetic aberrations are observed in approximately 40% of UL (Nibert and Heim, 1990; Meloni et al., 1992), potentially representing secondary changes in genetically susceptible cells (Gross and Morton, 2001). Recurrent chromosomal abnormalities identified in UL include t(12;14)(q14~15;q23~q24), del(7)(q22q32), rearrangements involving 6p21, 10q and 1q42, and deletions of 3q (Nilbert and Heim, 1990; Rein et al., 1998; Gross et al., 2004). To our knowledge, only three studies analyzing DPL at the cytogenetic level are reported (Mark et al., 1991; Quade et al., 1997; Miyake et al., 2009), and among these Miyake et al. (2009) also analyzed a DPL case occurring after laparoscopic myomectomy. Although a small number of DPL cases has been karyotyped, the rate of tumors with chromosomal aberrations is similar to UL and these abnormalities include relevant chromosomal rearrangements with UL, such as alterations in 7q22 and the long arm of chromosome 12 (Quade et al., 1997).
X chromosomal inactivation analysis of UL demonstrates that each leiomyoma has a nonrandom X inactivation pattern but varying parental origin of the inactivated X chromosome comparing multiple UL from the same individual. These findings suggest an independent monoclonal origin of each leiomyoma (Nilbert and Strombeck, 1992; Mashal et al., 1994). X inactivation in DPL also shows a monoclonal origin but, in contrast to UL, consistent inactivation of the same parental X chromosome is observed in all DPL tumorlets from the same patient. This can be either due to spread of a single clone or inactivation of the same parental X in multiple tumors. Low-level genetic and epigenetic instability characterized by loss of heterozygosity (LOH) and alteration in DNA methylation involving the X chromosome detected in DPL favor a clonal neoplastic process (Quade et al., 1997). Miyake et al. (2009) also confirmed the monoclonal origin of each DPL tumorlet with a nonrandom X chromosome inactivation pattern.
In the current study, we analyzed a DPL case presenting seven years after laparoscopic morcellation for UL. GTG-banding and aCGH studies of five different DPL nodules revealed the same chromosomal alterations including a r(1)(p34.3q41), a del(3)(q23q26.33) and a t(12;14)(q14.3;q24.1), all of which are characteristic chromosomal abnormalities observed in UL. In contrast, the del(9)(q2?2) reported by GTG-banding, which is not a typical finding in UL, was not observed by aCGH, prompting critical review of the karyotype. This discrepancy remains unresolved, and the apparently structurally abnormal chromosome 9 may represent either a balanced intrachromosomal or interchromosomal rearrangement. Further studies are required to understand whether the genetic abnormalities are particularly associated with “iatrogenic DPL” or can be observed in “sporadic DPL” cases as well. FISH analysis was performed to screen these chromosomal alterations in the UL from the initial surgery and only a deletion between 3q24 and 3q25.1 was detected. This finding can be interpreted as the sole chromosomal abnormality of the initial UL. Alternatively, BAC clones chosen for the del(1q) and t(12;14) were not located in the altered chromosomal regions, but this seems unlikely based on previous studies analyzing alterations in chromosome 1 and t(12;14)(q14.3;q24.1) in UL (Gross et al., 2004; Hodge et al., 2009b).
Heterozygous germline mutations in FH at 1q42.1 have been detected in two rare Mendelian syndromes establishing a link between UL and cancer: Reed syndrome, also called multiple cutaneous and uterine leiomyomatosis (MCUL1 or MCL, MIM 150800) and hereditary leiomyomatosis and renal cell cancer (HLRCC, MIM 605839). FH appears to act as a tumor suppressor and follow the classic Knudson ‘two-hit’ model as LOH was found in the tumors of affected individuals (Tomlinson et al., 2002; Alam et al., 2003). FH mutation and LOH for FH were detected in non-syndromic UL as well (Kiuru et al., 2002; Lehtonen et al., 2004). Also, evidence suggestive of genetic linkage to FH is found among women who are less than 40 years old at the diagnosis of non-syndromic UL (Gross et al., 2004). In the current DPL case, GTG-banding identified a r(1)(p34.3q41) rearrangement and aCGH analysis confirmed this alteration by detecting deletions on both ends of chromosome 1 with sizes of 35.4 and 31.5 Mb inclusive of the FH locus at 1q42.1. FISH analysis of the initial UL for this alteration was performed with a probe overlapping the FH region and the result was normal. The deletion involving the FH region detected in the DPL could be a “second hit”, were there to be a molecular mutation in FH in the initial UL not detectable by FISH as the “first hit”. Such findings would then warrant further investigation of FH in the development of DPL from UL.
Alterations in chromosome 3, particularly deletions of the long arm of chromosome 3 are described in UL (Nilbert et al., 1990; Dal Cin et al., 1995). Miyake et al. (2009) performed LOH analysis for 3q26.2, 3q28, 7p15.3 and 15q26.3 chromosomal regions in tumors obtained from three subsequent operations of the same patient: the first was a laparoscopy with morcellation for removal of UL, the second was two years later for removal of extrauterine fibroids again through laparoscopic morcellation, and the third was an open laparotomy for the removal of an intramural uterine fibroid and multiple DPL nodules six years after the initial surgery. LOH for 3q26.2 was present in all tumor samples. Additional 3q microsatellite markers were analyzed in tumor samples from all three surgeries and 3q allelic imbalances were detected of 19.2 Mb (between 3q26.1 and 3q26.3) and 47.1 Mb (between 3q24 and 3q26.2). Interestingly, the DPL reported herein had a del(3)(q23q26.33) confirmed by GTG-banding and aCGH. Also, the UL from the initial surgery had a deletion spanning a minimum of the 3q24-3q25.1 regions assessed by the FISH analysis. These findings suggest alteration of the function of a potential gene located in this chromosomal region may play a role in development of DPL after laparoscopic morcellation of UL.
The most common chromosomal translocation in UL is t(12;14)(q14~15;q23~q24) detected in 20% of karyotypically abnormal UL (Turc-Carel et al., 1988; Meloni et al., 1992) and is associated with elevated expression of HMGA2 (Gross et al., 2003). A related karyotype harboring a der(14)t(12;14)(q15;q24) is characteristic for IVL (Dal Cin et al., 2003). HMGA2 is an embryonic proliferation modulator that acts as an architectural factor to modulate transcription and belongs to the heterogeneous high-mobility group family of nonhistone DNA binding proteins (Fusco and Fedele, 2007). HMGA2 is expressed in a variety of fetal tissues but only lung and kidney in adults, and its chromosomal locus is involved in rearrangements in UL and other benign mesenchymal tumors (Gattas et al., 1999; Tallini and Dal Cin, 1999). In t(12;14)(q14~15;q23~q24) identified in UL, the breakpoint in 12q14~15 typically falls centromeric (5′) to HMGA2 but maybe found 3′, and the underlying molecular mechanism is dysregulation of HMGA2 (Schoenberg Fejzo et al., 1996; Quade et al., 2003). In the current DPL case, a t(12;14)(q14.3;q24.1) was detected by GTG-banding; no aberrations of chromosomal regions 12q14.3 or 14q24.1 were observed by aCGH, consistent with a balanced rearrangement. FISH for assessment of a t(12;14) in the initial UL, performed by testing for a fusion of probes overlapping the HMGA2 locus at 12q14.3 and a BAC in 14q24.1 within the RAD51L1 locus, gave a normal result. If the translocation breakpoint were distal to the 12q14.3 probe or proximal to the 14q24.1 probe, it would be possible to observe a normal hybridization pattern even in the presence of a translocation. But, because these are the commonly involved chromosomal regions, we may reasonably conclude that a t(12;14) was not present in the initial UL and occurred during the process of development of DPL from UL.
CNVs are chromosomal imbalances observed in karyotypically and phenotypically normal individuals and cover at least 10% of the genome (Sebat et al., 2004; Redon et al., 2006). In the aCGH analysis described herein, normal colon tissue of the patient was used as the reference DNA instead of DNA from a karyotypically normal individual to ascertain copy number aberrations truly specific to the tumor genome and to suppress detection of signals due to CNVs in the patient’s constitutional genome. Four copy number aberrations resembling CNVs were detected in the DNA of the current DPL: two deletions of chromosome 2 at 2p24.2-p24.1 and 2q32.3 with losses of 694 kb and 744 kb respectively, a 22.5 kb gain of 11p15.5, and a 65.5 kb deletion of 14q11.2. These aberrations are of potential pathogenetic significance in the DPL tumorigenesis, but could also represent CNVs present in different tissue types of the same individual. Studies of tissue specificity of CNVs are an active area of current investigation.
The pathogenesis of DPL remains ill defined in part because it is a rare condition. Nonetheless, there are several theories in the literature. One commonly accepted hypothesis is the independent metaplastic proliferation from subperitoneal mesenchymal cells (Drake et al., 2001). In such cases, each DPL nodule would be expected to be polyclonal populations of cells, but in fact they have been determined to represent monoclonal lesions with consistent inactivation of the same parental X chromosome (Quade et al., 1997). Another theory is that the primary tumor might be an inadequately diagnosed low-grade leiomyosarcoma with low malignant potential (Akkersdijk et al., 1990). However, because none of the characteristic findings of leiomyosarcoma such as coagulative tumor necrosis, severe nuclear or cytological atypia, elevated mitotic activity, or complex cytogenetic rearrangements have been observed in DPL, this is considered to be an unlikely scenario (Sutherland et al., 1980; Fletcher et al., 1990; Sreekantaiah et al., 1993; Bell et al., 1994). We favor a third interpretation of DPL etiology which is the metastatic scattering of a unicentric disease, most plausibly UL. This hypothesis is supported by the monoclonal origin of each tumorlet (Quade et al., 1997) and the correlation between molecular cytogenetic findings of DPL and UL, and the previous patient history of laparoscopic morcellation for UL.
Management of UL
Despite their benign nature, UL are a major clinical concern accounting for approximately 20% of visits to a gynecologist and resulting in expenditures in excess of 2.1 billion health care dollars annually in the US (Flynn et al., 2006; Hartmann et al., 2006). UL remain the single most common indication for hysterectomy in the United States. Moving from traditional “open” surgical techniques to laparoscopy requires the surgeon to morcellate the UL in order to remove a large volume of tissue through laparoscopic ports which are at most only 12–15 mm in diameter. Mechanical devices utilized for morcellation yield a large number of small tissue fragments which spill within the peritoneal cavity and are rarely completely recovered. This raises the possibility of inadvertent laparoscopic morcellation of a malignant tumor presumed to be a benign uterine disease (Schneider, 1997; Einstein et al., 2008).
Another less common, but important, complication of laparoscopic hysterectomy with morcellation is the seeding and proliferation of the tumor or uterine cells over abdominal organs, peritoneum, abdominal wall, and even the subcutaneous incision sites. Endometriosis or adenomyosis may occur after implantation and growth of the morcellated uterine tissue within the abdomen, umbilicus, or vagina, resulting in cyclic pelvic pain, or cyclical bleeding (Koninckx et al., 2000; Sepilian and Della Badia, 2003; Donnez et al., 2007). To date, more than 10 case studies reported the development of subcutaneous, parasitic or disseminated leiomyomas after laparoscopic approach to UL, most probably due to implantation and growth of the tumor particles throughout the abdomen or subcutaneous tissue (Ostrzenski, 1997; Paul and Koshy, 2006; Takeda et al., 2007; Kumar et al., 2008; Miyake et al., 2009; Thian et al., 2009; Al-Talib and Tulandi, 2010).
In sum, herein we report a DPL case occurring seven years after laparoscopic supracervical hysterectomy with morcellation for UL. By exploiting molecular cytogenetic analyses we conclude that the current DPL is likely the result of implantation and evolution of UL cells from the initial procedure. Although this complication is extremely rare, consideration should be given to informing patients about this risk prior to laparoscopic hysterectomy with morcellation.
Supplementary Material
Acknowledgments
The authors thank the Harvard-Partners Center for Genetics and Genomics for kindly providing the BAC clones and Cytogenetics Core of the Dana Farber Harvard Cancer Center (P30 CA006516) for assisting with the aCGH analysis.
Supported by: This research was supported by HD060530 (to C.C.M.). and HG004221 (to C.L.).
References
- Abramson S, Gilkeson RC, Goldstein JD, Woodard PK, Eisenberg R, Abramson N. Benign metastasizing leiomyoma: clinical, imaging, and pathologic correlation. AJR Am J Roentgenol. 2001;176:1409–1413. doi: 10.2214/ajr.176.6.1761409. [DOI] [PubMed] [Google Scholar]
- Akkersdijk GJ, Flu PK, Giard RW, van Lent M, Wallenburg HC. Malignant leiomyomatosis peritonealis disseminata. Am J Obstet Gynecol. 1990;163:591–593. doi: 10.1016/0002-9378(90)91205-q. [DOI] [PubMed] [Google Scholar]
- Al-Talib A, Tulandi T. Pathophysiology and possible iatrogenic cause of leiomyomatosis peritonealis disseminata. Gynecol Obstet Invest. 2010;69:239–244. doi: 10.1159/000274487. [DOI] [PubMed] [Google Scholar]
- Alam NA, Rowan AJ, Wortham NC, Pollard PJ, Mitchell M, Tyrer JP, Barclay E, Calonje E, Manek S, Adams SJ, Bowers PW, Burrows NP, Charles-Holmes R, Cook LJ, Daly BM, Ford GP, Fuller LC, Hadfield-Jones SE, Hardwick N, Highet AS, Keefe M, MacDonald-Hull SP, Potts ED, Crone M, Wilkinson S, Camacho-Martinez F, Jablonska S, Ratnavel R, MacDonald A, Mann RJ, Grice K, Guillet G, Lewis-Jones MS, McGrath H, Seukeran DC, Morrison PJ, Fleming S, Rahman S, Kelsell D, Leigh I, Olpin S, Tomlinson IP. Genetic and functional analyses of FH mutations in multiple cutaneous and uterine leiomyomatosis, hereditary leiomyomatosis and renal cancer, and fumarate hydratase deficiency. Hum Mol Genet. 2003;12:1241–1252. doi: 10.1093/hmg/ddg148. [DOI] [PubMed] [Google Scholar]
- Aruh L, Taskin O, Demir N. Recurrent leiomyomatosis peritonealis disseminata. Int J Gynaecol Obstet. 1993;43:330–331. doi: 10.1016/0020-7292(93)90528-5. [DOI] [PubMed] [Google Scholar]
- Aterman K, Fraser GM, Lea RH. Disseminated peritoneal leiomyomatosis. Virchows Arch A Pathol Anat Histol. 1977;374:13–26. doi: 10.1007/BF00430567. [DOI] [PubMed] [Google Scholar]
- Bekkers RL, Willemsen WN, Schijf CP, Massuger LF, Bulten J, Merkus JM. Leiomyomatosis peritonealis disseminata: does malignant transformation occur? A literature review. Gynecol Oncol. 1999;75:158–163. doi: 10.1006/gyno.1999.5490. [DOI] [PubMed] [Google Scholar]
- Bell SW, Kempson RL, Hendrickson MR. Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol. 1994;18:535–558. [PubMed] [Google Scholar]
- Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36:433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94:435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- Dal Cin P, Moerman P, Deprest J, Brosens I, Van den Berghe H. A new cytogenetic subgroup in uterine leiomyoma is characterized by a deletion of the long arm of chromosome 3. Genes Chromosomes Cancer. 1995;13:219–220. doi: 10.1002/gcc.2870130313. [DOI] [PubMed] [Google Scholar]
- Dal Cin P, Quade BJ, Neskey DM, Kleinman MS, Weremowicz S, Morton CC. Intravenous leiomyomatosis is characterized by a der(14)t(12;14)(q15;q24) Genes Chromosomes Cancer. 2003;36:205–206. doi: 10.1002/gcc.10159. [DOI] [PubMed] [Google Scholar]
- Donnez O, Squifflet J, Leconte I, Jadoul P, Donnez J. Posthysterectomy pelvic adenomyotic masses observed in 8 cases out of a series of 1405 laparoscopic subtotal hysterectomies. J Minim Invasive Gynecol. 2007;14:156–160. doi: 10.1016/j.jmig.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Drake A, Dhundee J, Buckley CH, Woolas R. Disseminated leiomyomatosis peritonealis in association with oestrogen secreting ovarian fibrothecoma. Bjog. 2001;108:661–664. doi: 10.1111/j.1471-0528.2001.00132.x. [DOI] [PubMed] [Google Scholar]
- Einstein MH, Barakat RR, Chi DS, Sonoda Y, Alektiar KM, Hensley ML, Abu-Rustum NR. Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int J Gynecol Cancer. 2008;18:1065–1070. doi: 10.1111/j.1525-1438.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- Fletcher JA, Morton CC, Pavelka K, Lage JM. Chromosome aberrations in uterine smooth muscle tumors: potential diagnostic relevance of cytogenetic instability. Cancer Res. 1990;50:4092–4097. [PubMed] [Google Scholar]
- Flynn M, Jamison M, Datta S, Myers E. Health care resource use for uterine fibroid tumors in the United States. Am J Obstet Gynecol. 2006;195:955–964. doi: 10.1016/j.ajog.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Fusco A, Fedele M. Roles of HMGA proteins in cancer. Nat Rev Cancer. 2007;7:899–910. doi: 10.1038/nrc2271. [DOI] [PubMed] [Google Scholar]
- Gattas GJ, Quade BJ, Nowak RA, Morton CC. HMGIC expression in human adult and fetal tissues and in uterine leiomyomata. Genes Chromosomes Cancer. 1999;25:316–322. [PubMed] [Google Scholar]
- Gross KL, Morton CC. Genetics and the development of fibroids. Clin Obstet Gynecol. 2001;44:335–349. doi: 10.1097/00003081-200106000-00020. [DOI] [PubMed] [Google Scholar]
- Gross KL, Neskey DM, Manchanda N, Weremowicz S, Kleinman MS, Nowak RA, Ligon AH, Rogalla P, Drechsler K, Bullerdiek J, Morton CC. HMGA2 expression in uterine leiomyomata and myometrium: quantitative analysis and tissue culture studies. Genes Chromosomes Cancer. 2003;38:68–79. doi: 10.1002/gcc.10240. [DOI] [PubMed] [Google Scholar]
- Gross KL, Panhuysen CI, Kleinman MS, Goldhammer H, Jones ES, Nassery N, Stewart EA, Morton CC. Involvement of fumarate hydratase in nonsyndromic uterine leiomyomas: genetic linkage analysis and FISH studies. Genes Chromosomes Cancer. 2004;41:183–190. doi: 10.1002/gcc.20079. [DOI] [PubMed] [Google Scholar]
- Halama N, Grauling-Halama SA, Daboul I. Familial clustering of Leiomyomatosis peritonealis disseminata: an unknown genetic syndrome? BMC Gastroenterol. 2005;5:33. doi: 10.1186/1471-230X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales HA, Peterson CM, Jones KP, Quinn JD. Leiomyomatosis peritonealis disseminata treated with a gonadotropin-releasing hormone agonist. A case report. Am J Obstet Gynecol. 1992;167:515–516. doi: 10.1016/s0002-9378(11)91445-8. [DOI] [PubMed] [Google Scholar]
- Hardman WJ, 3rd, Majmudar B. Leiomyomatosis peritonealis disseminata: clinicopathologic analysis of five cases. South Med J. 1996;89:291–294. [PubMed] [Google Scholar]
- Hartmann KE, Birnbaum H, Ben-Hamadi R, Wu EQ, Farrell MH, Spalding J, Stang P. Annual costs associated with diagnosis of uterine leiomyomata. Obstet Gynecol. 2006;108:930–937. doi: 10.1097/01.AOG.0000234651.41000.58. [DOI] [PubMed] [Google Scholar]
- Hodge JCT, Cuenco K, Huyck KL, Somasundaram P, Panhuysen CI, Stewart EA, Morton CC. Uterine leiomyomata and decreased height: a common HMGA2 predisposition allele. Hum Genet. 2009b;125:257–263. doi: 10.1007/s00439-008-0621-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge JC, Park PJ, Dreyfuss JM, Assil-Kishawi I, Somasundaram P, Semere LG, Quade BJ, Lynch AM, Stewart EA, Morton CC. Identifying the molecular signature of the interstitial deletion 7q subgroup of uterine leiomyomata using a paired analysis. Genes Chromosomes Cancer. 2009a;48:865–885. doi: 10.1002/gcc.20692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuru M, Lehtonen R, Arola J, Salovaara R, Jarvinen H, Aittomaki K, Sjoberg J, Visakorpi T, Knuutila S, Isola J, Delahunt B, Herva R, Launonen V, Karhu A, Aaltonen LA. Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res. 2002;62:4554–4557. [PubMed] [Google Scholar]
- Koninckx PR, Donders G, Vandecruys H. Umbilical endometriosis after unprotected removal of uterine pieces through the umbilicus. J Am Assoc Gynecol Laparosc. 2000;7:227–232. doi: 10.1016/s1074-3804(00)80045-6. [DOI] [PubMed] [Google Scholar]
- Kuchinka BD, Kalousek DK, Lomax BL, Harrison KJ, Barrett IJ. Interphase cytogenetic analysis of single cell suspensions prepared from previously formalin-fixed and paraffin-embedded tissues. Mod Pathol. 1995;8:183–186. [PubMed] [Google Scholar]
- Kumar S, Sharma JB, Verma D, Gupta P, Roy KK, Malhotra N. Disseminated peritoneal leiomyomatosis: an unusual complication of laparoscopic myomectomy. Arch Gynecol Obstet. 2008;278:93–95. doi: 10.1007/s00404-007-0536-9. [DOI] [PubMed] [Google Scholar]
- Lausen I, Jensen OJ, Andersen E, Lindahl F. Disseminated peritoneal leiomyomatosis with malignant change, in a male. Virchows Arch A Pathol Anat Histopathol. 1990;417:173–175. doi: 10.1007/BF02190536. [DOI] [PubMed] [Google Scholar]
- Lehtonen R, Kiuru M, Vanharanta S, Sjoberg J, Aaltonen LM, Aittomaki K, Arola J, Butzow R, Eng C, Husgafvel-Pursiainen K, Isola J, Jarvinen H, Koivisto P, Mecklin JP, Peltomaki P, Salovaara R, Wasenius VM, Karhu A, Launonen V, Nupponen NN, Aaltonen LA. Biallelic inactivation of fumarate hydratase (FH) occurs in nonsyndromic uterine leiomyomas but is rare in other tumors. Am J Pathol. 2004;164:17–22. doi: 10.1016/S0002-9440(10)63091-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepine LA, Hillis SD, Marchbanks PA, Koonin LM, Morrow B, Kieke BA, Wilcox LS. Hysterectomy surveillance--United States, 1980–1993. MMWR CDC Surveill Summ. 1997;46:1–15. [PubMed] [Google Scholar]
- Mark J, Havel G, Dahlenfors R, Wedell B. Cytogenetics of multiple uterine leiomyomas, parametrial leiomyoma and disseminated peritoneal leiomyomatosis. Anticancer Res. 1991;11:33–39. [PubMed] [Google Scholar]
- Mashal RD, Fejzo ML, Friedman AJ, Mitchner N, Nowak RA, Rein MS, Morton CC, Sklar J. Analysis of androgen receptor DNA reveals the independent clonal origins of uterine leiomyomata and the secondary nature of cytogenetic aberrations in the development of leiomyomata. Genes Chromosomes Cancer. 1994;11:1–6. doi: 10.1002/gcc.2870110102. [DOI] [PubMed] [Google Scholar]
- Meloni AM, Surti U, Contento AM, Davare J, Sandberg AA. Uterine leiomyomas: cytogenetic and histologic profile. Obstet Gynecol. 1992;80:209–217. [PubMed] [Google Scholar]
- Miyake T, Enomoto T, Ueda Y, Ikuma K, Morii E, Matsuzaki S, Murata Y. A case of disseminated peritoneal leiomyomatosis developing after laparoscope-assisted myomectomy. Gynecol Obstet Invest. 2009;67:96–102. doi: 10.1159/000164949. [DOI] [PubMed] [Google Scholar]
- Mulvany NJ, Slavin JL, Ostor AG, Fortune DW. Intravenous leiomyomatosis of the uterus: a clinicopathologic study of 22 cases. Int J Gynecol Pathol. 1994;13:1–9. doi: 10.1097/00004347-199401000-00001. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Kitamura S, Kawachi K, Kawata T, Fukutomi M, Hasegawa J, Morita R. Intravenous leiomyomatosis extending into the right atrium. Cardiovasc Surg. 1994;2:642–645. [PubMed] [Google Scholar]
- Nilbert M, Heim S. Uterine leiomyoma cytogenetics. Genes Chromosomes Cancer. 1990;2:3–13. doi: 10.1002/gcc.2870020103. [DOI] [PubMed] [Google Scholar]
- Nilbert M, Heim S, Mandahl N, Floderus UM, Willen H, Mitelman F. Characteristic chromosome abnormalities, including rearrangements of 6p, del(7q), +12, and t(12;14), in 44 uterine leiomyomas. Hum Genet. 1990;85:605–611. doi: 10.1007/BF00193583. [DOI] [PubMed] [Google Scholar]
- Nilbert M, Strombeck B. Independent origin of uterine leiomyomas with karyotypically identical alterations. Gynecol Obstet Invest. 1992;33:246–248. doi: 10.1159/000294895. [DOI] [PubMed] [Google Scholar]
- Ostrzenski A. Uterine leiomyoma particle growing in an abdominal-wall incision after laparoscopic retrieval. Obstet Gynecol. 1997;89:853–854. doi: 10.1016/s0029-7844(97)81428-7. [DOI] [PubMed] [Google Scholar]
- Paul PG, Koshy AK. Multiple peritoneal parasitic myomas after laparoscopic myomectomy and morcellation. Fertil Steril. 2006;85:492–493. doi: 10.1016/j.fertnstert.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Peters WA, 3rd, Howard DR, Andersen WA, Figge DC. Uterine smooth-muscle tumors of uncertain malignant potential. Obstet Gynecol. 1994;83:1015–1020. doi: 10.1097/00006250-199406000-00023. [DOI] [PubMed] [Google Scholar]
- Quade BJ, McLachlin CM, Soto-Wright V, Zuckerman J, Mutter GL, Morton CC. Disseminated peritoneal leiomyomatosis. Clonality analysis by X chromosome inactivation and cytogenetics of a clinically benign smooth muscle proliferation. Am J Pathol. 1997;150:2153–2166. [PMC free article] [PubMed] [Google Scholar]
- Quade BJ, Weremowicz S, Neskey DM, Vanni R, Ladd C, Dal Cin P, Morton CC. Fusion transcripts involving HMGA2 are not a common molecular mechanism in uterine leiomyomata with rearrangements in 12q15. Cancer Res. 2003;63:1351–1358. [PubMed] [Google Scholar]
- Rajab KE, Aradi AN, Datta BN. Postmenopausal leimyomatosis peritonealis disseminata. Int J Gynaecol Obstet. 2000;68:271–272. doi: 10.1016/s0020-7292(99)00203-9. [DOI] [PubMed] [Google Scholar]
- Redon R, Ishikawa S, Fitch KR, Feuk L, Perry GH, Andrews TD, Fiegler H, Shapero MH, Carson AR, Chen W, Cho EK, Dallaire S, Freeman JL, Gonzalez JR, Gratacos M, Huang J, Kalaitzopoulos D, Komura D, MacDonald JR, Marshall CR, Mei R, Montgomery L, Nishimura K, Okamura K, Shen F, Somerville MJ, Tchinda J, Valsesia A, Woodwark C, Yang F, Zhang J, Zerjal T, Zhang J, Armengol L, Conrad DF, Estivill X, Tyler-Smith C, Carter NP, Aburatani H, Lee C, Jones KW, Scherer SW, Hurles ME. Global variation in copy number in the human genome. Nature. 2006;444:444–454. doi: 10.1038/nature05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein MS, Friedman AJ, Barbieri RL, Pavelka K, Fletcher JA, Morton CC. Cytogenetic abnormalities in uterine leiomyomata. Obstet Gynecol. 1991;77:923–926. [PubMed] [Google Scholar]
- Rein MS, Powell WL, Walters FC, Weremowicz S, Cantor RM, Barbieri RL, Morton CC. Cytogenetic abnormalities in uterine myomas are associated with myoma size. Mol Hum Reprod. 1998;4:83–86. doi: 10.1093/molehr/4.1.83. [DOI] [PubMed] [Google Scholar]
- Schneider A. Recurrence of unclassifiable uterine cancer after modified laparoscopic hysterectomy with morcellation. Am J Obstet Gynecol. 1997;177:478–479. doi: 10.1016/s0002-9378(97)70226-6. [DOI] [PubMed] [Google Scholar]
- Schoenberg Fejzo M, Ashar HR, Krauter KS, Powell WL, Rein MS, Weremowicz S, Yoon SJ, Kucherlapati RS, Chada K, Morton CC. Translocation breakpoints upstream of the HMGIC gene in uterine leiomyomata suggest dysregulation of this gene by a mechanism different from that in lipomas. Genes Chromosomes Cancer. 1996;17:1–6. doi: 10.1002/(SICI)1098-2264(199609)17:1<1::AID-GCC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Marshall LM, Baird DD. Epidemiologic contributions to understanding the etiology of uterine leiomyomata. Environ Health Perspect. 2000;108(Suppl 5):821–827. doi: 10.1289/ehp.00108s5821. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Troge J, Alexander J, Young J, Lundin P, Maner S, Massa H, Walker M, Chi M, Navin N, Lucito R, Healy J, Hicks J, Ye K, Reiner A, Gilliam TC, Trask B, Patterson N, Zetterberg A, Wigler M. Large-scale copy number polymorphism in the human genome. Science. 2004;305:525–528. doi: 10.1126/science.1098918. [DOI] [PubMed] [Google Scholar]
- Sepilian V, Della Badia C. Iatrogenic endometriosis caused by uterine morcellation during a supracervical hysterectomy. Obstet Gynecol. 2003;102:1125–1127. doi: 10.1016/s0029-7844(03)00683-5. [DOI] [PubMed] [Google Scholar]
- Sharma P, Chaturvedi KU, Gupta R, Nigam S. Leiomyomatosis peritonealis disseminata with malignant change in a post-menopausal woman. Gynecol Oncol. 2004;95:742–745. doi: 10.1016/j.ygyno.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Sreekantaiah C, Davis JR, Sandberg AA. Chromosomal abnormalities in leiomyosarcomas. Am J Pathol. 1993;142:293–305. [PMC free article] [PubMed] [Google Scholar]
- Sutherland JA, Wilson EA, Edger DE, Powell D. Ultrastructure and steroid-binding studies in leiomyomatosis peritonealis disseminata. Am J Obstet Gynecol. 1980;136:992–996. doi: 10.1016/0002-9378(80)90624-9. [DOI] [PubMed] [Google Scholar]
- Takeda A, Mori M, Sakai K, Mitsui T, Nakamura H. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14:770–775. doi: 10.1016/j.jmig.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Tallini G, Dal Cin P. HMGI(Y) and HMGI-C dysregulation: a common occurrence in human tumors. Adv Anat Pathol. 1999;6:237–246. [PubMed] [Google Scholar]
- Thian YL, Tan KH, Kwek JW, Wang J, Chern B, Yam KL. Leiomyomatosis peritonealis disseminata and subcutaneous myoma--a rare complication of laparoscopic myomectomy. Abdom Imaging. 2009;34:235–238. doi: 10.1007/s00261-008-9379-5. [DOI] [PubMed] [Google Scholar]
- Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomaki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- Turc-Carel C, Dal Cin P, Boghosian L, Terk-Zakarian J, Sandberg AA. Consistent breakpoints in region 14q22-q24 in uterine leiomyoma. Cancer Genet Cytogenet. 1988;32:25–31. doi: 10.1016/0165-4608(88)90307-x. [DOI] [PubMed] [Google Scholar]
- Vikhlyaeva EM, Khodzhaeva ZS, Fantschenko ND. Familial predisposition to uterine leiomyomas. Int J Gynaecol Obstet. 1995;51:127–131. doi: 10.1016/0020-7292(95)02533-i. [DOI] [PubMed] [Google Scholar]
- Weremowicz S, Schofield DE. Preparation of cells from formalin-fixed, paraffin-embedded tissue for use in fluorescence in situ hybridization (FISH) experiments. Curr Protoc Hum Genet. 2007;Chapter 8(Unit 8.8) doi: 10.1002/0471142905.hg0808s52. [DOI] [PubMed] [Google Scholar]
- Wise LA, Palmer JR, Harlow BL, Spiegelman D, Stewart EA, Adams-Campbell LL, Rosenberg L. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol. 2004;159:113–123. doi: 10.1093/aje/kwh016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Imamura Y, Yamamoto T, Fukuda M. Leiomyomatosis peritonealis disseminata with malignant change in a man. Pathol Int. 2003;53:179–185. doi: 10.1046/j.1440-1827.2003.01452.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


