Abstract
We developed extraction and analysis protocols for element detection in neonatal blood spots (NBSs) using sector-field inductively coupled plasma-mass spectrometry (SF-ICP-MS). A 5% (v/v) nitric acid element extraction protocol was optimized and used to simultaneously measure 28 elements in NBS card filter paper and 150 NBSs. NBS element concentrations were corrected for filter paper background contributions estimated from measurements in samples obtained from either unspotted or spotted NBS cards. A lower 95% uncertainty limit (UL) that accounted for ICP-MS method, filter paper element concentration, and element recovery uncertainties was calculated by standard methods for each individual’s NBS element concentration. Filter paper median element levels were highly variable within and between lots for most elements. After accounting for measurement uncertainties, 11 elements (Ca, Cs, Cu, Fe, K, Mg, Na, P, Rb, S, Zn) had lower 95% ULs >0 ng/spot with estimated concentrations ranging from 0.05->50,000 ng/spot in ≥50% of NBS samples in both correction methods. In a NBS sample minority, Li, Cd, Cs, Cr, Ni, Mo, and Pb had estimated concentrations ≥20-fold higher than the respective median level. Taking measurement uncertainties into account, this assay could be used for semiquantitative newborn blood element measurement and for detection of individuals exposed to supraphysiologic levels of some trace elements. Adequate control of filter paper element contributions remains the primary obstacle to fully quantitative element measurement in newborn blood using NBSs.
Keywords: Inductively Coupled Plasma Mass Spectrometry, Guthrie cards, neonatal blood spots, metals, elements, measurement
Introduction
Neonatal blood spots (NBS) are collected from virtually every infant in the United States. Typically five spots, each consisting of approximately 80 microliters of blood, are collected on a Guthrie card (Guthrie and Susi, 1963) via a heel stick within a few days of birth. With only a few of these spots being used by health departments to screen for newborn diseases, the remainder could potentially be used for exposure assessment in epidemiological studies. Several states store remaining NBSs and make them available to researchers after appropriate institutional review board (IRB) review (Olshan, 2007). Since there is evidence that perinatal exposures may affect health both early and later in life (Smith et al., 2006; Waterland and Michels, 2007) and neonatal venous blood samples (the optimal material for blood measurement assays) are not routinely archived, developing methods to measure specific contaminant exposures and/or variations in blood chemistry using NBS may prove fruitful for studies of the developmental origins of disease (Waterland and Michels, 2007). For example, environmental exposure to lead, one of the few elements that have been measured in NBS (Schwartz 1994), is of great interest due to its neurological toxicity (Jarup, 2003; O'Broin, 1993; Schonfeld et al., 1994; Verebey et al., 1995; Wang and Demshar, 1992). Many other elements that have been noted as probable carcinogens or factors that can contribute to carcinogenesis (e.g. arsenic, cadmium, chromium, copper, nickel, vanadium) (Beyersmann, 2002; Desoize, 2003; Jarup, 2003; Pesch et al., 2000; Silbergeld, 2003) can be measured in venous blood with current multi-element instrument technology, but application to NBSs has been limited as indicated by the paucity of published studies on this topic.
A variety of instrumental techniques have been used to measure select elements in human biological material including (primarily) single-element techniques such as flame atomic absorption spectroscopy and graphite furnace atomic absorption spectroscopy; and multi-element tools such as, inductively coupled plasma atomic emission spectrometry, and ICP-MS. For analysis of elements in small samples the advantages of ICP-MS include detection limits (<0.001–10 ngmL−1) that are orders-of-magnitude lower than the other techniques, simultaneous measurement of multiple elements, and relatively high throughput (Bolann et al., 2007). In applying ICP-MS methods to clinical sciences, the sample matrix presents substantive analytical challenges and in general the matrix ultimately controls the precision and accuracy of the method. For example, for blood both physical and spectral matrix-based interferences limit ICP-MS performance, especially traditional unit-resolution quadrupole-based ICP-MS. Sector-field (SF) ICP-MS (i.e. magnetic-sector) effectively mass-resolves the spectral interferences that severely compromise quantification of many elements in complex blood matrices when quadrupole-based ICP-MS is utilized (Krachler, 2007), yet still provides comparable to superior sensitivity. The greatly enhanced signal/noise and accuracy of SF-ICP-MS makes it the technique of choice for building a method for quantification of a large suite of elements in NBS (Bocca et al., 2004; Rodushkin et al., 2000).
A key challenge to utilizing this technique to measure elements in NBS compared with other clinical matrices (i.e. hair, venous blood, and urine) is the development of extraction protocols that maximize the number of elements that can be extracted from the spotted blood with adequate recovery rates, while simultaneously minimizing the contribution of background element contamination to the measurement from the filter paper. In this paper we describe a method to extract and quantify multiple elements (metals and non-metals) in NBS using SF-ICP-MS and present a detailed account of method uncertainties derived from background filter paper element levels and other sources.
METHODS
Samples
Following exempt approval from the University of Minnesota Institutional Review Board, over 1000 de-identified NBS were obtained from the Minnesota Department of Health (MDH) in 2000. NBS cards were stored in boxes at MDH in rubber banded groups of 20–25 at ambient temperature for up to a year. The blood spot portion of the card was folded over to prevent it from touching other cards. A square that encompassed the spot and included a small amount of surrounding filter paper was cut from each card (Figure 1) and placed into a plastic sleeve followed by storage at 4°C for ≥7 years before testing. One hundred fifty NBS that met the valid specimen criteria specified by the manufacturer (GE Healthcare Inc., 2009) were chosen for elemental characterization, transferred to trace metal cleaned polystyrene petri dishes, and sent from the University of Minnesota to the University of Wisconsin State Laboratory of Hygiene (WSLH) for analysis. Three different lots of unspotted NBS cards (W-031 (n=5), W-041 (n=5) and W-051 (n=15)) that were taken directly from the supplier’s packaging (Schleicher & Schuell 903 grade paper, Whatman Inc, Piscataway, NJ) were also sent to the WSLH for elemental analysis.
Figure 1.
New born screening card (Schleicher & Schuell 903 grade paper, Whatman Inc, Piscataway, NJ)
Extraction protocol
Three different general extraction protocols were evaluated to determine optimal extraction conditions for a large number of elements from NBS as detailed in the supplementary methods. Briefly, SF-ICP-MS was used to measure 35 elements (those listed in Table 1 with the addition of Ag, As, Hg, Rh, Se, Sn, Pt) under each protocol. The latter seven elements, due either to extremely low blood levels, poor analytical recoveries/reproducibility, or lack of suitable external reference materials, were excluded from further analyses. All of the extraction/digestion protocols were applied to both (a) blank filter paper and (b) blood reference materials as part of the optimization procedures. A 5% (v/v) nitric acid/0.1% (v/v) Triton X-100 extraction protocol was chosen for element measurement for this study based on acceptable recovery rates (80–120%) for the largest number of elements and levels of elements extracted from unspotted (blank) filter paper that were among the lowest of the evaluated protocols. Element recoveries were, on average, poorer and more variable, and method blanks higher, in the two other general types of extraction protocols evaluated; a TMAH/ETOH/APDC/Triton extraction and a TMAH digestion followed by ETOH/APDC extraction – refer to the supplementary methods for details of these extraction methods).
Table 1.
ICP-MS measured element concentrations in blank filter paper by lot (ng/spot).
| W-0311 | W-0411 | W-0511 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Median | Mean | Std Dev | %CV | Median | Mean | Std Dev | %CV | Median | Mean | Std Dev | %CV | p-value2 |
| Al | 105.78 | 112.57 | 57.54 | 51% | 20.35 | 23.67 | 6.88 | 29% | 25.81 | 56.95 | 70.25 | 123% | 0.0376 |
| Ba | 14.76 | 14.84 | 2.51 | 17% | 11.66 | 11.39 | 1.07 | 9% | 3.14 | 3.06 | 1.02 | 33% | 0.0002 |
| Ca | 7 338.50 | 7 445.68 | 495.36 | 7% | 6 846.11 | 6 686.19 | 748.40 | 11% | 4 892.98 | 4 733.23 | 647.97 | 14% | 0.0002 |
| Cd | 0.14 | 0.15 | 0.02 | 14% | 0.13 | 0.13 | 0.01 | 10% | 0.02 | 0.02 | 0.01 | 57% | 0.0002 |
| Ce | 0.21 | 0.22 | 0.03 | 13% | 0.16 | 0.16 | 0.03 | 18% | 0.02 | 0.04 | 0.05 | 152% | 0.0002 |
| Co | 1.48 | 1.61 | 0.23 | 14% | 1.17 | 1.26 | 0.28 | 22% | 1.89 | 1.90 | 0.28 | 15% | 0.0376 |
| Cr | 0.27 | 0.36 | 0.22 | 61% | 0.28 | 0.30 | 0.11 | 38% | 0.26 | 0.36 | 0.25 | 70% | 0.8145 |
| Cs3 | 3.22 | 5.41 | 4.83 | 89% | 1.55 | 2.33 | 1.86 | 80% | 2.52 | 10.51 | 29.07 | 277% | 0.2921 |
| Cu | 13.19 | 14.53 | 3.93 | 27% | 3.43 | 4.17 | 1.29 | 31% | 3.14 | 3.70 | 2.06 | 56% | 0.0376 |
| Fe | 79.65 | 84.10 | 16.86 | 20% | 76.61 | 77.95 | 6.09 | 8% | 49.41 | 53.27 | 23.30 | 44% | 0.0029 |
| K | 67.51 | 71.86 | 10.81 | 15% | 111.65 | 110.74 | 61.83 | 56% | 256.93 | 259.56 | 54.06 | 21% | 0.0006 |
| Li | 0.06 | 0.12 | 0.08 | 69% | 0.09 | 0.09 | 0.03 | 35% | 0.21 | 0.27 | 0.13 | 48% | 0.0376 |
| Mg | 1 095.89 | 1 072.89 | 98.17 | 9% | 1 322.12 | 1 300.07 | 104.43 | 8% | 1501.26 | 1 523.28 | 72.37 | 5% | 0.0006 |
| Mn | 34.07 | 34.36 | 3.13 | 9% | 21.40 | 21.35 | 1.36 | 6% | 5.37 | 5.82 | 1.10 | 19% | 0.0002 |
| Mo | 0.06 | 0.16 | 0.17 | 106% | 0.02 | 0.06 | 0.13 | 227% | 0.19 | 0.22 | 0.24 | 110% | 0.2921 |
| Na | 1 919.67 | 1 921.40 | 100.31 | 5% | 2 497.40 | 2 385.04 | 382.10 | 16% | 3 321.83 | 3 116.59 | 809.64 | 26% | 0.0006 |
| Ni | 0.89 | 0.90 | 0.14 | 15% | 0.62 | 0.60 | 0.15 | 24% | 0.57 | 0.55 | 0.36 | 67% | 0.0376 |
| P | 314.67 | 309.09 | 38.59 | 12% | 373.30 | 386.53 | 58.43 | 15% | 45.82 | 50.22 | 80.98 | 126% | 0.0002 |
| Pb | 0.75 | 24.81 | 53.33 | 3% | 0.51 | 0.57 | 0.33 | 57% | 0.21 | 0.86 | 1.23 | 142% | 0.2921 |
| Rb | 0.09 | 0.08 | 0.04 | 46% | 0.11 | 0.11 | 0.04 | 33% | 0.26 | 0.27 | 0.05 | 19% | 0.0006 |
| S | 317.47 | 317.55 | 49.99 | 16% | 355.16 | 363.54 | 65.65 | 18% | 646.41 | 649.67 | 85.90 | 13% | 0.0006 |
| Sb3 | 3.94 | 44.90 | 100.07 | 223% | −1.31 | 1.16 | 21.95 | 1900% | 6.82 | 8.53 | 36.72 | 430% | 0.8145 |
| Sr | 13.67 | 13.46 | 1.04 | 8% | 11.08 | 11.36 | 1.41 | 12% | 7.26 | 7.95 | 1.64 | 21% | 0.0029 |
| Ti | 1.31 | 1.32 | 0.83 | 63% | 0.50 | 0.64 | 0.75 | 117% | 0.63 | 2.86 | 7.16 | 250% | 0.1749 |
| Tl3 | 0.00 | 0.81 | 2.11 | 260% | 1.14 | 1.55 | 1.76 | 113% | 1.44 | 1.88 | 1.76 | 93% | 0.2921 |
| U3 | 9.70 | 10.35 | 1.71 | 17% | 5.59 | 5.90 | 0.78 | 13% | 4.19 | 4.64 | 2.93 | 63% | 0.0174 |
| V | 0.08 | 0.09 | 0.03 | 32% | 0.09 | 0.09 | 0.02 | 24% | 0.12 | 0.15 | 0.10 | 66% | 0.2921 |
| Zn | 18.81 | 17.76 | 5.17 | 29% | 13.95 | 0.24 | 25.50 | 10836% | 6.30 | 7.08 | 37.38 | 528% | 0.0174 |
The number of samples for each of the three lots: W-031, W-041, and W-051, was 5, 5, and 15 respectively.
Calculated by Brown-Mood median test
Expressed as picograms/spot
Spotted and unspotted card circles (~8 mm diameter) were vertically bisected (Figure 1) with a ceramic blade on a clean Teflon cutting surface. An approximately equal size filter paper sample adjacent to the NBS was cut from 15 of the 150 NBS cards for measurement of the elemental background in spotted cards. Sections were weighed on an analytical balance (Mettler AE163, precision 0.01 mg) in clean plastic weigh boats, placed at the bottom of an acid-leached 15 ml polypropylene extraction tube to which 5 ml of extraction solution (5% (v/v) Optima® grade 16M nitric acid (Fisher Scientific) + 0.1% (v/v) Triton X-100) was subsequently added. Samples were then vortexed briefly and sonicated for 10 minutes followed by incubation for 30 minutes, brief vortexing, and an additional 5 minute sonication. They were then filtered through a 25 mm diameter 0.45 µm Whatman Puradisc polypropylene syringe filter using polypropylene acid-leached 20 mL syringes (Norm-Jet; Henke Sass Wolf, Tuttlingen Germany) to remove undigested material, and placed into clean polypropylene auto-sampler tubes, diluted one to one (v/v) with 2% (v/v) (0.32 M) Optima® grade nitric acid immediately before SF-ICP-MS analysis. All processing steps were conducted at room temperature.
Contamination Control Procedures
To minimize any laboratory contamination of NBSs with elements, all preparation steps (supply and equipment cleaning), sample extraction, and analyses were performed in a trace element clean laboratory by personnel with extensive experience in trace level techniques. Filter substrates were handled with Teflon forceps and gloved hands and critical sample and equipment handling were performed under polypropylene or acrylic laminar-flow benches. Precautions were taken to minimize contamination during chemical extractions including the use of only ultra-high purity reagents. Samples contacted only trace metal compatible plastic materials (Teflon, polypropylene, or polyethylene) that were prepared in multi-step acid (2M nitric, 2M hydrochloric, and 0.2M nitric; each step for 2-days) leachings. Equipment/supplies were protected from environmental contamination by double-bagging in plastic. Further details of the contamination control protocols that are routinely used at the WSLH have been published previously (Shafer and Overdier, 1996; McElroy et al., 2007; McElroy et al., 2008; Shafer et al., 2008).
SF-ICP-MS Procedures
Sample extract element concentrations were determined with a single-collector magnetic sector ICP-MS instrument (Element 2- Thermo-Finnigan, Bremen Germany). The Finnigan Element 2 (with fast scanning magnet and Pt guard electrode) is interfaced to an ESI low-flow Teflon micro-concentric nebulizer (MCN) and quartz cyclonic spray chamber and can be operated in three resolution modes: low, medium, and high with the mass resolution for each element chosen based on consideration of resolution of spectral interferences and sensitivity. In general, we applied the resolution mode that provided the necessary mass resolution of all known molecular interferences, but no higher. Sensitivities ranging from 1500 to 4000 megaHertz (MHz)/parts per million (ppm) (low resolution mode for mono-isotopic element), backgrounds less than 2 counts per second (cps), and instrumental detection limits (3-standard deviations) ranging from ~0.001 to 0.1 ng ml−1 are typically observed. Three internal standards (gallium, indium, bismuth) were spiked into the diluted sample extracts (and standards) prior to analysis to correct for matrix and transport-driven sensitivity variation and long-term drift. Quantification was performed using external standards (multi-element mixes with element-appropriate concentration ranges) with internal normalization to the internal standards. Isotopes were acquired in peak jumping mode and multiple isotopes (where feasible) of many target elements were acquired as part of the overall data quality assessment. A minimum of three replicate analyses, from which instrumental measurement reproducibility estimates in ng element (μ1 below) were obtained, were performed on each sample after a 45 second uptake and stabilization period. A long rinse with 2% (v/v) high purity nitric acid was performed between samples to minimize carry-over and to recondition the sampler cone.
Quality assurance/control samples analyzed along with each batch of samples included externally certified blood reference materials (CRM) (Metals Level 1 blood, catalogue no. 44521, UTAK Laboratories Inc., Valencia, CA, USA), and Toxic Metals in Bovine Blood, NIST 966), element-spiked reference blood, digestion matrix element spikes, sample element spikes, analytical and sample replicates, method blanks, and isotope and resolution checks. Supplementary Table 1 summarizes general and element specific SF-ICP-MS operating parameters, as well as acceptance criteria for QC samples and supplementary table 2 outlines QA/QC metrics and acceptance criteria for samples.
Data analysis
Element concentrations in NBS sample extracts were first corrected for any element contribution from reagents, handling, and the instrument by subtracting the batch-specific, analytical method blank (typically the mean of 3–4 blanks). Measurements were then corrected for the estimated contribution of elements from the filter paper using two approaches. In the first approach (correction method A), which was applied to all 150 NBS samples, we estimated the filter paper elemental background as the mean level in blank (unspotted) filter paper samples obtained from three different lots (W-031, W-041, W-051). In the second approach (correction method B), which was applied to a subset of the NBS samples (n=15), we estimated the filter paper blank from the element levels measured in a sample of filter paper adjacent to the NBS (spotted card filter paper). Calculation details of the filter blank correction protocols can be found in the supplementary material.
We estimated the total uncertainty (μc) in ng/spot associated with each element concentration value (y) by taking the square root of the sum of the individual squared uncertainties according to the formula:
where μ1 is the ng uncertainty associated with the SF-ICP-MS measurement as estimated from the three replicate analyses described above, μ2 is the uncertainty in ng element in the filter paper blanks per section assayed estimated from lots W-031, W-041, and W-051 (correction method A) or the adjacent filter paper (correction method B) (μ2 calculation details are provided in section III of the supplementary material), μ3 is the uncertainty in element recovery that was estimated from the variation in element recovery in the CRMs and element-spiked CRM spotted on filter paper (percent recovery × ng element detected in sample), and f is the spot fraction (mass of spot section analyzed / total mass of spot) assayed. A lower 95% uncertainty limit was calculated as y − 2 μc to characterize the lower limit of the range of values where the true blood element concentration was likely to lie (Seiler et al. 1994; Taylor et al. 1994).
NBS element measurement reproducibility and repeatability were assessed in 15 randomly selected NBS by comparing ICP-MS measurements between two approximately half spot samples obtained from a single NBS (reproducibility), and two repeat runs of the same NBS extract (repeatability). The percent coefficient of variation (CV) was used to express variation between duplicate samples and was calculated according to the formula: 100 × standard deviation/mean ng element/spot. The mean CV across pairs was calculated to estimate the overall reproducibility between duplicate measurements for each element for both metrics of reproducibility described above. Interassay CVs were assessed using blank filter paper spotted with 80 µl of CRM that was assayed in five analytical batches that included three replicate ~half spot samples/batch and was calculated according to the formula: 100*standard deviation of the batch mean element concentration/batch mean element concentration.
Statistical analysis
Microsoft Excel 2003 and SAS version 9.1 (Cary, NC) were used to perform all calculations and statistical tests. Statistical differences in element concentrations between blank filter paper lots were determined using the Brown-Mood nonparametric test in the SAS procedure Npar1way that is equivalent to a one way ANOVA F statistic using median scores. For each element, we calculated the percentage of NBS with lower 95% uncertainty interval limits >0 ng/spot to determine the elements that could be reliably detected in the majority of NBS (i.e. ≥50%) Correlations were assessed using Pearson’s correlation coefficient. Statistical differences (p<0.05) between batches in sample element measurements were assessed using the ANOVA F test.
RESULTS
ICP-MS element measurement in NBS cards
Element levels varied considerably within lots with CVs >20% between blank filter paper samples within lots for at least one lot for most elements. Element concentrations were also highly variable between blank filter paper lots, with significant differences in median element levels for most elements (20/28) (Table 1). Element concentrations in filter paper from blank NBS cards ranged from 0.0001–~10,000 ng/spot with Al, Ca, Fe, K, Mg, Na, P, S, and Zn detected at the highest levels and Cd, Cs, Sb, and Tl detected at the lowest levels. Of note is that outliers (defined as >3 standard deviations from the mean) were detected for a few element measurements in single filter paper samples for Pb (lot W-031; 120.2 ng/spot), Na (lot W-051; 388.8 ng/spot), and Ti (lot W-051; 28.1 ng/spot) and were excluded from correction method A calculations.
After accounting for estimated measurement uncertainties, 11 of 28 elements (Ca, Cs, Cu, Fe, K, Mg, Na, P, Rb, S and Zn) had lower 95% uncertainty limits >0 ng/spot in the majority of NBS samples in both correction methods (Table 2). An additional five elements (Ce, Mn, Sr, Li, Co) met this criterion in one of the correction methods. Estimated median element concentrations for the 11 elements that could be detected in most samples spanned 6 orders of magnitude ranging from ~0.05 ng/spot (Cs) to >50,000 ng/spot (Na and K). It is important to note that some elements, although not detected in the majority of NBSs, could be detected in a minority of samples at levels that were orders of magnitude higher than the median level. For example, lithium was detected in 4/150 NBSs (correction method A) and 1/15 NBSs that was also used in correction method B at levels >500 times the estimated corresponding median NBS concentration. Additionally, the elements Cd (n=1/150), Cs (n=1/150), Cr (n=22/150), Ni (n=4/150), Mo (n=51/150) using correction method A and Pb (n=1/15) using correction method B were detected in individual NBS at levels ≥20 times the corresponding median NBS concentration (data not shown). Although most elements showed a generally consistent pattern between correction methods with regard to whether they could be detected in most samples, it is of interest to note that a few elements showed different patterns depending on which correction method was used. For example, Cd was detected in 100% of samples using correction method A and 0% of samples using correction method B. In contrast, an opposite pattern was observed for Co, which was detected in 100% of samples using correction method B and 0% of samples using correction method A.
Table 2.
Estimated element concentrations in NBS samples (ng/spot).
| Correction method A1 | Correction method B1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | Median uncorrected concentration (n=150) |
median | mean | sd | Samples with detectable element (%) |
Median uncorrected concentration (n=15) |
median | mean | sd | Samples with detectable element (%) |
R2 |
| Al | 72.13 | −8.13 | 14.35 | 59.60 | 1 | 61.40 | −10.85 | −12.02 | 20.26 | 0 | 0.39 |
| Ba | 19.89 | 11.43 | 11.74 | 3.62 | 99 | 20.03 | 0.96 | 0.69 | 2.62 | 13 | 0.51 |
| Ca | 13 159.67 | 6 042.05 | 6 062.44 | 2 003.74 | 83 | 13 265.95 | 5 699.21 | 5 462.04 | 1 713.48 | 73 | 0.85 |
| Cd | 0.33 | 0.24 | 0.33 | 0.46 | 100 | 0.31 | −0.07 | −0.15 | 0.18 | 0 | 0.76 |
| Ce | 0.37 | 0.24 | 0.24 | 0.16 | 76 | 0.41 | −0.03 | −0.04 | 0.10 | 7 | 0.19 |
| Co | 1.43 | −0.73 | −0.76 | 0.67 | 0 | 1.48 | 1.31 | 1.29 | 0.25 | 100 | 0.64 |
| Cr | 0.43 | 0.01 | 0.05 | 0.28 | 3 | 0.40 | −0.16 | −0.43 | 1.03 | 7 | 0.45 |
| Cs | 0.07 | 0.06 | 0.10 | 0.30 | 61 | 0.06 | 0.03 | 0.03 | 0.03 | 80 | 0.47 |
| Cu | 56.45 | 48.99 | 50.63 | 14.12 | 100 | 60.01 | 46.53 | 45.45 | 12.30 | 100 | 0.91 |
| Fe | 9 317.13 | 9 237.96 | 9 797.48 | 3 481.14 | 100 | 11 526.76 | 11 261.81 | 10 124.47 | 3 679.06 | 100 | 1.00 |
| K | 87 441.07 | 87 202.97 | 95 169.20 | 37 889.52 | 100 | 92 189.82 | 58 085.18 | 58 665.40 | 18 807.63 | 100 | 0.96 |
| Li | 0.53 | 0.28 | 13.42 | 84.72 | 32 | 0.78 | 0.63 | 35.65 | 135.66 | 87 | 1.00 |
| Mg | 4 672.12 | 2 943.92 | 3 016.80 | 666.84 | 99 | 5 027.94 | 3 559.58 | 3 570.04 | 803.53 | 100 | 0.95 |
| Mn | 35.83 | 19.31 | 18.53 | 5.89 | 97 | 38.34 | 0.49 | 0.33 | 4.55 | 0 | 0.26 |
| Mo | 0.20 | 0.02 | 0.43 | 1.66 | 19 | 0.56 | −0.25 | −0.22 | 0.34 | 0 | 0.05 |
| Na | 74 889.37 | 71 689.86 | 75 469.07 | 26 607.75 | 100 | 80 687.55 | 51 973.43 | 52 866.97 | 15 005.55 | 100 | 0.94 |
| Ni | 1.27 | 0.46 | 1.58 | 5.98 | 18 | 1.65 | −0.72 | 3.37 | 11.97 | 13 | 1.00 |
| P | 25 068.88 | 24 841.72 | 26 051.10 | 7 178.15 | 100 | 27 198.43 | 25 971.20 | 25 773.40 | 6 260.10 | 100 | 0.99 |
| Pb | 1.83 | 0.76 | 1.33 | 1.87 | 10 | 1.61 | 0.30 | 0.88 | 2.90 | 27 | 0.95 |
| Rb | 84.83 | 84.59 | 96.18 | 45.31 | 100 | 96.30 | 59.57 | 57.23 | 21.04 | 100 | 0.96 |
| S | 3 644.95 | 3 011.47 | 3 169.25 | 1 446.07 | 100 | 3 897.68 | 2 647.47 | 2 572.00 | 602.73 | 100 | 0.97 |
| Sb | 0.09 | 0.07 | 0.11 | 0.15 | 9 | 0.07 | −0.09 | −0.06 | 0.23 | 0 | 0.70 |
| Sr | 18.59 | 6.65 | 6.77 | 2.58 | 56 | 19.98 | 4.58 | 4.47 | 2.98 | 33 | 0.94 |
| Ti | 0.82 | −0.46 | −0.24 | 1.23 | 0 | 0.62 | −1.10 | −1.07 | 1.65 | 0 | 0.62 |
| Tl3 | 6.00 | 4.20 | 3.78 | 3.11 | 16 | 6.67 | 4.72 | 4.05 | 2.24 | 20 | 0.41 |
| U3 | 7.75 | −0.37 | 0.15 | 3.64 | 2 | 5.51 | −3.53 | −3.74 | 2.58 | 0 | 0.40 |
| V | 0.16 | −0.01 | 0.00 | 0.07 | 1 | 0.14 | −0.01 | −0.04 | 0.11 | 0 | 0.37 |
| Zn | 213.59 | 203.26 | 217.82 | 91.48 | 87 | 226.43 | 142.16 | 119.67 | 121.15 | 80 | 0.82 |
Statistics for correction methods A and B are based on 150 and 15 samples respectively.
Pearson’s correlation coefficient between NBS element concentrations calculated using method A and B on the same samples (n=15)
Expressed in picograms/spot
To address reasons for these differences in element detection between filter paper blank correction methods, we compared element concentrations between unspotted and spotted NBS card filter paper (Table 3). Median element levels were higher in spotted than unspotted NBS card filter paper from all three tested lots for 16 of 28 elements. For Cd that was detected in all samples using correction method A but not B, the adjacent filter paper median was higher than in all three of the tested blank filter paper lots. For Co, the opposite pattern was observed with a higher level detected in all three lots of blank filter paper than in the adjacent filter paper.
Table 3.
Comparison of background element levels in blank filter paper vs. adjacent filter paper.
| Element | Median lot W-031 |
Median lot W-041 |
Median lot W-051 |
Median adjacent paper |
Median ratio adjacent paper/lot W-031 |
Median ratio adjacent paper /lot W-041 |
Median ratio adjacent paper /lot W-051 |
|---|---|---|---|---|---|---|---|
| Al1 | 3.01 | 0.61 | 0.75 | 1.62 | 0.54 | 2.65 | 2.16 |
| Ba1 | 0.44 | 0.36 | 0.09 | 0.46 | 1.05 | 1.27 | 5.14 |
| Ca1 | 220.28 | 212.48 | 136.82 | 181.54 | 0.82 | 0.85 | 1.33 |
| Cd2 | 4.66 | 3.90 | 0.63 | 9.20 | 1.97 | 2.36 | 14.70 |
| Ce2 | 6.06 | 4.83 | 0.57 | 9.50 | 1.57 | 1.97 | 16.53 |
| Co2 | 47.81 | 35.30 | 53.12 | 3.50 | 0.07 | 0.10 | 0.07 |
| Cr2 | 8.75 | 8.84 | 7.34 | 14.10 | 1.61 | 1.59 | 1.92 |
| Cs2 | 0.10 | 0.05 | 0.07 | 0.90 | 8.65 | 16.43 | 12.29 |
| Cu1 | 0.40 | 0.12 | 0.09 | 0.28 | 0.72 | 2.39 | 3.16 |
| Fe1 | 2.49 | 2.45 | 1.39 | 4.29 | 1.72 | 1.75 | 3.10 |
| K1 | 2.06 | 3.20 | 7.35 | 948.04 | 459.11 | 296.51 | 129.06 |
| Li2 | 1.85 | 2.62 | 6.00 | 3.40 | 1.83 | 1.30 | 0.57 |
| Mg1 | 31.87 | 40.95 | 43.37 | 30.63 | 0.96 | 0.75 | 0.71 |
| Mn1 | 1.02 | 0.67 | 0.16 | 0.90 | 0.88 | 1.34 | 5.75 |
| Mo2 | 1.75 | 0.74 | 5.28 | 23.30 | 13.32 | 31.67 | 4.41 |
| Na | 58.30 | 72.31 | 94.20 | 646.28 | 11.09 | 8.94 | 6.86 |
| Ni2 | 26.07 | 17.79 | 15.16 | 58.70 | 2.25 | 3.30 | 3.87 |
| P1 | 9.60 | 11.94 | 1.32 | 34.52 | 3.60 | 2.89 | 26.23 |
| P22 | 24.24 | 15.61 | 5.47 | 40.10 | 1.65 | 2.57 | 7.33 |
| R22 | 2.89 | 3.09 | 7.27 | 1010.90 | 349.84 | 327.20 | 139.03 |
| S1 | 8.57 | 11.36 | 19.07 | 25.45 | 2.97 | 2.24 | 1.33 |
| S22 | 0.11 | −0.04 | 0.21 | 3.40 | 30.35 | −90.31 | 16.29 |
| Sr1 | 0.39 | 0.35 | 0.21 | 0.35 | 0.91 | 1.00 | 1.67 |
| Ti2 | 37.23 | 15.66 | 18.84 | 32.40 | 0.87 | 2.07 | 1.72 |
| Tl2 | −0.01 | 0.04 | 0.04 | 0.04 | −7.97 | 1.00 | 0.99 |
| U2 | 0.28 | 0.19 | 0.12 | 0.20 | 0.72 | 1.05 | 1.66 |
| V2 | 2.27 | 2.80 | 3.42 | 3.70 | 1.63 | 1.32 | 1.08 |
| Zn1 | 0.51 | 0.43 | 0.18 | 1.77 | 3.49 | 4.09 | 10.02 |
Concentration in ng/mg paper extracted
Concentration of element in pg/mg paper extracted)
Measurement reproducibility and repeatability
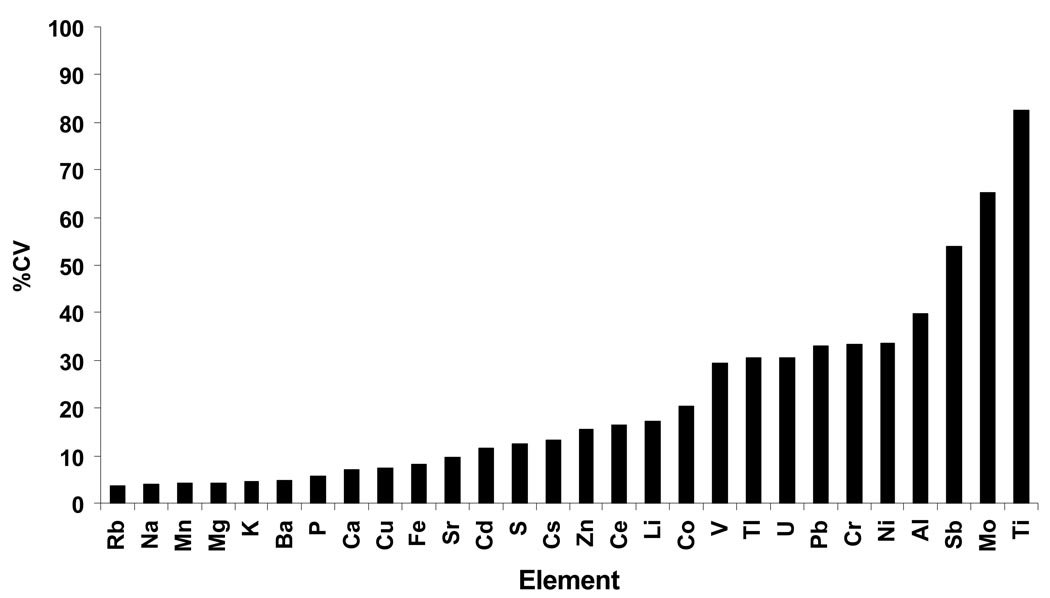
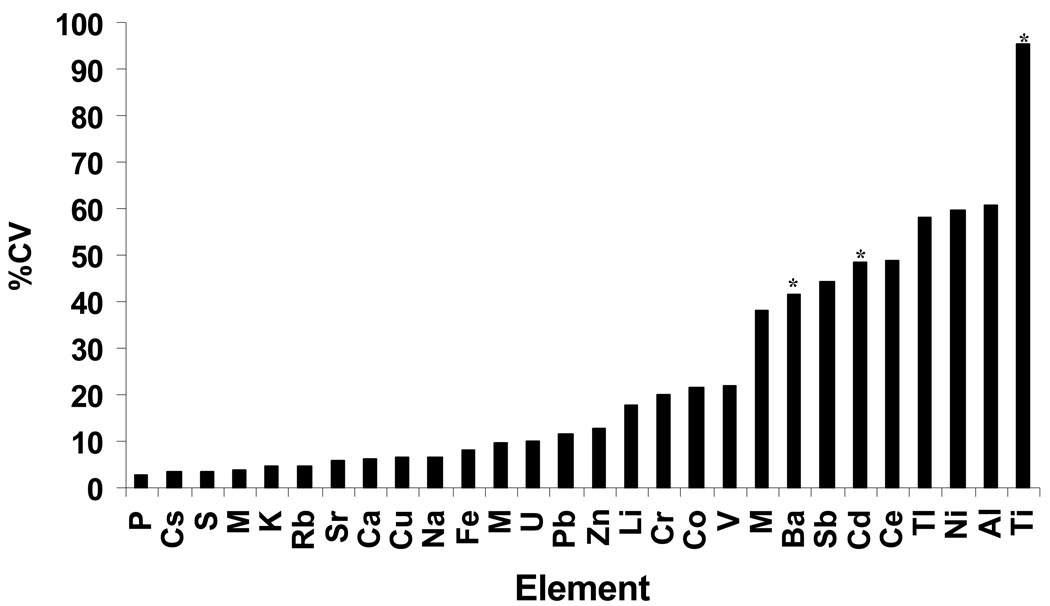
Intra-assay reproducibility of NBS element measurements was assessed in 15 paired half spot samples from the same NBS card. Seventeen of the twenty-eight elements had CVs <20% with the lowest CVs for Rb, Na, and Mn (Figure 2). Elements with poor reproducibility (i.e. the highest CVs) were Mo, Ti and Sb. As would be expected, reproducibility decreased with decreasing element concentration, with a Pearson’s correlation coefficient of r=0.55 (data not shown). Element measurement repeatability was also assessed in 15 NBS sample extracts that were run twice on the ICP-MS instrument. CVs for most elements were <10% (data not shown), implying that extraction variability was the largest component to overall variability in individual element measurements. Interassay reproducibility of NBS measurements was assessed in triplicate CRM spot samples that were run in each of five analytical batches. For elements determined to be quantifiable in NBS with both correction methods (Ca, Cs, Cu, Fe, K, Mg, Na, P, Rb, S, Zn), CVs were acceptable ranging from 2.6% (P) to 12.6% (Zn) (Figure 3).
Figure 2.
Reproducibility of element measurements in NBS. The figure shows a bar graph of the natural log of the median element concentration (ng/spot) vs. the mean CV of duplicate one half spot NBS samples.
Figure 3.
Interassay element reproducibility in SRM blood spots. The figure shows a bar graph of element vs. the average CV from 5 assays of triplicate SRM spotted filter paper samples per assay. *Significantly different (Tukey’s test p<0.05) between at least 2 of the 5 batches.
Discussion
It is becoming increasingly clear that early-life exposures may be etiologically relevant to disease later in life (Waterland and Michels, 2007). Electrolyte and trace metal disturbances are known to be involved in the pathogenesis of many diseases with some evidence suggesting that prenatal exposure to lead and lithium is associated with an increased risk of adverse outcomes in the offspring (Bellinger, 2005; Giles and Bannigan, 2006; Seiler et al., 1994). Therefore, an important aspect of conducting studies that aim to determine relationships between diseases and prenatal exposures is the development of assays to measure analytes in samples collected around the time of birth. Although venous blood samples would be the preferred sample for measuring neonatal exposures, these samples are not routinely available retrospectively. Since NBSs are collected on most newborns and retained by some states that also permit their use in research (Olshan, 2007), our objective was to develop an assay to measure elements in NBS samples using SF-ICP-MS.
Previous studies have simultaneously measured up to 37 elements in whole blood, plasma, urine, or hair by ICP-MS (Barbany E, 1997; Goulle et al., 2005; Heitland and Koster, 2006a; Heitland and Koster, 2006b; Muniz et al., 2001). To our knowledge, this is the first study that has attempted to measure a large panel of elements in NBS. Using our optimized extraction protocol that we developed specifically for this application, coupled with SF-ICP-MS analysis, we were able to quantify 11 clinically relevant elements (Ca, Cs, Cu, Fe, K, Mg, Na, P, Rb, S, Zn) in the majority of NBS samples after accounting for measurement uncertainties. In addition, some elements (Li, Cd, Cs, Cr, Ni, Mo, and Pb) were detected at high levels in a minority of NBSs indicating that the assay may be useful for determining infants who have been exposed to supraphysiologic levels of trace elements while in utero.
We are aware of only two other studies that have used ICP-MS for element detection in dried blood spots (Chaudhuri et al., 2008; Cizdziel, 2007), only one of which measured elements using an extraction-based protocol (Chaudhuri et al., 2008). In the first study, the authors reported successful measurement of lead and mercury but not cadmium (Chaudhuri et al., 2008). In the second study, that introduced sample material into the ICP-MS by laser ablation, Pb, Ca, V, Fe, Cu, and Zn were measured in dried blood samples (Cizdziel, 2007). This technique has the advantage over solution-based ICP-MS of measuring elements in solid matrices and thus requires no extraction/digestion procedures. However, limitations of this method include filter-blank and sample heterogeneity issues, matrix effects, and calibration challenges that limit quantitative analysis, particularly for multi-element applications (Pisonero J, 2009).
Our assay was optimized to measure elements in a half blood spot, allowing the remainder to be used for other analyses (e.g. genotyping, measurement of other analytes, etc). Element measurement in a half spot (in contrast to a single punch) should theoretically improve assay reproducibility that can be influenced by sample location on the filter paper and hematocrit level (Carter, 1978; Holub et al., 2006; O'Broin, 1993; Mei et al., 2001) that has been shown to be variable among infants (Bizzarro et al., 2004; Kayiran et al., 2003). CVs of <10% were obtained between half spots from the same NBS for most elements in the majority of NBS indicating that the assay is highly reproducible.
Our results indicated some significant limitations to using NBSs as the source material for blood element quantification. Chief among these is adequate control of extraneous non-blood element. Our study and a similar study (Chaudhuri et al., 2008) found significant differences in element levels between NBS card lots for cadmium and lead. Both studies and one additional study (Cizdziel, 2007) noted the presence of high outliers for lead in some of the samples of blank filter paper that were tested. Thus, stochastic contamination of the NBS from field processing as well as filter paper lot variation in background element levels could have a substantial impact on the ability to quantify selected elements in NBS. For other elements (e.g. K, Fe, Na, P, Rb, and Zn), the filter paper contributed very little to the total element amount, and therefore quantification would not be substantially impacted by lot variation in filter paper element
Contamination of NBS samples from environmental sources (e.g. handling, heel sweat), however, could still be an issue, but the magnitude of this factor remains unclear. Whether the generally higher element levels observed in the “blank” region of spotted paper in comparison to that in unspotted cards was the result of environmental contamination, filter heterogeneity, or “bleed” from the blood spot is uncertain. Bleed from the spot could be due to diffusion of plasma or serum past the designated spot area. Although we did not visually observe any contamination, our data indicate that this may have occurred. For example, very low levels of Fe were measured in the “blank” spot sampled adjacent to the blood spot relative to the blood spot, as expected, because Fe resides mainly in red blood cells that would not be expected to diffuse to any large degree outside the spot area. In contrast, Na, which also had very low levels in blank unspotted filter paper relative to the blood spot, and is predominantly found in the extracellular compartment of blood, had a markedly lower corrected median concentration when corrected for element using correction method B (36% lower), but not correction method A (4% lower), which suggests some plasma migration. Further research is needed to address whether a sample of filter paper that is further away from the blood sample has lower background element than one adjacent to the spot, and might provide an ideal correction that better reflects extraneous non-blood element sources.
Another limitation to fully quantitative element measurement in NBSs is with regard to the volume of blood spotted on NBS cards. We assumed in our background element correction calculations (as described in the supplementary material) an 80 µl spotted blood volume based on experiments that showed this blood volume completely filled the inscribed NBS circle without saturating it (data not shown). A similar volume assumption has been made by others (Chaudhuri et al., 2008). This assumption may be problematic if blood is improperly spotted. However, several recent studies have suggested that spotted blood volume variation may not be a large concern in properly spotted samples (see GE Healthcare, 2009 for links to images of valid NBS specimens). In a study of phenylalanine measurement in NBS, concentrations varied by only 11% between punches from NBSs spotted with either 35 or 100 µl of blood (Adam et al., 2000). Other recent studies have suggested no difference in the concentrations of small molecules measured in same sized punches from filter paper spotted with different blood volumes (Li and Tse, 2010). In practice, researchers may want to include an additional volume uncertainty component in their calculation of NBS element concentrations, especially if improperly spotted cards are used in their study.
A further suggestion for improvement in measurement precision caused by spotted blood volume variation is the “use of a ratio element such as magnesium or calcium, which has a narrower range in cord blood, to normalize other element values” (Olshan, 2007). However, our data indicate that Mg and Ca may be inappropriate for normalization purposes due to both their high level and variability in blank filter paper samples. Other elements, such as potassium, which was found at low levels in unspotted filter paper relative to NBS, could be explored as a candidate to normalize for volume differences. The ability to quantify a number of elements that might prove useful in normalizing elemental values in the NBS is a notable feature of the method described here. Although we could not address this question with our data, this is an important area for further research.
Conclusions
In conclusion, our data indicated that at least 11 elements can be quantified in most NBSs. In addition, seven trace elements (Li, Cd, Cs, Cr, Ni, Mo, and Pb) were detected in a minority of NBSs. Thus, this assay could also be useful for semiquantitative measurement of elements that are found at relatively high levels in blood to classify individuals to exposure categories (e.g. high, medium, low) and it could also be used to screen for highly elevated levels of some trace elements. However, the fundamental issue that limits the scope of the technique is uncertainty in element contributions from non-blood sources. Further improvement in blood element quantification could be gained from studies that determine the optimal location for filter paper sampling from the NBS card for use in control of non-blood sources of element.
Supplementary Material
Acknowledgments
Financial support: NIH grant T32 CA099936 and the Children’s Cancer Research Fund, Minneapolis, MN.
Abbreviations
- CV
percent coefficient of variation
- SF-ICP-MS
sector-field inductively coupled plasma-mass spectrometry
- Al
aluminum
- Ba
barium
- Ca
calcium
- Cd
cadmium
- Ce
cerium
- Co
cobalt
- Cr
chromium
- Cs
Cesium
- Cu
copper
- Fe
iron
- K
potassium
- MDH
Minnesota Department of Health
- Mg
magnesium
- Mn
maganese
- Na
sodium
- NBS
neonatal blood spot
- ng
nanograms
- Ni
nickel
- P
phosphorous
- Pb
lead
- Rb
rubidium
- Sb
antimony
- Sr
strontium
- sd
standard deviation
- Ti
titanium
- Tl
thallium
- V
vanadium
- WSLH
Wisconsin State Laboratory of Hygiene
- Zn
zinc
References
- Adam BW, Alexander JR, Smith SJ, Chace DH, Loeber JG, Elvers LH, et al. Recoveries of phenylalanine from two sets of dried-blood-spot reference materials: prediction from hematocrit, spot volume, and paper matrix. Clin Chem. 2000;46(1):126–128. [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) Atlanta, Georgia: US Department of Health and Human Services (Public Health Service); Toxicological profile for lead. 2007 August;
- Barbany E, Bergdahl IA, Schutz A, Skerfving S, Oskarsson A. Inductively coupled plasma mass spectrometry for direct multi-element analysis of diluted blood and serum. Journal of Analytical Atomic Spectrometry. 1997;12:1005–1009. [Google Scholar]
- Bellinger DC. Teratogen update: lead and pregnancy. Birth Defects Res A Clin Mol Teratol. 2005;73(6):409–420. doi: 10.1002/bdra.20127. [DOI] [PubMed] [Google Scholar]
- Beyersmann D. Effects of carcinogenic metals on gene expression. Toxicol Lett. 2002;127(1–3):63–68. doi: 10.1016/s0378-4274(01)00484-2. [DOI] [PubMed] [Google Scholar]
- Bizzarro MJ, Colson E, Ehrenkranz RA. Differential diagnosis and management of anemia in the newborn. Pediatr Clin North Am. 2004;51(4):1087–1107. doi: 10.1016/j.pcl.2004.03.006. xi. [DOI] [PubMed] [Google Scholar]
- Bocca B, Alimonti A, Petrucci F, Violante N, Sancesario G, Forte G, et al. Quantification of trace elements by sector field inductively coupled plasma mass spectrometry in urine, serum, blood and cerebrospinal fluid of patients with Parkinson's disease. Spectrochim Acta B. 2004;59:559–566. [Google Scholar]
- Bolann BJ, Rahil-Khazen R, Henriksen H, Isrenn R, Ulvik RJ. Evaluation of methods for trace-element determination with emphasis on their usability in the clinical routine laboratory. Scand J Clin Lab Invest. 2007;67(4):353–366. doi: 10.1080/00365510601095281. [DOI] [PubMed] [Google Scholar]
- Carter GF. The paper punched disc technique for lead in blood samples with abnormal haemoglobin values. Br J Ind Med. 1978;35(3):235–240. doi: 10.1136/oem.35.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri SN, Butala SJ, Ball RW, Braniff CT. Pilot study for utilization of dried blood spots for screening of lead, mercury and cadmium in newborns. J Expo Sci Environ Epidemiol. 2008 doi: 10.1038/jes.2008.19. [DOI] [PubMed] [Google Scholar]
- Cizdziel JV. Determination of lead in blood by laser ablation ICP-TOF-MS analysis of blood spotted and dried on filter paper: a feasibility study. Anal Bioanal Chem. 2007;388(3):603–611. doi: 10.1007/s00216-007-1242-y. [DOI] [PubMed] [Google Scholar]
- Desoize B. Metals and metal compounds in carcinogenesis. In Vivo. 2003;17(6):529–539. [PubMed] [Google Scholar]
- Giles JJ, Bannigan JG. Teratogenic and developmental effects of lithium. Curr Pharm Des. 2006;12(12):1531–1541. doi: 10.2174/138161206776389804. [DOI] [PubMed] [Google Scholar]
- GE Healthcare. Neonatal screening simple spot check. [accessed 18 February 2010];2009 Available at http://www.whatman.com/NeonatalScreeningProducts.aspx.
- Goulle JP, Mahieu L, Castermant J, Neveu N, Bonneau L, Laine G, et al. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci Int. 2005;153(1):39–44. doi: 10.1016/j.forsciint.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Guthrie R, Susi A. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics. 1963;32:338–343. [PubMed] [Google Scholar]
- Heitland P, Koster HD. Biomonitoring of 30 trace elements in urine of children and adults by ICP-MS. Clin Chim Acta. 2006a;365(1–2):310–318. doi: 10.1016/j.cca.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Heitland P, Koster HD. Biomonitoring of 37 trace elements in blood samples from inhabitants of northern Germany by ICP-MS. J Trace Elem Med Biol. 2006b;20(4):253–262. doi: 10.1016/j.jtemb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Holub M, Tuschl K, Ratschmann R, Strnadova KA, Muhl A, Heinze G, et al. Influence of hematocrit and localisation of punch in dried blood spots on levels of amino acids and acylcarnitines measured by tandem mass spectrometry. Clin Chim Acta. 2006;373(1–2):27–31. doi: 10.1016/j.cca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Jarup L. Hazards of heavy metal contamination. Br Med Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- Kayiran SM, Ozbek N, Turan M, Gurakan B. Significant differences between capillary and venous complete blood counts in the neonatal period. Clin Lab Haematol. 2003;25(1):9–16. doi: 10.1046/j.1365-2257.2003.00484.x. [DOI] [PubMed] [Google Scholar]
- Krachler M. Environmental applications of single collector high resolution ICP-MS. J Environ Monit. 2007;9(8):790–804. doi: 10.1039/b703823m. [DOI] [PubMed] [Google Scholar]
- Li W, Tse FLS. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Clin Chem. 2010;24:49–65. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- McElroy JA, Shafer MM, Hampton JM, Newcomb PA. Predictors of urinary cadmium levels in adult females. Science of the Total Environment. 2007;382(2–3):214–223. doi: 10.1016/j.scitotenv.2007.04.015. [DOI] [PubMed] [Google Scholar]
- McElroy JA, Shafer MM, Gangnon RE, Crouch LA, Newcomb PA. Urinary lead exposure and breast cancer risk in a population-based case-control study. Cancer Epidemiology Biomarkers & Prevention. 2008;17(9):2311–2317. doi: 10.1158/1055-9965.EPI-08-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane WJ, Pappas RS, Wilson-McElprang V, Paschal D. A rugged and transferable method for determining blood cadmium, mercury, and lead with inductively coupled plasma mass spectrometry. Spectrochimica Acta B – Atomic Spectroscopy. 2008;63:638–644. [Google Scholar]
- Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131(5):1631S–1636S. doi: 10.1093/jn/131.5.1631S. [DOI] [PubMed] [Google Scholar]
- Muniz CS, Fernandez-Martin JL, Marchante-Gayon JM, Garcia Alonso JI, Cannata-Andia JB, Sanz-Medel A. Reference values for trace and ultratrace elements in human serum determined by double-focusing ICP-MS. Biol Trace Elem Res. 2001;82(1–3):259–272. doi: 10.1385/bter:82:1-3:259. [DOI] [PubMed] [Google Scholar]
- O'Broin S. Influence of hematocrit on quantitative analysis of "blood spots" on filter paper. Clin Chem. 1993;39(6):1354–1355. [PubMed] [Google Scholar]
- Olshan AF. Meeting report: the use of newborn blood spots in environmental research: opportunities and challenges. Environ Health Perspect. 2007;115(12):1767–1779. doi: 10.1289/ehp.10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesch B, Haerting J, Ranft U, Klimpel A, Oelschlagel B, Schill W. Occupational risk factors for renal cell carcinoma: agent-specific results from a case-control study in Germany. MURC Study Group. Multicenter urothelial and renal cancer study. Int J Epidemiol. 2000;29(6):1014–1024. doi: 10.1093/ije/29.6.1014. [DOI] [PubMed] [Google Scholar]
- Pisonero JFB, Gunther D. Critical revision of GD-MS, LA-ICP-MS and SIMS as inorganic mass spectrometric techniques for direct solid analysis. J Anal At Spectrom. 2009;24:1145–1160. [Google Scholar]
- Rodushkin I, Odman F, Olofsson R, Axelsson MD. Determination of 60 elemetns in whole blood by sector field inductively coupled plasma mass spectrometry. J Anal At Spectrom. 2000;15:937–944. [Google Scholar]
- SAS Institute. SAS 9.1 Documentation. SAS Institute. 2009 [Google Scholar]
- Schonfeld DJ, Cullen MR, Rainey PM, Berg AT, Brown DR, Hogan JC, et al. Screening for lead poisoning in an urban pediatric clinic using samples obtained by fingerstick. Pediatrics. 1994;94(2 Pt 1):174–179. [PubMed] [Google Scholar]
- Schwartz J. Low-level lead exposure and children's IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65(1):42–55. doi: 10.1006/enrs.1994.1020. [DOI] [PubMed] [Google Scholar]
- Seiler HG, Sigel A, Sigel H. Handbook on metals in clinical and analytical chemistry. New York: M. Dekker; 1994. p. 753. xx. [Google Scholar]
- Shafer MM, Overdier JT USEPA. Analysis of Surface Waters for Trace Elements by Inductively-Coupled Plasma Mass Spectrometry. US Environmental Protection Agency Lake Michigan Mass Balance Methods Compendium. 1996;Volume 3 Metals: LMMB 057. [Google Scholar]
- Shafer MM, Overdier JT, Ramsl PC, Teschler-Nicola M, Farrell PM. Enhanced methods for assessment of the trace element composition of Iron Age bone. Science of the Total Environment. 2008;401(1–3):144–161. doi: 10.1016/j.scitotenv.2008.02.063. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK. Facilitative mechanisms of lead as a carcinogen. Mutat Res. 2003;533(1–2):121–133. doi: 10.1016/j.mrfmmm.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, et al. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114(8):1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BN, Kuyatt CE National Institute of Standards and Technology (U.S.) Guidelines for evaluating and expressing the uncertainty of NIST measurement results [microform] Gaithersburg, MD: U.S. Dept. of Commerce, Technology Administration, National Institute of Standards and Technology; 1994
- Verebey K, Rosen JF, Schonfeld DJ, Carriero D, Eng YM, Deutsch J, et al. Blood collection and analytical considerations in blood lead screening in children. Clin Chem. 1995;41(3):469–470. [PubMed] [Google Scholar]
- Wang ST, Demshar HP. Determination of blood lead in dried blood-spot specimens by Zeeman-effect background corrected atomic absorption spectrometry. The Analyst. 1992;117(6):959–961. doi: 10.1039/an9921700959. [DOI] [PubMed] [Google Scholar]
- Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.