Abstract
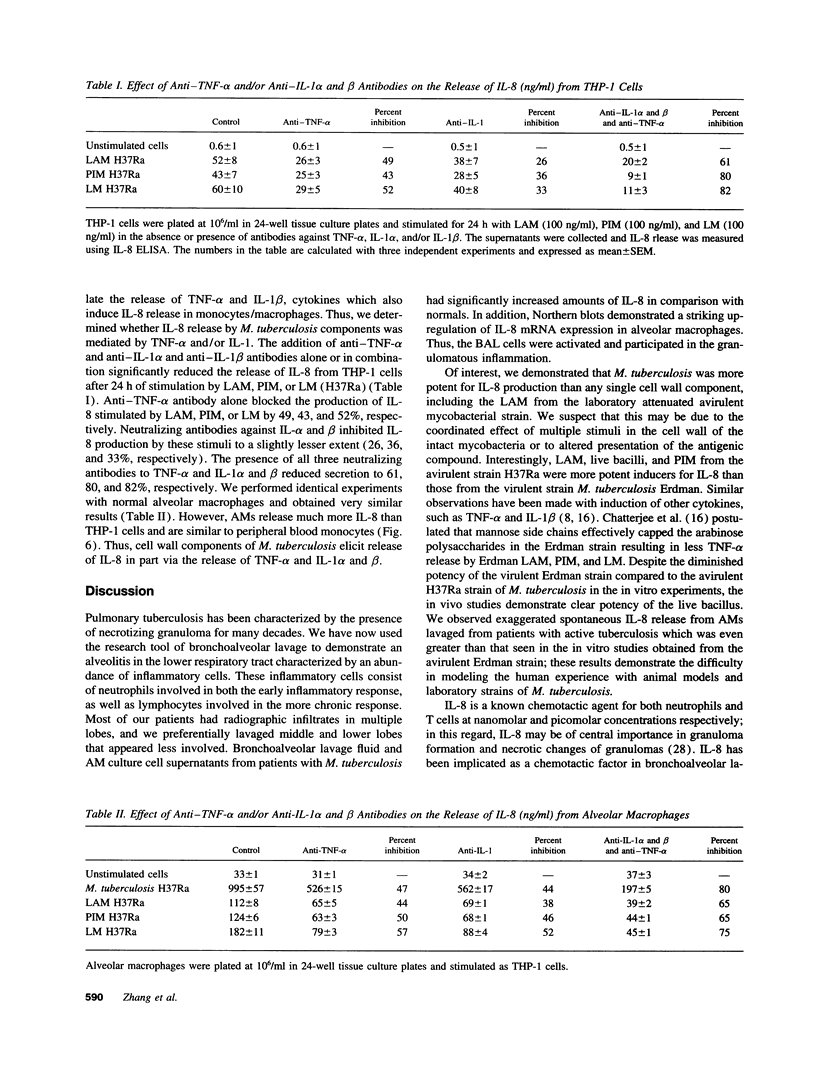
Mycobacterium tuberculosis infection is accompanied by acute and chronic inflammatory infiltrates associated with necrotizing granulomas in lung tissue. The cellular infiltrate is characterized by inflammatory cells which include neutrophils, lymphocytes, and macrophages. In animal and in vitro models of mycobacterial infection, cytokines including tumor necrosis factor-alpha (TNF-alpha), interferon gamma (IFN-gamma), and interleukin-1 beta (IL-1 beta) participate in granulomatous inflammation. We hypothesized that interleukin-3, a potent chemoattractant for neutrophils and lymphocytes, could be released by activated alveolar macrophages after exposure to M. tuberculosis or its components and contribute to granulomatous lung inflammation. A quantitative immunoassay revealed that IL-8 protein release was significantly elevated in supernatants of macrophages and in lavage fluid obtained from patients with pulmonary tuberculosis compared to normal controls. In addition, Northern blots demonstrated striking up-regulation of IL-8 mRNA in macrophages from these patients. M. tuberculosis and its cell wall components lipoarabinomannan (LAM), lipomannan (LM), and phosphoinositolmannoside (PIM) stimulated IL-8 protein release and mRNA expression in vitro from alveolar macrophages, but deacylated LAM did not. Neutralizing antibodies to TNF-alpha and/or IL-1-alpha and beta blocked 83% of the stimulation. IL-8 synthesis and release is an early response of macrophages after phagocytosis of M. tuberculosis. Its production serves to attract both acute and chronic inflammatory cells of active infection and thus participates in the process of containment of the pathogen.
Full text
PDF

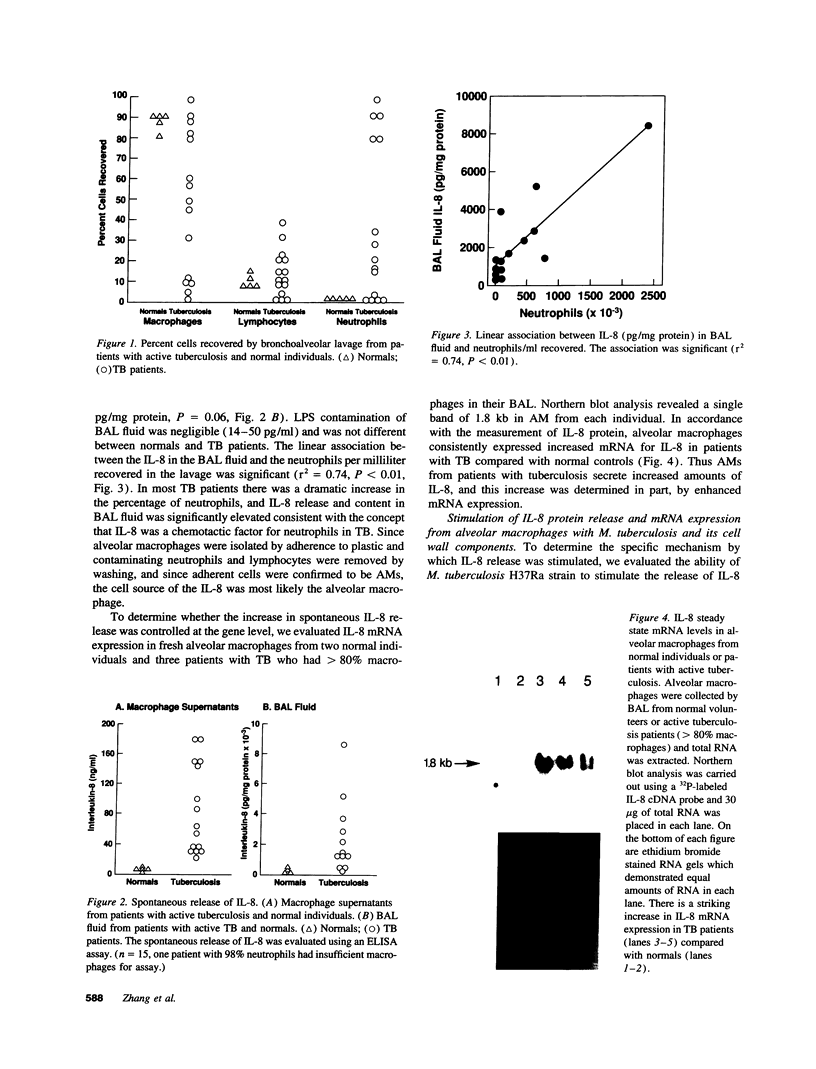
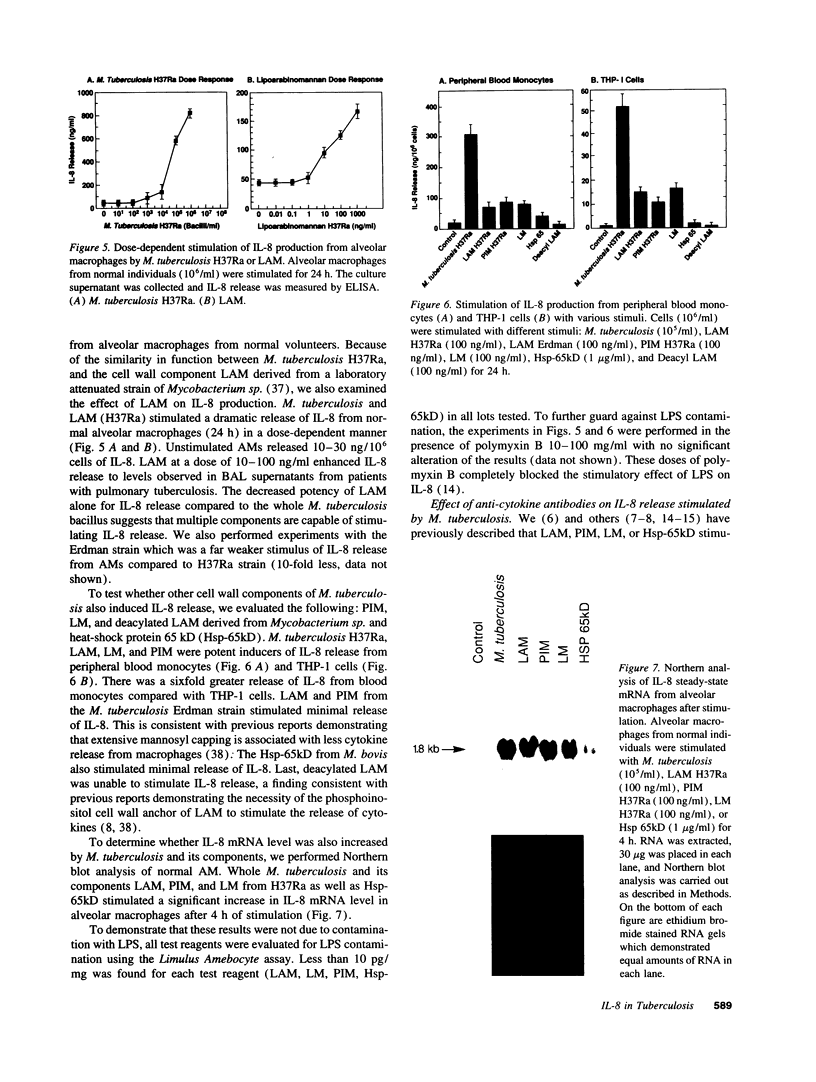


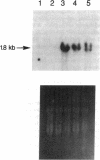
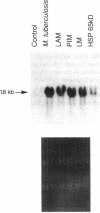
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antony V. B., Sahn S. A., Antony A. C., Repine J. E. Bacillus Calmette-Guérin-stimulated neutrophils release chemotaxins for monocytes in rabbit pleural spaces and in vitro. J Clin Invest. 1985 Oct;76(4):1514–1521. doi: 10.1172/JCI112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Walz A., Kunkel S. L. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989 Oct;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes P. F., Bloch A. B., Davidson P. T., Snider D. E., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991 Jun 6;324(23):1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Chatterjee D., Abrams J. S., Lu S., Wang E., Yamamura M., Brennan P. J., Modlin R. L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992 Jul 15;149(2):541–547. [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Barnes P. F., Mistry S. D., Cooper C. L., Pirmez C., Rea T. H., Modlin R. L. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989 Feb 15;142(4):1114–1119. [PubMed] [Google Scholar]
- Bazzoni F., Cassatella M. A., Rossi F., Ceska M., Dewald B., Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991 Mar 1;173(3):771–774. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R., Murray C. J. Tuberculosis: commentary on a reemergent killer. Science. 1992 Aug 21;257(5073):1055–1064. doi: 10.1126/science.257.5073.1055. [DOI] [PubMed] [Google Scholar]
- Cadranel J., Philippe C., Perez J., Milleron B., Akoun G., Ardaillou R., Baud L. In vitro production of tumour necrosis factor and prostaglandin E2 by peripheral blood mononuclear cells from tuberculosis patients. Clin Exp Immunol. 1990 Aug;81(2):319–324. doi: 10.1111/j.1365-2249.1990.tb03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré P. C., Mortenson R. L., King T. E., Jr, Noble P. W., Sable C. L., Riches D. W. Increased expression of the interleukin-8 gene by alveolar macrophages in idiopathic pulmonary fibrosis. A potential mechanism for the recruitment and activation of neutrophils in lung fibrosis. J Clin Invest. 1991 Dec;88(6):1802–1810. doi: 10.1172/JCI115501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Fan X. D., Hunter S. W., Brennan P. J., Bloom B. R. Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun. 1991 May;59(5):1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Roberts A. D., Lowell K., Brennan P. J., Orme I. M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992 Mar;60(3):1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Roberts A. D., Lowell K., Brennan P. J., Orme I. M. Structural basis of capacity of lipoarabinomannan to induce secretion of tumor necrosis factor. Infect Immun. 1992 Mar;60(3):1249–1253. doi: 10.1128/iai.60.3.1249-1253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Davey M. P., Remick D. G., Kunkel S. L. Release of interleukin-1 by peripheral blood mononuclear cells in patients with tuberculosis and active inflammation. Infect Immun. 1986 Apr;52(1):341–343. doi: 10.1128/iai.52.1.341-343.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge L. E., Kenney J. S., Jones M. L., Warren J. S., Remick D. G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS- and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol. 1992 Apr 1;148(7):2133–2141. [PubMed] [Google Scholar]
- Elliott A. M., Luo N., Tembo G., Halwiindi B., Steenbergen G., Machiels L., Pobee J., Nunn P., Hayes R. J., McAdam K. P. Impact of HIV on tuberculosis in Zambia: a cross sectional study. BMJ. 1990 Sep 1;301(6749):412–415. doi: 10.1136/bmj.301.6749.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Friedland J. S., Remick D. G., Shattock R., Griffin G. E. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur J Immunol. 1992 Jun;22(6):1373–1378. doi: 10.1002/eji.1830220607. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Lawley T. J., Crystal R. G. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1981 Jul;68(1):259–269. doi: 10.1172/JCI110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Garrett K. C., Richerson H. B., Fantone J. C., Ward P. A., Rennard S. I., Bitterman P. B., Crystal R. G. Pathogenesis of the granulomatous lung diseases. Am Rev Respir Dis. 1984 Sep;130(3):476–496. doi: 10.1164/arrd.1984.130.3.476. [DOI] [PubMed] [Google Scholar]
- Jones G. S., Amirault H. J., Andersen B. R. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990 Sep;162(3):700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Appella E., Oppenheim J. J., Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989 Mar 17;243(4897):1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A., Yoshimura T., Noer K., Kutvirt S., Van Epps D. Leukocyte specificity and binding of human neutrophil attractant/activation protein-1. J Immunol. 1990 Feb 15;144(4):1323–1330. [PubMed] [Google Scholar]
- Lipschik G. Y., Doerfler M. E., Kovacs J. A., Travis W. D., Andrawis V. A., Lawrence M. G., Dichter J. R., Ognibene F. P., Shelhamer J. H. Leukotriene B4 and interleukin-8 in human immunodeficiency virus-related pulmonary disease. Chest. 1993 Sep;104(3):763–769. doi: 10.1378/chest.104.3.763. [DOI] [PubMed] [Google Scholar]
- Lynch J. P., 3rd, Standiford T. J., Rolfe M. W., Kunkel S. L., Strieter R. M. Neutrophilic alveolitis in idiopathic pulmonary fibrosis. The role of interleukin-8. Am Rev Respir Dis. 1992 Jun;145(6):1433–1439. doi: 10.1164/ajrccm/145.6.1433. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Baldwin E. T., Mukaida N. Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines. Chem Immunol. 1992;51:236–265. [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989 Nov;1(1):2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- McCain R. W., Holden E. P., Blackwell T. R., Christman J. W. Leukotriene B4 stimulates human polymorphonuclear leukocytes to synthesize and release interleukin-8 in vitro. Am J Respir Cell Mol Biol. 1994 Jun;10(6):651–657. doi: 10.1165/ajrcmb.10.6.8003341. [DOI] [PubMed] [Google Scholar]
- Moreno C., Taverne J., Mehlert A., Bate C. A., Brealey R. J., Meager A., Rook G. A., Playfair J. H. Lipoarabinomannan from Mycobacterium tuberculosis induces the production of tumour necrosis factor from human and murine macrophages. Clin Exp Immunol. 1989 May;76(2):240–245. [PMC free article] [PubMed] [Google Scholar]
- Moser B., Barella L., Mattei S., Schumacher C., Boulay F., Colombo M. P., Baggiolini M. Expression of transcripts for two interleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J. 1993 Aug 15;294(Pt 1):285–292. doi: 10.1042/bj2940285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville K., Bromberg A., Bromberg R., Bonk S., Hanna B. A., Rom W. N. The third epidemic--multidrug-resistant tuberculosis. Chest. 1994 Jan;105(1):45–48. doi: 10.1378/chest.105.1.45. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Prinzis S., Chatterjee D., Brennan P. J. Structure and antigenicity of lipoarabinomannan from Mycobacterium bovis BCG. J Gen Microbiol. 1993 Nov;139(11):2649–2658. doi: 10.1099/00221287-139-11-2649. [DOI] [PubMed] [Google Scholar]
- Reibman J., Meixler S., Lee T. C., Gold L. I., Cronstein B. N., Haines K. A., Kolasinski S. L., Weissmann G. Transforming growth factor beta 1, a potent chemoattractant for human neutrophils, bypasses classic signal-transduction pathways. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6805–6809. doi: 10.1073/pnas.88.15.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom W. N., Zhang Y. The rising tide of tuberculosis and the human host response to Mycobacterium tuberculosis. J Lab Clin Med. 1993 Jun;121(6):737–741. [PubMed] [Google Scholar]
- Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983 May 6;220(4597):568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Chensue S. W., Basha M. A., Standiford T. J., Lynch J. P., Baggiolini M., Kunkel S. L. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1990 Apr;2(4):321–326. doi: 10.1165/ajrcmb/2.4.321. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Takashima T., Ueta C., Tsuyuguchi I., Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990 Oct;58(10):3286–3292. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Amir-Tahmasseb M., Ellner J. J. Induction of interleukin 1 and tumor necrosis factor by mycobacterial proteins: the monocyte western blot. Proc Natl Acad Sci U S A. 1990 May;87(9):3348–3352. doi: 10.1073/pnas.87.9.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis R. S., Fujiwara H., Ellner J. J. Direct stimulation of monocyte release of interleukin 1 by mycobacterial protein antigens. J Immunol. 1986 Jan;136(1):193–196. [PubMed] [Google Scholar]
- Weiland J. E., Davis W. B., Holter J. F., Mohammed J. R., Dorinsky P. M., Gadek J. E. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986 Feb;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C., Newman I. Identification of IL-8 as a locomotor attractant for activated human lymphocytes in mononuclear cell cultures with anti-CD3 or purified protein derivative of Mycobacterium tuberculosis. J Immunol. 1992 Oct 15;149(8):2689–2694. [PubMed] [Google Scholar]
- Wolpe S. D., Cerami A. Macrophage inflammatory proteins 1 and 2: members of a novel superfamily of cytokines. FASEB J. 1989 Dec;3(14):2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Doerfler M., Lee T. C., Guillemin B., Rom W. N. Mechanisms of stimulation of interleukin-1 beta and tumor necrosis factor-alpha by Mycobacterium tuberculosis components. J Clin Invest. 1993 May;91(5):2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lathigra R., Garbe T., Catty D., Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991 Feb;5(2):381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Rom W. N. Regulation of the interleukin-1 beta (IL-1 beta) gene by mycobacterial components and lipopolysaccharide is mediated by two nuclear factor-IL6 motifs. Mol Cell Biol. 1993 Jun;13(6):3831–3837. doi: 10.1128/mcb.13.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]