Abstract
Idiosyncratic adverse drug reactions (IADRs) occur in a minority of patients yet account for the majority of postmarketing use restrictions by the Food and Drug Administration. Despite the impact of these toxicities, the underlying mechanisms are still poorly understood. Animal models of IADRs would be beneficial in understanding mechanisms and in developing assays with predictive potential. Recent work exploring the interactions between inflammatory stress and drugs associated with human idiosyncratic drug-induced liver injury (IDILI) has led to the development of the first animal models that apply to a range of drugs. Here, we discuss hypotheses for the mechanisms of IDILI and focus on a murine model of trovafloxacin-induced hepatotoxicity as an example related to the inflammatory stress hypothesis.
Keywords: idiosyncratic, adverse drug reaction, trovafloxacin, hepatotoxicity, inflammation
Adverse Drug Reactions
Adverse drug reactions (ADRs) are a serious problem not only for public health but also for pharmaceutical companies and regulatory agencies. In a study in the United Kingdom, ADRs accounted for more than 6% of hospital admissions. Of these, the mortality rate was 2% (Pirmohamed et al., 2004). In addition to the risk to public health, these reactions are a major issue in drug development. A significant amount of time and money is expended in the effort to predict the risk of adverse reactions from drug candidates in order to eliminate candidates likely to cause these reactions before they reach the market. Despite comprehensive preclinical drug testing and clinical trials, over 10% of drugs approved during 1975–2000 were either withdrawn from the market or were restricted in use (Lasser et al., 2002). In 1998, the pharmaceutical industry spent over 20 billion dollars on drug discovery and development, with screening assays and toxicity testing accounting for about 20% of the total amount spent (Michelson and Joho, 2000). Despite such extensive efforts, in 1999, 258,000 adverse drug events were reported in the United States and over 440,000 worldwide (Sibbald, 2001), suggesting that this is a major and persistent problem.
ADRs can affect various tissues, but the liver is often a target organ. Of the 28 drugs removed from the U.S. market between 1976 and 2005, 6 were withdrawn due to hepatotoxicity (Kaitin, 2005). Drug-induced liver injury (DILI) accounts for more than 50% of acute liver failure cases (Ostapowicz et al., 2002). It is associated with significant mortality, and consequently, a number of drugs that have been associated with DILI have been removed from the market. For example, bromfenac (Hunter et al., 1999), troglitazone (Graham et al., 2003; Murphy et al., 2000), and tienilic acid (Bernuau et al., 1981) have been completely removed due to hepatotoxicity. In addition, DILI induced by drugs such as trovafloxacin (TVX) (Lazarczyk et al., 2001; Nicholson et al., 2002), nefazodone (Choi, 2003), and nevirapine (Maniar et al., 2006; Sanne et al., 2005) has led to “black box” warnings, limiting their use. DILI is the leading cause for the withdrawal of drugs from the market by either the U.S. Food and Drug Administration (FDA) or pharmaceutical companies (Temple and Himmel, 2002).
Idiosyncratic Adverse Drug Reactions
An important subset of ADRs that cause DILI are idiosyncratic adverse drug reactions (IADRs), which account for about 13% of all cases of acute liver failure (Ostapowicz et al., 2002). Although IADRs are a relatively small subset of ADRs, the majority of postmarketing restrictions by the FDA on use of drugs has been due to IADRs (Lasser et al., 2002). Idiosyncratic drug-induced liver injury (IDILI) often occurs in a small fraction (< 1%) of patients during drug therapy. The mechanisms underlying IDILI are unknown, but they typically do not involve the pharmacological actions of the drug. In addition, IDILI occurs at doses that are generally safe to the majority of patients and with an inconsistent temporal relationship to exposure. That is, some patients may develop toxicities soon after the onset of drug administration, whereas reactions in others may occur after a month or more on maintenance therapy. Finally, there is a wide range in the severity of the reactions depending on the drug and the affected individual. The implication drawn from these characteristics is that occurrence of an IADR is governed by susceptibility factor(s) within individuals. These factors may be either genetic or environmental or both.
The development of animal models is needed to predict those drugs that cause IADRs in order to halt their development and/or understand which individuals are likely to be susceptible. Animal models could provide mechanistic insight about IADR-causing drugs still in use and thereby help clinicians prevent and/or treat the reactions. Additionally, the insights provided by animal models of IADRs could lead to the development of high throughput, in vitro predictive tests based on the elucidated mechanisms of action. Such preclinical tests could help pharmaceutical companies prevent the sending of candidates with a high IADR potential to clinical trials or to market, thereby averting human suffering and saving money spent on clinical trials, marketing, and potential lawsuits from patients affected by IADRs.
Despite extensive research, few animal models exist that produce liver injury from IDILI-associated drugs. The inability of current animal tests to predict IDILI is likely due, in part, to the reaction being idiosyncratic and usually rare in animals, as it is in humans; thus, an extremely large number of animals would be needed to detect toxicity. It has been estimated that 30,000 patients are required to detect an IADR that occurs in 1 in 10,000 patients (Lee, 2003). Assuming that similar incidence occurs in animals and that animals respond like humans, toxicity testing would require 30,000 animals for a single drug to predict such IDILI confidently. In addition, the current animal testing paradigms might not include sufficient biological (e.g., genetic and environmental) diversity to elucidate IDILI responses. Because such large studies are not possible for drug candidates, it is critical that the modes and mechanisms of action of IDILI become better understood to develop predictive models.
Exploration of the pathogenesis of IDILI in humans is limited by the often infrequent occurrence of the reactions, the fact that patients are typically seen only after the injury is well established, and the difficulty in obtaining tissue for study. The obstacle of human tissue availability is currently being addressed by the National Institutes of Health-supported DILI Network. However, the study of human liver tissue taken after injury will likely provide only limited insight about early pathogenic mechanisms. For this insight to occur, animal models will be needed that are based on the modes of action of IDILI. As noted above, these modes of action are incompletely understood, but several reasonable hypotheses have arisen in recent years. In this review, we will summarize briefly and provide perspectives on the major hypotheses for modes of action of IDILI. Additionally, we will describe recent results with TVX as an example of an animal model based on the inflammatory stress hypothesis.
MODES OF ACTION OF IDILI
Despite the limitations and difficulties described above, some progress has been made in understanding IDILI. This has led to the development of several theories about IDILI pathogenesis. It is important to emphasize that to date none of the hypotheses to explain IDILI pathogenesis has been proven or disproven. Indeed, it is possible that various combinations of modes of action may be involved, even with IDILI from a single drug.
Reactive Intermediate Hypothesis
One theory to explain IADRs is that a drug is metabolized into a reactive metabolite that binds covalently to important cellular proteins, damages membrane integrity, and/or alters calcium homeostasis or other intracellular signaling in ways that lead to cell death (Kaplowitz et al., 1986). Susceptible individuals might have polymorphisms in the bioactivating enzyme(s), and this could explain why IDILI is often quite rare. There are many examples in which a drug linked with IDILI has the ability to form a metabolite that is reactive (Walgren et al., 2005). One such example is troglitazone (TGZ), an antidiabetic drug linked with serious idiosyncratic hepatotoxicity (Murphy et al., 2000). TGZ is metabolized in the rat to five intermediates with the ability to form glutathione conjugates that appear in bile (Kassahun et al., 2001). Metabolic activation of TGZ by cytochrome P450 3A4 (CYP3A4) results in reactive metabolites capable of binding to proteins and other nucleophiles (He et al., 2004). It is important to note that TGZ reactive metabolites have not been causally linked to liver injury. Moreover, numerous drugs that form reactive metabolites are not associated with an increased risk of IDILI (Uetrecht, 2006). Indeed, formation of a reactive metabolite may be a necessary but insufficient condition for liver pathogenesis for many drugs that cause IDILI. Thus, although the reactive intermediate hypothesis is a reasonable one, a causal link between reactive metabolite generation and hepatotoxicity has not been established conclusively for many drugs that cause IDILI.
Genetic Polymorphism Hypothesis
A related hypothesis is that genetic polymorphisms cause differences in the toxic responses to drugs. Polymorphisms in drug-metabolizing enzymes can lead to differences among individuals in pharmacokinetics as well as in reactive intermediate formation (Williams and Park, 2003). Human polymorphisms in genes encoding CYP drug-metabolizing enzymes have been identified. Such polymorphisms could result in potentially toxic plasma concentrations of the drug. Alternatively, a polymorphism in a gene encoding a cytoprotective factor might render individuals susceptible to therapeutic doses of drugs, resulting in IDILI. For example, a genetic polymorphism in an anti-inflammatory cytokine or antiapoptotic protein could alter the toxicity threshold in individuals for some drugs, thereby contributing to IDILI. For the genetic polymorphism hypothesis to explain IDILI, the polymorphism in people on drug therapy would have to be as rare as the IADR itself or the IADR would have to result from a combination of several more common polymorphisms. The genetic polymorphism hypothesis does provide some explanation for the variability in the timing of onset of IADRs: if individuals with different polymorphisms experienced different rates of metabolic activation from the same drug dosage regimen, then a critical threshold needed for toxicity would be reached by some individuals sooner than others.
An example often referenced in support of the polymorphism hypothesis is hepatotoxicity caused by isoniazid, a drug used in the prevention and treatment of tuberculosis (Mitchell et al., 1976). It was hypothesized that a polymorphism creating a rapid acetylator phenotype produced more of a reactive metabolite capable of causing hepatocellular necrosis in susceptible individuals (Huang, 2007; Mitchell et al., 1976). However, several epidemiological studies failed to find an association between the rapid acetylation polymorphism and liver injury (Gurumurthy et al., 1984). Polymorphisms in other genes may be important as well. For example, human leukocyte antigen polymorphisms have been linked to IDILI from some drugs (Andrade et al., 2004; Grattagliano et al., 2009). Thus, an association between IDILI and genetic polymorphisms exists for some drugs; however, the association of a specific polymorphism with an IADR to a drug does not by itself constitute proof of cause and effect. Also, whether a polymorphic phenotype interacting with drug exposure is sufficient to precipitate an IDILI reaction or whether other interacting factors are required remains to be elucidated.
Hapten Hypothesis
A widely accepted theory to explain IADRs is that they result from an adaptive immune response to an altered self-protein. Clinical characteristics of some IDILI reactions, such as the delayed onset of toxicity, an obscure relationship to drug dose, and the occurrence of eosinophilia and accompanying skin rash, have led to the hypothesis that IDILI reactions are mediated by an adaptive immune response (Uetrecht, 2003). The hapten hypothesis states that a chemically reactive drug or a reactive metabolite binds to an endogenous protein. This protein adduct is then seen as a foreign antigen that initiates immunological recognition (Park et al., 1987). According to this hypothesis, the drug-modified protein must be recognized by B cells or processed by antigen-presenting cells and presented to T cells. This results in the expansion of effector T cells and B cells reactive to the adducted protein. The immune system then recognizes the adduct as a foreign antigen, and upon prolonged or subsequent exposure to the drug, robust antigen-specific cell expansion occurs, resulting in the formation of autoantibodies by memory B cells and/or the activation of cytotoxic T cells targeting adducted proteins and initiating a reaction that culminates in tissue injury (Robin et al., 1997).
In support of the hapten hypothesis, the presence of autoantibodies has been detected in patients with IDILI after exposure to several drugs, including diclofenac, troglitazone, halothane, and tienilic acid (Aithal et al., 2004; Obermayer-Straub et al., 2000). In further support of this hypothesis, repeated exposure to halothane is a risk factor for the development of halothane-induced liver injury (Bird and Williams, 1989). However, a recent study found that 39% of patients who developed halothane hepatitis were not previously exposed to halothane (Eghtesadi-Araghi et al., 2008), raising doubt about whether sensitization and challenge exposures are needed to precipitate severe halothane hepatotoxicity. Additionally, autoantibodies were also found in patients treated with diclofenac or halothane who did not develop hepatotoxicity (Aithal et al., 2004; Kitteringham et al., 1995). Thus, from case reports, firm evidence for a cause-and-effect relationship between autoantibodies and IDILI is lacking. Efforts have been undertaken in animal experiments to show the involvement of the specific immune system in IDILI; however, in all the current animal models of drug immunogenicity, an adaptive immune response was detected in the absence of significant liver damage (Park et al., 1998). Accordingly, experimental support for this hypothesis is incomplete, and an animal model of liver injury that requires drug sensitization and challenge and the involvement of antibodies or sensitized T cells (i.e., an adaptive immune response) has not emerged so far.
The Danger Hypothesis
A theory closely related to the hapten hypothesis is the danger hypothesis, which proposes that a damaging immune system activation occurs only when the drug or its metabolite binds to a protein and causes a stress response or in the presence of a concurrent independent stressor, resulting in a “danger signal” (Naisbitt et al., 2000; Uetrecht, 1999). Thus, according to the danger hypothesis, the formation of a drug-protein adduct is insufficient to cause injury and a secondary signal is needed that acts as an adjuvant during initial immunization. This signal could be mild cell death or cytokine release, which triggers the production of effector lymphocytes necessary for damaging adaptive immune system activation upon subsequent drug exposure (Kaplowitz, 2005). It has been postulated that some reactive drug metabolites themselves could cause this danger signal, leading to IDILI (Seguin and Uetrecht, 2003; Uetrecht, 1999). However, the danger signal could result from a number of independent factors including intestinal microbial disturbances or an infection causing an innate immune response resulting in inflammatory stress.
Mitochondrial Dysfunction Hypothesis
Another hypothesis to explain IDILI is that drug-induced mitochondrial toxicity or drug interaction with compromised mitochondria is an underlying cause. Differences in rates of accumulation of dysfunctional mitochondria among individuals on drug therapy could explain variations in sensitivity and in times to onset of overt toxicity (Boelsterli and Lim, 2007). Mitochondrial dysfunction can encompass several changes such as decreased adenosine triphosphate production, mitochondrial reactive oxygen species (ROS) production, and/or dissipation of the mitochondrial membrane potential. Either an exogenously imposed mitochondrial disease or a polymorphism could alter mitochondrial function and render cells sensitive to a drug, resulting in idiosyncratic toxicity (Waring and Anderson, 2005).
There is extensive evidence linking some IDILI-associated drugs with mitochondrial alterations. Troglitazone, tolcapone, diclofenac, valproic acid, and isoniazid are some of the drugs that cause IDILI and that also cause mitochondrial dysfunction in hepatocytes in vitro (Boelsterli and Lim, 2007; Haasio et al., 2002; Keller et al., 1992; Petrescu and Tarba, 1997; Schwab and Tuschl, 2003; Shishido et al., 2003). In addition, diclofenac and troglitazone are cytotoxic to HepG2 cells (a human-derived cell line) through a mitochondria-dependent mechanism (Boelsterli, 2003; Tirmenstein et al., 2002). However, there are several drugs that cause mitochondrial alterations in vitro but have not resulted in ADRs in people. In one study, superoxide dismutase 2 (SOD2) heterozygous mice, a model of silent mitochondrial abnormality, were treated chronically (4 weeks) with troglitazone. This treatment had no effect on wild-type mice but resulted in mild hepatocellular necrosis in SOD2+/− mice (Ong et al., 2007). It is important to note that mitochondrial dysfunction can be induced by a number of independent factors such as concurrent xenobiotic exposure, hypoxia, or inflammation. Accordingly, it is possible that alterations in mitochondrial function play a role in other hypothesized modes of action of IDILI.
Failure-to-Adapt Hypothesis
According to this hypothesis, a relatively large fraction of people develop minor and reversible liver toxicity in response to a drug. Most of these individuals “adapt,” experiencing resolution of liver injury even with continued exposure to the drug. It is proposed that a small fraction of these people fail to adapt, and the injury then progresses to overt toxicity that appears as an idiosyncratic reaction (Watkins, 2005). Reports of isoniazid hepatotoxicity seem to support this theory, inasmuch as 15% of patients taking isoniazid experience minor alanine aminotransferase (ALT) elevations, but less than 1% develop symptomatic hepatitis with continued treatment (Black et al., 1975). Similarly, 20% of patients anesthetized with halothane develop mild, reversible hepatotoxicity and about 1 in 20,000 progresses to potentially fatal halothane hepatitis (Mushin et al., 1971).
The mechanisms underlying the adaptation phenomenon are unknown. The inability to adapt could involve either environmental or genetic factors or both. This hypothesis could fit with the genetic polymorphism hypothesis, in that a polymorphism in an anti-inflammatory cytokine or antiapoptotic protein might render certain individuals unable to adapt and control minor tissue damage. Alternatively, a liver with limited capacity for replicative repair might not be able to adapt to a drug-induced insult, resulting in otherwise minor and reversible hepatotoxicity progressing to serious injury.
Adaptation may not be recognized in clinical trials because drug treatment is usually stopped when the serum ALT activity rises to eight times the upper limit of normal or greater than three times the upper limit of normal when there is a concurrent elevation in bilirubin, making it impossible to distinguish between patients who would and would not adapt with continued drug exposure. In addition, there are currently no animal models in which adaptation has been studied with drugs that cause IDILI in humans. It is also important to note that the failure-to-adapt hypothesis does not discount other IDILI hypotheses, insofar as toxicity may be due to any number of mechanisms to which certain individuals cannot adapt and therefore experience an IDILI.
Multiple Determinant Hypothesis
The multiple determinant hypothesis proposes that idiosyncratic reactions are the result of several discrete but required factors or processes all occurring simultaneously (Li, 2002). Each factor has an independent probability of occurring, but all of them are required to precipitate an IADR. The probability of occurrence is then related to the product of the individual probabilities, thus accounting for the rare occurrence of many IADRs. According to this hypothesis, an idiosyncratic reaction would only occur in an individual if all the critical factors occur within an appropriate and limited timeframe.
The multiple determinant hypothesis is a rather general and encompassing one that is consistent with most of the other hypotheses mentioned above. Inasmuch as environmental and genetic factors as well as physical-chemical properties of the drug itself might influence the probability that a specific drug will cause a hepatotoxic response, it is important to determine which factors are critical to toxicity and why. Moreover, understanding in more detail the mechanistic aspects of IDILI is critical to developing predictive animal models. The hypothesis embraces the concept that underlying factors have the potential to lower the toxicity threshold to a drug, rendering a normally therapeutic dose toxic. Several factors have the potential to affect the susceptibility of an individual to drug toxicity, including age, gender, coexposure to other pharmacological agents, drug metabolism differences, state of health, and various homeostasis-altering stresses (Aldridge et al., 2003; Amacher, 2006; Cunningham, 1997; Rademaker, 2001; Wilke et al., 2007).
Inflammatory Stress Hypothesis
In the multiple determinant hypothesis, one environmental factor that might render an individual sensitive to a normally nontoxic drug administration is inflammatory stress. The observation that a mild inflammatory episode can alter hepatotoxicity has led to the inflammatory stress hypothesis, which states that an acute episode of inflammation has the potential to interact with concurrent drug therapy to precipitate IDILI (Roth et al., 2003).
A desirable quality of a drug is a large difference between the therapeutic dose and the smallest toxic dose (i.e., the therapeutic window). The hypothesis is that a modest inflammatory stress can decrease the threshold for hepatotoxicity, thereby shrinking the therapeutic window and resulting in a toxic response at an otherwise safe and pharmacologically effective dose of the drug. For most drugs that cause IDILI, doses normally needed to injure the liver may exceed those that result in death, thus a hepatotoxic reaction would not be observed, but an inflammatory stress could increase sensitivity of the liver so that hepatotoxicity occurs at doses in the therapeutic range (Roth and Ganey, 2010). Conversely, a drug could magnify an otherwise innocuous inflammatory episode, rendering it injurious. In either case, IDILI would occur at a drug dose that is nontoxic to individuals not experiencing a concurrent inflammatory stress. Inflammatory episodes are commonplace in people and occur erratically throughout life, which can explain the unpredictable nature of IADRs.
Inflammatory stress can be induced by a number of mechanisms including infection, intestinal microbial disturbances, cell death, etc. (Ganey et al., 2004). Lipopolysaccharide (LPS), a component of gram-negative bacterial cell walls, is one agent that can induce an inflammatory stress in vivo. The first animal models of idiosyncratic hepatotoxicity have been developed in which nontoxic doses of IDILI-causing drugs are rendered hepatotoxic upon coexposure to a nontoxic but modestly inflammatory dose of LPS. Several drugs known to cause IDILI in humans, such as TVX, ranitidine, sulindac, chlorpromazine, halothane, amiodarone, and diclofenac, have been evaluated, and all were hepatotoxic to rodents when coupled with a nontoxic dose of an inflammagen (Buchweitz et al., 2002; Cheng et al., 2009; Deng et al., 2006; Luyendyk et al., 2003; Waring et al., 2006; Zou et al., 2009). Drugs in the same pharmacologic class that were not associated with IADRs in humans were used when available, and these did not interact with inflammatory stress to cause hepatotoxicity in animal models (Luyendyk et al., 2003; Waring et al., 2006). The selectivity of this model to identify IDILI-causing drugs suggested a potential role for inflammatory stress in the mechanism of human IDILI.
INVOLVEMENT OF INFLAMMATORY STRESS IN TVX-INDUCED IDILI
A challenge still lies in understanding mechanisms of the hepatotoxicity observed with coexposure to LPS and an IDILI-causing drug. To explore these mechanisms, TVX and its interactions with inflammatory mediators have been explored recently in animal models, and the results of these studies will be summarized in this section.
Overview of TVX
TVX is an example of a drug linked to human IDILI that was not predicted by preclinical testing or evident in clinical trials. It is a broad-spectrum fluoroquinolone antibiotic with excellent Gram-negative activity and a greater spectrum of bactericidal activity against gram-positive pathogens compared with other fluoroquinolones. In addition to its increased bactericidal spectrum, TVX has high bioavailability after oral dosing and a relatively long elimination half-life, allowing for once-daily dosing and rendering it a drug with distinct therapeutic advantage (Fischman et al., 1997).
The bactericidal activity of TVX is apparently exerted by the parent compound, not by a metabolite. Approximately 50% of a dose of TVX in humans is recovered unchanged in the feces (43%) and urine (6%). For the portion of TVX that is metabolized, phase II conjugation, specifically glucuronidation, plays a major role (Dalvie et al., 1997). Metabolic studies with TVX did not reveal a reactive intermediate generated during its metabolism. However, a recent study using a cyclopropylamine-containing surrogate molecule for TVX suggested that TVX might be metabolized to a reactive α,β-unstaurated aldehyde (Sun et al., 2007). The reactive intermediate was only found in systems in vitro with active Cyp1A2 or myeloperoxidase (MPO). MPO is released by activated neutrophils (polymorphonuclear leukocytes [PMNs]), suggesting that this surrogate molecule and perhaps TVX itself might be metabolized differently in a proinflammatory environment to a reactive species that could contribute to TVX toxicity.
TVX-Induced Hepatotoxicity in Human Patients
TVX was released in February 1998, as a potent new fluoroquinolone antibiotic. In 1999, reports surfaced linking TVX with hepatotoxicity, which led to severe restriction of TVX use and prescription. Before restrictions were placed on TVX usage, ∼2.5 million prescriptions were filled. A total of 140 severe hepatic reactions were reported, making the incidence of TVX-induced severe hepatotoxicity ∼1 in 18,000 prescriptions. Of these 140 severe hepatic reactions, 14 resulted in liver failure, i.e., ∼1 in 178,000 prescriptions (Stahlmann, 2002). The hepatotoxic events linked with TVX were equal in males and females and were seen in individuals ranging in age from 21 to 91 years old. Examination of the case reports revealed that the duration of TVX therapy in patients did not correlate with a toxic response. This clinical profile classified TVX hepatotoxicity as idiosyncratic.
TVX was the first fluoroquinolone to have its use severely restricted because of liver toxicity, and the majority of drugs in this class has not been associated with IDILI (De and De, 2001). Therefore, it was concluded that TVX IDILI arises independently of the pharmacologic properties of the drug. In retrospect, the hepatotoxic potential of TVX was seen in dogs at doses exceeding therapeutic doses (Stahlmann, 2002). Some dogs developed elevated liver enzyme signals and centrilobular necrosis. However, because the toxicity was species-specific, reversible, and inconsistent in animal studies and was not seen in clinical trials, it was not considered relevant for patients treated with therapeutic doses or a reason to halt development of the drug.
The pathology of TVX IDILI in human liver was similar in several reported cases. Viral, metabolic, and autoimmune causes of hepatitis were excluded (Chen et al., 2000; Lazarczyk et al., 2001; Lucena et al., 2000). Liver biopsy revealed centrilobular hepatocellular necrosis with collapsed sinusoids around the central vein, whereas the portal tracts appeared normal. In addition, parenchyma-based lymphocytes, plasma cells, and eosinophils were present, and these inflammatory cells were highly concentrated at the peripheral edges of the centrilobular necrosis (Chen et al., 2000; Lazarczyk et al., 2001; Lucena et al., 2000).
Interactions between TVX and Inflammatory Stress in Animal Models
TVX did not cause liver damage in rodents, even after large doses (e.g., 1 g/kg in mice) (Shaw et al., 2007). However, in both rats and mice, a normally nontoxic dose of TVX synergized with an inflammatory stress induced by LPS to cause acute liver injury (Shaw et al., 2007; Waring et al., 2006). TVX/LPS-treated mice developed lesions of hepatocellular oncotic necrosis and apoptosis, which were primarily located in midzonal regions with some centrilobular involvement. The reason for the difference in the localization of lesions primarily to midzonal regions in mice compared with centrilobular regions in human clinical toxicities is unknown but could be due to species differences in the location of metabolizing enzymes, inflammatory cells, Toll-like receptor (TLR) expression, or other factors. Nevertheless, the hepatotoxic interaction between TVX and inflammatory stress was not specific to LPS-induced activation of TLR4, inasmuch as coexposure of mice to TVX and gram-positive bacterial cell wall components (TLR2 agonists) also induced liver injury (Shaw et al., 2009d). Levofloxacin, a fluoroquinolone that is not associated with idiosyncratic hepatotoxicity in human patients, failed to synergize with LPS to cause liver injury (Shaw et al., 2007). The synergistic hepatotoxic interactions between TVX and bacterial cell wall components were the first models in vivo to reveal consistently the potential of TVX to cause severe liver damage. Additionally, the underlying interaction between TVX and inflammatory stress that precipitates liver injury is neither species-specific nor TLR4-specific. These observations are important, inasmuch as they suggest that TVX interacts with a common mediator of inflammatory stress and that this interaction translates across species, possibly even to humans.
TVX/LPS coexposure induced a unique hepatic gene expression profile at a time before the onset of liver injury (Shaw et al., 2009b). This suggests that global gene expression in the liver could be an early biomarker of hepatotoxicity in mice, a finding that might also hold true in humans. A number of the genes selectively changed by TVX/LPS treatment were downregulated; however, several genes involved in the JAK/STAT and interferon (IFN) signaling pathways were selectively increased (Shaw et al., 2009b). In accordance with this finding, both interleukin (IL)-18−/− (a cytokine that stimulates IFNγ production) and IFNγ−/− mice were protected from TVX/LPS-induced liver injury. This was the first drug/inflammation model of liver injury in which the role of IFNγ was shown to be involved. Additionally, the study supported the use of early gene expression analysis to identify a mechanistic pathway involved in the liver injury.
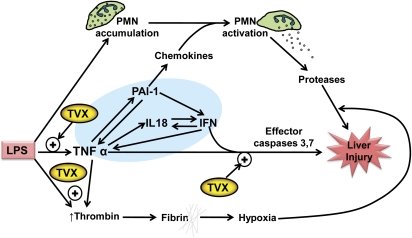
Inflammatory stress is a general response that results in cytokine production, leukocyte recruitment, hemostatic system activation and complement activation. Subsequent studies explored the roles of specific inflammatory mediators involved in TVX/LPS-induced liver injury in mice, summarized below and in Figure 1. PMN recruitment and activation is a common event in acute inflammation. PMN accumulation into liver in response to LPS was not enhanced by TVX, but liver injury caused by either TVX/LPS or TVX/peptidoglycan-lipoteichoic acid coexposure was dependent on PMN activation (Shaw et al., 2009d). Moreover, PMN elastase–deficient mice were protected from TVX/LPS-induced hepatocellular injury, suggesting that toxic proteases released upon PMN activation contributed to the liver damage (Fig. 1). Although liver biopsies from patients suffering from TVX hepatotoxicity did not reveal a neutrophilic infiltrate, the biopsies were not taken until the liver injury was well developed (Chen et al., 2000; Lazarczyk et al., 2001; Lucena et al., 2000). Therefore, it is possible that neutrophils are involved in the initial injury in humans, and the secondary wave of infiltrating cells seen in biopsies reflects the response to this injury.
FIG. 1.
Multiple mechanisms in an TVX/LPS murine model of IDILI. Treatment of mice with LPS results in PMN accumulation in liver, thrombin generation, and TNF production that are benign. TVX enhances the appearance of TNF by increasing its biosynthesis and slowing its elimination. TNF activates a series of events that include increasing the production of other cytokines, such as IL-18 and PAI-1. These cytokines increase IFN production, and a vicious cycle is established in which IFN and PAI-1 further enhance TNF production (shaded area). IFN can synergize with TNF to cause caspase-dependent hepatocyte injury in vitro. TVX can enhance this TNF-mediated cytotoxicity. The increase in TNF also acts by indirect mechanisms to promote hepatocellular injury in vivo. Perhaps through secondary production of chemokines, TNF causes activation of PMNs, which release toxic proteases that contribute to hepatocellular injury. TVX also enhances production of thrombin, which results in the deposition of insoluble fibrin clots that are associated with liver hypoxia. One way in which hypoxia can contribute to hepatocellular necrosis is by increasing the killing activity of proteases released by activated PMNs. This working hypothesis is based on results described in publications cited in the text.
Inflammatory stress can also induce alterations in the hemostatic system, which can play a role in liver injury (Ganey et al., 2007). The hemostatic system exists as a balance between the opposing effects of coagulation and fibrinolysis. This balance can be altered clinically by TVX and, therefore, might be a contributor to TVX-induced hepatotoxicity in humans (Goel et al., 1999). In mice, TVX/LPS cotreatment resulted in the early deposition of fibrin in liver sinusoids, and liver injury induced by TVX/LPS depended on the activation of thrombin, an activator of coagulation (Shaw et al., 2009c) (Fig. 1). Additionally, plasminogen activator inhibitor-1 (PAI-1), which inhibits fibrinolysis, was selectively increased in plasma after TVX/LPS coexposure, creating a procoagulant environment (Shaw et al., 2009c). Inhibition of either coagulation system activation or PAI-1 protected mice from TVX/LPS-induced liver injury (Shaw et al., 2009c).
Surprisingly, PAI-1 inhibition did not protect mice via reducing fibrin deposition in the liver; rather, it reduced inflammatory cytokine production (Shaw et al., 2009c). The observation that PAI-1 contributed to acute liver injury through regulation of proinflammatory cytokines was novel. A central upstream player in TVX/LPS hepatotoxicity proved to be tumor necrosis factor (TNF) -alpha (Fig. 1). This proinflammatory cytokine is a 17-kD protein that arises from a cell-associated pro-form that is cleaved by TNF-converting enzyme, giving rise to active TNF. The nonhepatotoxic dose of LPS used in this model caused the rapid appearance of TNF in plasma, which peaked within 2 h of administration and declined rapidly thereafter (Shaw et al., 2009e). TVX pretreatment prolonged the LPS-induced increase in plasma concentration of TNF (Shaw et al., 2009a). Neutralization of TNF with etanercept afforded mice complete protection from TVX/LPS-induced liver injury (Shaw et al., 2009b). Furthermore, the TVX-induced prolongation of the LPS-induced increase in the plasma concentration of TNF was critical to the development of hepatotoxicity. TNF neutralization after the initial peak of TNF, but before the prolongation, reduced TVX/LPS-induced hepatocellular damage (Shaw et al., 2009e).
Further analysis revealed that TNF neutralization reduced thrombin activation as well as the appearance of IL-18, IFNγ, PAI-1, and several leukocyte chemokines (MIP-1alpha, KC, and MIP-2), indicating that TNF was a critical and proximal mediator in the cascade of events that resulted in hepatocellular necrosis (Shaw et al. 2007, 2009e) (Fig. 1). This was supported by the observation that replacing LPS injection with a nontoxic dose of recombinant murine TNF produced liver injury with similar hepatocellular lesions as TVX/LPS coexposure (Shaw et al. 2009a,e).
Based on the central and proximal role of TNF, subsequent studies explored interactions between TVX and TNF. Following its injection, recombinant TNF is rapidly cleared from plasma. The concentration of TNF in the plasma of TVX/TNF-treated mice was prolonged due partly (∼1/3) to increased TNF biosynthesis but mostly to a decrease in TNF clearance (Shaw et al., 2009a). By incompletely understood mechanisms, TNF receptors are shed from cells into plasma as soluble receptors (sTNFRs) that bind TNF (Bemelmans et al., 1994, 1996). The TNF-sTNFR complexes are then removed primarily by the kidney (Bemelmans et al., 1993). How TVX reduces TNF clearance is unknown: the concentrations of sTNFRs were not increased in the plasma of TVX/TNF-treated mice, and renal function was not impaired (Shaw et al., 2009e), suggesting that neither enhanced removal of TNF by sTNFRs nor reduced renal clearance of TNF-sTNFR complexes were responsible. Nevertheless, the ability of a drug to reduce the clearance of TNF is a novel finding which could be of importance if this interaction also occurs in humans and translates to other IDILI-associated drugs.
In addition to reducing TNF clearance, TVX cotreatment enhanced the plasma concentrations of proinflammatory cytokines induced by TNF. The TVX/TNF interaction responsible for this enhancement likely occurs in Kupffer cells and/or endothelial cells, but further study is needed to confirm this. As noted above, neutralization of TNF reduced the appearance of cytokines, such as IL-18, IFNγ, and PAI-1; conversely, inactivation or knockout of these cytokines reduced the appearance of TNF (Shaw et al., 2009c,d,e). This suggests that a dysregulated cycle of cytokine amplification contributes to the liver injury (Fig. 1). Additionally, interactions between TVX and TNF were found in hepatocytes: TVX increased the potency and efficacy of TNF to induce effector caspase activation and cell death in vitro (Shaw et al., 2009a). These findings highlight that there are multiple mechanisms that contribute to the hepatotoxic interaction between TVX and TNF (Fig. 1).
THE POTENTIAL ROLE OF INFLAMMATORY STRESS IN VARIOUS MODES OF ACTION OF IDILI
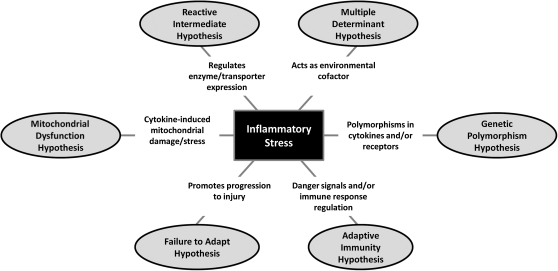
The role of inflammatory stress in human IADRs is unknown; however, substantial evidence for interactions between IDILI-causing drugs and inflammation has been reported in rodents (Table 1). The mounting evidence for these interactions suggests that inflammation plays a role in the idiosyncratic toxicities induced by some drugs. Nevertheless, it is important to understand that inflammatory stress is unlikely to be an exclusive mode of action. Rather, it is likely that various mechanisms, singly or in combination, are involved in idiosyncratic toxicities. Notably, the inflammatory stress hypothesis fits well with several of the other proposed hypotheses about modes of action involved in DILI as explained below and summarized in Figure 2.
TABLE 1.
Human IDILI-Associated Drugs for which Interaction with an Inflammagen Precipitates Liver Injury in Animals
| Drug | Pharmacologic class | Inflammagen | TLR activated | Reference |
| Diclofenac | NSAID | LPS | 4 | Deng et al. (2006) |
| Sulindac | NSAID | LPS | 4 | Zou et al. (2009) |
| Halothane | Volatile | Poly I:C | 3 | Cheng et al. (2009) |
| Anesthetic | LPS | 4 | Dugan et al. (2010) | |
| Chlorpromazine | Antipsychotic | LPS | 4 | Buchweitz et al. (2002) |
| TVX | Antibiotic | LPS | 4 | Waring et al. (2006); Shaw et al. (2007) |
| PGN/LTA | 2 | Shaw et al. (2009d) | ||
| TNF | — | Shaw et al. (2009e) | ||
| Ranitidine | H2 blocker | LPS | 4 | Luyendyk et al. (2003) |
| Amiodarone | Antiarrhythmic | LPS | 4 | Lu et al. (2010) |
Note. Drugs that cause IDILI in humans and that synergize with an inflammagen to precipitate liver injury in rodents are listed. Inflammagens: LPS (gram-negative bacterial stimulus); PGN/LTA, peptidoglycan/lipoteichoic acid (gram-positive bacterial stimulus); poly I:C, polyinosinic:polycytidylic acid (viral double-stranded RNA mimic). NSAID, nonsteroidal anti-inflammatory drug.
FIG. 2.
Links between inflammatory stress and several hypotheses for the pathogenesis of IDILI. According to the inflammatory stress hypothesis, IDILI-associated drugs interact with an otherwise benign episode of inflammation to precipitate liver injury. This hypothesis is consistent with several other proposed modes of action of IDILI. The figure depicts how inflammatory stress may link to these.
The roles of the innate and adaptive immune systems in IADRs are highly debated. The innate and adaptive branches of the immune system were once considered as two independent entities, but the two branches are highly interdependent (Iwasaki and Medzhitov, 2010; Sims and Smith, 2010). Furthermore, recent research has focused on the regulation of the adaptive immune responses by innate immunity (Iwasaki and Medzhitov, 2010). As an example, the innate immune cytokines IL-1β and IL-6 are involved in the development and expansion of Th17 cells (Kimura et al., 2007; Shainheit et al., 2008); conversely, Th17 cells are critical for PMN recruitment in some models (Huang et al., 2004). Animal models that provide evidence for the adaptive immune system involvement in liver injury from IDILI-associated drugs are lacking, but it is likely that even if an adaptive immune response occurs in response to a drug acting as a hapten, an inflammatory stress may be required for the expansion of effector lymphocytes or in providing the danger signal to overcome tolerance. Accordingly, inflammatory stress may be critical for an adaptive immune response to become damaging to the liver.
Regarding the mitochondrial dysfunction hypothesis, several cytokines such as TNF and IFNγ are known to cause mitochondrial stress in several cell types (Mariappan et al., 2009; Watanabe et al., 2003). Therefore, it is possible that inflammatory stress can cause minor and normally inconsequential mitochondrial production of ROS that renders individuals susceptible to idiosyncratic toxicities from some drugs.
The presence of an inflammatory stress could alter the intermediates formed in the metabolism of drugs and result in the formation of a reactive intermediate only in the presence of inflammation. For example, MPO, an enzyme released from activated PMNs can play a role in the metabolism of some drug structures (Hofstra and Uetrecht, 1993). As noted above, a recent study using a cyclopropylamine-containing surrogate molecule as a model for TVX suggested that TVX might be metabolized in the presence of MPO to a reactive species (Sun et al., 2007). In this case, the reactive intermediate would not be formed/detected in current preclinical animal testing (i.e., in the absence of concurrent hepatic PMN infiltration and activation).
Although drug metabolism and transporter genes have been emphasized in the current thinking about the role of polymorphisms in IDILI, the genetic polymorphism hypothesis might be considered more broadly. For example, it is possible that polymorphisms in anti- or proinflammatory cytokines or in antiapoptotic proteins could render individuals more susceptible to IDILI. Evidence for this exists for liver injury from diclofenac, for which patients who developed hepatotoxicity were more likely to have polymorphisms in IL-10 or IL-4 (Aithal et al., 2004).
Neither the failure-to-adapt hypothesis nor the multiple determinant hypothesis proposes specific mechanisms to explain IDILI. The inflammatory stress hypothesis is consistent with both of these. Drug-inflammation interaction models in animals such as the one with TVX described above suggest that inflammatory stress is a likely environmental factor to be considered in the multiple determinant hypothesis. Similarly, the potentiation of hepatotoxic responses by LPS in animal models can be interpreted as inflammatory stress preventing adaptation that would normally occur to hepatic stress imposed by drug treatment. The failure-to-adapt hypothesis of IDILI arose originally from the observation that therapeutic doses of acetaminophen in human volunteers commonly resulted in modest increases in serum ALT activity that returned to normal in most people upon continued administration of the drug, suggesting adaptation to the injury caused by the drug (Watkins, 2005). Excessive inflammation is one factor that could promote progression of injury, which would be seen as failure to adapt. Indeed, the recent observation that concurrent exposure to inflammagens can convert a nontoxic acetaminophen dose into one that is markedly hepatotoxic in mice can be interpreted as inflammation preventing the hepatic adaptive response to this drug (Maddox et al., 2010). Accordingly, it is possible that some individuals who fail to adapt are those who experience a concurrent inflammatory episode or who are unable to mount an anti-inflammatory response to the initial drug insult.
CONCLUDING REMARKS
The inflammatory stress hypothesis has provided some of the first animal models of idiosyncratic hepatotoxicity in which liver injury occurs. Furthermore, these models in rodents are the first that produce pronounced liver injury using a variety of drugs associated with IDILI. In exploring the LPS/TVX model, several factors and events important in the hepatotoxic interaction between TVX and inflammatory stress were found. PMN activation; cytokines such as IL-18, IFNγ, and PAI-1; and thrombin activation all proved to be important in the pathogenesis of liver injury, and they all were found to act downstream of TNF as a proximal mediator. Prolonged exposure to TNF appeared to be an important initiating factor, and impaired clearance of TNF by TVX exposure was largely responsible for this. In vitro, TVX also had direct actions on hepatocytes that increased their sensitivity to injury caused directly by TNF. Thus, TVX interacted with TNF at several levels, and it is clear that numerous inflammatory factors and mechanisms are involved in the toxic interaction of this drug with the innate immune response. Importantly, many of the factors critically involved in the LPS/TVX interaction are also important in other drug/inflammation interaction models of IDILI (e.g., see Deng et al., 2009). These findings might prove helpful in understanding the modes of action and mechanisms underlying human IDILI. Additionally, further understanding of interactions between IDILI-associated drugs and the innate immune system could provide the basis for more effective preclinical tests that may reduce development of drug candidates with IDILI potential.
FUNDING
National Institutes of Health (R01DK061315 and R01ES004139).
References
- Aithal GP, Ramsay L, Daly AK, Sonchit N, Leathart JB, Alexander G, Kenna JG, Caldwell J, Day CP. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology. 2004;39:1430–1440. doi: 10.1002/hep.20205. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Gibbons JA, Flaherty MM, Kreider ML, Romano JA, Levin ED. Heterogeneity of toxicant response: sources of human variability. Toxicol. Sci. 2003;76:3–20. doi: 10.1093/toxsci/kfg204. [DOI] [PubMed] [Google Scholar]
- Amacher DE. Reactive intermediates and the pathogenesis of adverse drug reactions: the toxicology perspective. Curr. Drug Metab. 2006;7:219–229. doi: 10.2174/138920006776359284. [DOI] [PubMed] [Google Scholar]
- Andrade RJ, Lucena MI, Alonso A, Garcia-Cortes M, Garcia-Ruiz E, Benitez R, Fernandez MC, Pelaez G, Romero M, Corpas R, et al. HLA class II genotype influences the type of liver injury in drug-induced idiosyncratic liver disease. Hepatology. 2004;39:1603–1612. doi: 10.1002/hep.20215. [DOI] [PubMed] [Google Scholar]
- Bemelmans MH, Gouma DJ, Buurman WA. Influence of nephrectomy on tumor necrosis factor clearance in a murine model. J. Immunol. 1993;150:2007–2017. [PubMed] [Google Scholar]
- Bemelmans MH, Gouma DJ, Buurman WA. Tissue distribution and clearance of soluble murine TNF receptors in mice. Cytokine. 1994;6:608–615. doi: 10.1016/1043-4666(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Bemelmans MH, van Tits LJ, Buurman WA. Tumor necrosis factor: function, release and clearance. Crit. Rev. Immunol. 1996;16:1–11. doi: 10.1615/critrevimmunol.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- Bernuau J, Mallet L, Benhamou JP. [Hepatotoxicity due to tienilic acid] Gastroenterol. Clin. Biol. 1981;5:692–693. [PubMed] [Google Scholar]
- Bird GL, Williams R. Detection of antibodies to a halothane metabolite hapten in sera from patients with halothane-associated hepatitis. J. Hepatol. 1989;9:366–373. doi: 10.1016/0168-8278(89)90147-5. [DOI] [PubMed] [Google Scholar]
- Black M, Mitchell JR, Zimmerman HJ, Ishak KG, Epler GR. Isoniazid-associated hepatitis in 114 patients. Gastroenterology. 1975;69:289–302. [PubMed] [Google Scholar]
- Boelsterli UA. Diclofenac-induced liver injury: a paradigm of idiosyncratic drug toxicity. Toxicol. Appl. Pharmacol. 2003;192:307–322. doi: 10.1016/s0041-008x(03)00368-5. [DOI] [PubMed] [Google Scholar]
- Boelsterli UA, Lim PL. Mitochondrial abnormalities—a link to idiosyncratic drug hepatotoxicity? Toxicol. Appl. Pharmacol. 2007;220:92–107. doi: 10.1016/j.taap.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Buchweitz JP, Ganey PE, Bursian SJ, Roth RA. Underlying endotoxemia augments toxic responses to chlorpromazine: is there a relationship to drug idiosyncrasy? J. Pharmacol. Exp. Ther. 2002;300:460–467. doi: 10.1124/jpet.300.2.460. [DOI] [PubMed] [Google Scholar]
- Chen HJ, Bloch KJ, Maclean JA. Acute eosinophilic hepatitis from trovafloxacin. N. Engl. J. Med. 2000;342:359–360. doi: 10.1056/NEJM200002033420517. [DOI] [PubMed] [Google Scholar]
- Cheng L, You Q, Yin H, Holt M, Franklin C, Ju C. Effect of polyI: C cotreatment on halothane-induced liver injury in mice. Hepatology. 2009;49:215–226. doi: 10.1002/hep.22585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. Nefazodone (Serzone) withdrawn because of hepatotoxicity. CMAJ. 2003;169:1187. [PMC free article] [PubMed] [Google Scholar]
- Cunningham G. Adverse drug reactions in the elderly and their prevention. Scott. Med. J. 1997;42:136–137. doi: 10.1177/003693309704200506. [DOI] [PubMed] [Google Scholar]
- Dalvie DK, Khosla N, Vincent J. Excretion and metabolism of trovafloxacin in humans. Drug Metab. Dispos. 1997;25:423–427. [PubMed] [Google Scholar]
- De SA, De SG. Adverse reactions to fluoroquinolones. An overview on mechanistic aspects. Curr. Med. Chem. 2001;8:371–384. doi: 10.2174/0929867013373435. [DOI] [PubMed] [Google Scholar]
- Deng X, Luyendyk JP, Ganey PE, Roth RA. Inflammatory stress and idiosyncratic hepatotoxicity: hints from animal models. Pharmacol. Rev. 2009;61:262–282. doi: 10.1124/pr.109.001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Stachlewitz RF, Liguori MJ, Blomme EA, Waring JF, Luyendyk JP, Maddox JF, Ganey PE, Roth RA. Modest inflammation enhances diclofenac hepatotoxicity in rats: role of neutrophils and bacterial translocation. J. Pharmacol. Exp. Ther. 2006;319:1191–1199. doi: 10.1124/jpet.106.110247. [DOI] [PubMed] [Google Scholar]
- Dugan CM, Macdonald AE, Roth RA, Ganey PE. A mouse model of severe halothane hepatitis based on human risk factors. J. Pharmacol. Exp. Ther. 2010;333:364–372. doi: 10.1124/jpet.109.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghtesadi-Araghi P, Sohrabpour A, Vahedi H, Saberi-Firoozi M. Halothane hepatitis in Iran: a review of 59 cases. World J. Gastroenterol. 2008;14:5322–5326. doi: 10.3748/wjg.14.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman AJ, Babich JW, Alpert NM, Vincent J, Wilkinson RA, Callahan RJ, Correia JA, Rubin RH. Pharmacokinetics of 18F-labeled trovafloxacin in normal and Escherichia coli-infected rats and rabbits studied with positron emission tomography. Clin. Microbiol. Infect. 1997;3:379. doi: 10.1111/j.1469-0691.1997.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Ganey PE, Luyendyk JP, Maddox JF, Roth RA. Adverse hepatic drug reactions: inflammatory episodes as consequence and contributor. Chem. Biol. Interact. 2004;150:35–51. doi: 10.1016/j.cbi.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Ganey PE, Luyendyk JP, Newport SW, Eagle TM, Maddox JF, Mackman N, Roth RA. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology. 2007;46:1177–1186. doi: 10.1002/hep.21779. [DOI] [PubMed] [Google Scholar]
- Goel K, Menzies D, Cunha BA. Elevated international normalized ratio associated with trovafloxacin. Ann. Intern. Med. 1999;131:72. doi: 10.7326/0003-4819-131-1-199907060-00024. [DOI] [PubMed] [Google Scholar]
- Graham DJ, Green L, Senior JR, Nourjah P. Troglitazone-induced liver failure: a case study. Am. J. Med. 2003;114:299–306. doi: 10.1016/s0002-9343(02)01529-2. [DOI] [PubMed] [Google Scholar]
- Grattagliano I, Bonfrate L, Diogo CV, Wang HH, Wang DQ, Portincasa P. Biochemical mechanisms in drug-induced liver injury: certainties and doubts. World J. Gastroenterol. 2009;15:4865–4876. doi: 10.3748/wjg.15.4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurumurthy P, Krishnamurthy MS, Nazareth O, Parthasarathy R, Sarma GR, Somasundaram PR, Tripathy SP, Ellard GA. Lack of relationship between hepatic toxicity and acetylator phenotype in three thousand South Indian patients during treatment with isoniazid for tuberculosis. Am. Rev. Respir. Dis. 1984;129:58–61. doi: 10.1164/arrd.1984.129.1.58. [DOI] [PubMed] [Google Scholar]
- Haasio K, Koponen A, Penttila KE, Nissinen E. Effects of entacapone and tolcapone on mitochondrial membrane potential. Eur. J. Pharmacol. 2002;453:21–26. doi: 10.1016/s0014-2999(02)02383-x. [DOI] [PubMed] [Google Scholar]
- He K, Talaat RE, Pool WF, Reily MD, Reed JE, Bridges AJ, Woolf TF. Metabolic activation of troglitazone: identification of a reactive metabolite and mechanisms involved. Drug Metab. Dispos. 2004;32:639–646. doi: 10.1124/dmd.32.6.639. [DOI] [PubMed] [Google Scholar]
- Hofstra AH, Uetrecht JP. Myeloperoxidase-mediated activation of xenobiotics by human leukocytes. Toxicology. 1993;82:221–242. doi: 10.1016/0300-483x(93)90066-2. [DOI] [PubMed] [Google Scholar]
- Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- Huang YS. Genetic polymorphisms of drug-metabolizing enzymes and the susceptibility to antituberculosis drug-induced liver injury. Expert. Opin. Drug Metab. Toxicol. 2007;3:1–8. doi: 10.1517/17425255.3.1.1. [DOI] [PubMed] [Google Scholar]
- Hunter EB, Johnston PE, Tanner G, Pinson CW, Awad JA. Bromfenac (Duract)-associated hepatic failure requiring liver transplantation. Am. J. Gastroenterol. 1999;94:2299–2301. doi: 10.1111/j.1572-0241.1999.01321.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaitin KI. Drug safety withdrawals in the U.S. not linked to speed of FDA approval. Tufts Center for the Study of Drug Development Impact Report 2005. 2005;7 [Google Scholar]
- Kaplowitz N. Idiosyncratic drug hepatotoxicity. Nat. Rev. Drug Discov. 2005;4:489–499. doi: 10.1038/nrd1750. [DOI] [PubMed] [Google Scholar]
- Kaplowitz N, Aw TY, Simon FR, Stolz A. Drug-induced hepatotoxicity. Ann. Intern. Med. 1986;104:826–839. doi: 10.7326/0003-4819-104-6-826. [DOI] [PubMed] [Google Scholar]
- Kassahun K, Pearson PG, Tang W, McIntosh I, Leung K, Elmore C, Dean D, Wang R, Doss G, Baillie TA. Studies on the metabolism of troglitazone to reactive intermediates in vitro and in vivo. Evidence for novel biotransformation pathways involving quinone methide formation and thiazolidinedione ring scission. Chem. Res. Toxicol. 2001;14:62–70. doi: 10.1021/tx000180q. [DOI] [PubMed] [Google Scholar]
- Keller BJ, Yamanaka H, Thurman RG. Inhibition of mitochondrial respiration and oxygen-dependent hepatotoxicity by six structurally dissimilar peroxisomal proliferating agents. Toxicology. 1992;71:49–61. doi: 10.1016/0300-483x(92)90053-h. [DOI] [PubMed] [Google Scholar]
- Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitteringham NR, Kenna JG, Park BK. Detection of autoantibodies directed against human hepatic endoplasmic reticulum in sera from patients with halothane-associated hepatitis. Br. J. Clin. Pharmacol. 1995;40:379–386. doi: 10.1111/j.1365-2125.1995.tb04560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287:2215–2220. doi: 10.1001/jama.287.17.2215. [DOI] [PubMed] [Google Scholar]
- Lazarczyk DA, Goldstein NS, Gordon SC. Trovafloxacin hepatotoxicity. Dig. Dis. Sci. 2001;46:925–926. doi: 10.1023/a:1010741510046. [DOI] [PubMed] [Google Scholar]
- Lee WM. Drug-induced hepatotoxicity. N. Engl. J. Med. 2003;349:474–485. doi: 10.1056/NEJMra021844. [DOI] [PubMed] [Google Scholar]
- Li AP. A review of the common properties of drugs with idiosyncratic hepatotoxicity and the “multiple determinant hypothesis” for the manifestation of idiosyncratic drug toxicity. Chem. Biol. Interact. 2002;142:7–23. doi: 10.1016/s0009-2797(02)00051-0. [DOI] [PubMed] [Google Scholar]
- Lu J, Roth RA, Ganey PE. Characterization of an inflammatory stress model of amiodarone idiosyncratic hepatotoxicity in rats. Toxicologist. 2010;114:159. (Abstract) [Google Scholar]
- Lucena MI, Andrade RJ, Rodrigo L, Salmeron J, Alvarez A, Lopez-Garrido MJ, Camargo R, Alcantara R. Trovafloxacin-induced acute hepatitis. Clin. Infect. Dis. 2000;30:400–401. doi: 10.1086/313680. [DOI] [PubMed] [Google Scholar]
- Luyendyk JP, Maddox JF, Cosma GN, Ganey PE, Cockerell GL, Roth RA. Ranitidine treatment during a modest inflammatory response precipitates idiosyncrasy-like liver injury in rats. J. Pharmacol. Exp. Ther. 2003;307:9–16. doi: 10.1124/jpet.103.054288. [DOI] [PubMed] [Google Scholar]
- Maddox JF, Amuzie CJ, Li M, Newport SW, Sparkenbaugh E, Cuff CF, Pestka JJ, Cantor GH, Roth RA, Ganey PE. Bacterial- and viral-induced inflammation increases sensitivity to acetaminophen hepatotoxicity. J. Toxicol. Environ. Health A. 2010;73:58–73. doi: 10.1080/15287390903249057. [DOI] [PubMed] [Google Scholar]
- Maniar JK, Shah SR, Verma R, Kamath R, Gupte P, Maniar A. Nevirapine-induced fulminant hepatitis. J. Assoc. Physicians India. 2006;54:957–958. [PubMed] [Google Scholar]
- Mariappan N, Elks CM, Fink B, Francis J. TNF-induced mitochondrial damage: a link between mitochondrial complex I activity and left ventricular dysfunction. Free Radic. Biol. Med. 2009;46:462–470. doi: 10.1016/j.freeradbiomed.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson S, Joho K. Drug discovery, drug development and the emerging world of pharmacogenomics: prospecting for information in a data-rich landscape. Curr. Opin. Mol. Ther. 2000;2:651–654. [PubMed] [Google Scholar]
- Mitchell JR, Zimmerman HJ, Ishak KG, Thorgeirsson UP, Timbrell JA, Snodgrass WR, Nelson SD. Isoniazid liver injury: clinical spectrum, pathology, and probable pathogenesis. Ann. Intern. Med. 1976;84:181–192. doi: 10.7326/0003-4819-84-2-181. [DOI] [PubMed] [Google Scholar]
- Murphy EJ, Davern TJ, Shakil AO, Shick L, Masharani U, Chow H, Freise C, Lee WM, Bass NM. Troglitazone-induced fulminant hepatic failure. Acute Liver Failure Study Group. Dig. Dis. Sci. 2000;45:549–553. doi: 10.1023/a:1005405526283. [DOI] [PubMed] [Google Scholar]
- Mushin WW, Rosen M, Jones EV. Post-halothane jaundice in relation to previous administration of halothane. Br. Med. J. 1971;3:18–22. doi: 10.1136/bmj.3.5765.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt DJ, Gordon SF, Pirmohamed M, Park BK. Immunological principles of adverse drug reactions: the initiation and propagation of immune responses elicited by drug treatment. Drug Saf. 2000;23:483–507. doi: 10.2165/00002018-200023060-00002. [DOI] [PubMed] [Google Scholar]
- Nicholson SC, Webb CD, Moellering RC., Jr Antimicrobial-associated acute hepatitis. Pharmacotherapy. 2002;22:794–796. doi: 10.1592/phco.22.9.794.34066. [DOI] [PubMed] [Google Scholar]
- Obermayer-Straub P, Strassburg CP, Manns MP. Autoimmune hepatitis. J. Hepatol. 2000;32(1 Suppl.):181–197. doi: 10.1016/s0168-8278(00)80425-0. [DOI] [PubMed] [Google Scholar]
- Ong MM, Latchoumycandane C, Boelsterli UA. Troglitazone-induced hepatic necrosis in an animal model of silent genetic mitochondrial abnormalities. Toxicol. Sci. 2007;97:205–213. doi: 10.1093/toxsci/kfl180. [DOI] [PubMed] [Google Scholar]
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 2002;137:947–954. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- Park BK, Coleman JW, Kitteringham NR. Drug disposition and drug hypersensitivity. Biochem. Pharmacol. 1987;36:581–590. [PubMed] [Google Scholar]
- Park BK, Pirmohamed M, Kitteringham NR. Role of drug disposition in drug hypersensitivity: a chemical, molecular, and clinical perspective. Chem. Res. Toxicol. 1998;11:969–988. doi: 10.1021/tx980058f. [DOI] [PubMed] [Google Scholar]
- Petrescu I, Tarba C. Uncoupling effects of diclofenac and aspirin in the perfused liver and isolated hepatic mitochondria of rat. Biochim. Biophys. Acta. 1997;1318:385–394. doi: 10.1016/s0005-2728(96)00109-0. [DOI] [PubMed] [Google Scholar]
- Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademaker M. Do women have more adverse drug reactions? Am. J. Clin. Dermatol. 2001;2:349–351. doi: 10.2165/00128071-200102060-00001. [DOI] [PubMed] [Google Scholar]
- Robin MA, Le RM, Descatoire V, Pessayre D. Plasma membrane cytochromes P450 as neoantigens and autoimmune targets in drug-induced hepatitis. J. Hepatol. 1997;26(Suppl. 1):23–30. doi: 10.1016/s0168-8278(97)82329-x. [DOI] [PubMed] [Google Scholar]
- Roth RA, Ganey PE. Intrinsic versus idiosyncratic drug-induced hepatotoxicity—two villains or one? J. Pharmacol. Exp. Ther. 2010;332:692–697. doi: 10.1124/jpet.109.162651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RA, Luyendyk JP, Maddox JF, Ganey PE. Inflammation and drug idiosyncrasy—is there a connection? J. Pharmacol. Exp. Ther. 2003;307:1–8. doi: 10.1124/jpet.102.041624. [DOI] [PubMed] [Google Scholar]
- Sanne I, Mommeja-Marin H, Hinkle J, Bartlett JA, Lederman MM, Maartens G, Wakeford C, Shaw A, Quinn J, Gish RG, et al. Severe hepatotoxicity associated with nevirapine use in HIV-infected subjects. J. Infect. Dis. 2005;191:825–829. doi: 10.1086/428093. [DOI] [PubMed] [Google Scholar]
- Schwab CE, Tuschl H. In vitro studies on the toxicity of isoniazid in different cell lines. Hum. Exp. Toxicol. 2003;22:607–615. doi: 10.1191/0960327103ht401oa. [DOI] [PubMed] [Google Scholar]
- Seguin B, Uetrecht J. The danger hypothesis applied to idiosyncratic drug reactions. Curr. Opin. Allergy Clin. Immunol. 2003;3:235–242. doi: 10.1097/00130832-200308000-00001. [DOI] [PubMed] [Google Scholar]
- Shainheit MG, Smith PM, Bazzone LE, Wang AC, Rutitzky LI, Stadecker MJ. Dendritic cell IL-23 and IL-1 production in response to schistosome eggs induces Th17 cells in a mouse strain prone to severe immunopathology. J. Immunol. 2008;181:8559–8567. doi: 10.4049/jimmunol.181.12.8559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Beggs KM, Sparkenbaugh EM, Dugan CM, Ganey PE, Roth RA. Trovafloxacin enhances TNF-induced inflammatory stress and cell death signaling and reduces TNF clearance in a murine model of idiosyncratic hepatotoxicity. Toxicol. Sci. 2009a;111:288–301. doi: 10.1093/toxsci/kfp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Ditewig AC, Waring JF, Liguori MJ, Blomme EA, Ganey PE, Roth RA. Coexposure of mice to trovafloxacin and lipopolysaccharide, a model of idiosyncratic hepatotoxicity, results in a unique gene expression profile and interferon gamma-dependent liver injury. Toxicol. Sci. 2009b;107:270–280. doi: 10.1093/toxsci/kfn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Fullerton AM, Scott MA, Ganey PE, Roth RA. The role of the hemostatic system in murine liver injury induced by coexposure to lipopolysaccharide and trovafloxacin, a drug with idiosyncratic liability. Toxicol. Appl. Pharmacol. 2009c;236:293–300. doi: 10.1016/j.taap.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Ganey PE, Roth RA. Trovafloxacin enhances the inflammatory response to a Gram-negative or a Gram-positive bacterial stimulus, resulting in neutrophil-dependent liver injury in mice. J. Pharmacol. Exp. Ther. 2009d;330:72–78. doi: 10.1124/jpet.109.151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Ganey PE, Roth RA. Tumor necrosis factor alpha is a proximal mediator of synergistic hepatotoxicity from trovafloxacin/lipopolysaccharide coexposure. J. Pharmacol. Exp. Ther. 2009e;328:62–68. doi: 10.1124/jpet.108.143792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Hopfensperger MJ, Ganey PE, Roth RA. Lipopolysaccharide and trovafloxacin coexposure in mice causes idiosyncrasy-like liver injury dependent on tumor necrosis factor-alpha. Toxicol. Sci. 2007;100:259–266. doi: 10.1093/toxsci/kfm218. [DOI] [PubMed] [Google Scholar]
- Shishido S, Koga H, Harada M, Kumemura H, Hanada S, Taniguchi E, Kumashiro R, Ohira H, Sato Y, Namba M, et al. Hydrogen peroxide overproduction in megamitochondria of troglitazone-treated human hepatocytes. Hepatology. 2003;37:136–147. doi: 10.1053/jhep.2003.50014. [DOI] [PubMed] [Google Scholar]
- Sibbald B. Cisapride, before and after: still waiting for ADE-reporting reform. CMAJ. 2001;165:1370. [Google Scholar]
- Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- Stahlmann R. Clinical toxicological aspects of fluoroquinolones. Toxicol. Lett. 2002;127:269–277. doi: 10.1016/s0378-4274(01)00509-4. [DOI] [PubMed] [Google Scholar]
- Sun Q, Zhu R, Foss FW, Jr., Macdonald TL. Mechanisms of trovafloxacin hepatotoxicity: studies of a model cyclopropylamine-containing system. Bioorg. Med. Chem. Lett. 2007;17:6682–6686. doi: 10.1016/j.bmcl.2007.10.070. [DOI] [PubMed] [Google Scholar]
- Temple RJ, Himmel MH. Safety of newly approved drugs: implications for prescribing. JAMA. 2002;287:2273–2275. doi: 10.1001/jama.287.17.2273. [DOI] [PubMed] [Google Scholar]
- Tirmenstein MA, Hu CX, Gales TL, Maleeff BE, Narayanan PK, Kurali E, Hart TK, Thomas HC, Schwartz LW. Effects of troglitazone on HepG2 viability and mitochondrial function. Toxicol. Sci. 2002;69:131–138. doi: 10.1093/toxsci/69.1.131. [DOI] [PubMed] [Google Scholar]
- Uetrecht J. Screening for the potential of a drug candidate to cause idiosyncratic drug reactions. Drug Discov. Today. 2003;8:832–837. doi: 10.1016/s1359-6446(03)02816-2. [DOI] [PubMed] [Google Scholar]
- Uetrecht J. Evaluation of which reactive metabolite, if any, is responsible for a specific idiosyncratic reaction. Drug Metab. Rev. 2006;38:745–753. doi: 10.1080/03602530600959615. [DOI] [PubMed] [Google Scholar]
- Uetrecht JP. New concepts in immunology relevant to idiosyncratic drug reactions: the “danger hypothesis” and innate immune system. Chem. Res. Toxicol. 1999;12:387–395. doi: 10.1021/tx980249i. [DOI] [PubMed] [Google Scholar]
- Walgren JL, Mitchell MD, Thompson DC. Role of metabolism in drug-induced idiosyncratic hepatotoxicity. Crit. Rev. Toxicol. 2005;35:325–361. doi: 10.1080/10408440590935620. [DOI] [PubMed] [Google Scholar]
- Waring JF, Anderson MG. Idiosyncratic toxicity: mechanistic insights gained from analysis of prior compounds. Curr. Opin. Drug Discov. Devel. 2005;8:59–65. [PubMed] [Google Scholar]
- Waring JF, Liguori MJ, Luyendyk JP, Maddox JF, Ganey PE, Stachlewitz RF, North C, Blomme EA, Roth RA. Microarray analysis of lipopolysaccharide potentiation of trovafloxacin-induced liver injury in rats suggests a role for proinflammatory chemokines and neutrophils. J. Pharmacol. Exp. Ther. 2006;316:1080–1087. doi: 10.1124/jpet.105.096347. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Suzuki O, Haruyama T, Akaike T. Interferon-gamma induces reactive oxygen species and endoplasmic reticulum stress at the hepatic apoptosis. J. Cell Biochem. 2003;89:244–253. doi: 10.1002/jcb.10501. [DOI] [PubMed] [Google Scholar]
- Watkins PB. Idiosyncratic liver injury: challenges and approaches. Toxicol. Pathol. 2005;33:1–5. doi: 10.1080/01926230590888306. [DOI] [PubMed] [Google Scholar]
- Wilke RA, Lin DW, Roden DM, Watkins PB, Flockhart D, Zineh I, Giacomini KM, Krauss RM. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat. Rev. Drug Discov. 2007;6:904–916. doi: 10.1038/nrd2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DP, Park BK. Idiosyncratic toxicity: the role of toxicophores and bioactivation. Drug Discov. Today. 2003;8:1044–1050. doi: 10.1016/s1359-6446(03)02888-5. [DOI] [PubMed] [Google Scholar]
- Zou W, Devi SS, Sparkenbaugh E, Younis HS, Roth RA, Ganey PE. Hepatotoxic interaction of sulindac with lipopolysaccharide: role of the hemostatic system. Toxicol. Sci. 2009;108:184–193. doi: 10.1093/toxsci/kfn259. [DOI] [PMC free article] [PubMed] [Google Scholar]