Abstract
Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) are widespread environmental contaminants associated with changes in behavior and neurochemical function in laboratory animals and behavioral deficits in children. PCBs and PBDEs are found in food, especially in seafood and dairy products, and coexposure to these contaminants is likely. We examined the effects of an environmentally relevant mixture of PCBs (Fox River Mix [FRM]) and a PBDE mixture (DE-71) alone and in combination on synaptosomal and medium dopamine (DA) levels and the levels of the DA metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) in striatal synaptosomes derived from postnatal days (PND) 7, PND14, or PND21 rats. FRM elevated medium DA and reduced synaptosomal DA concentrations with greater potency than equimolar concentrations of DE-71. The effects of FRM, but not DE-71, were dependent on the age of the animals from which the synaptosomes were derived, with greater effects observed in synaptosomes from the youngest animals. We used Bliss’ model of independence to assess the possible interaction(s) of a 1:1 mixture of FRM and DE-71 on synaptosomal DA function and found that the effects of the FRM/DE-71 mixture were additive. Furthermore, as for FRM alone, the effects of the FRM/DE71 mixture were greater in synaptosomes prepared from PND7 rats than in synaptosomes from PND14 and PND21 rats. Because the effects of these contaminants are additive, it is necessary to take into account the cumulative exposure to organohalogen contaminants such as PCBs and PBDEs during risk assessment.
Keywords: dopamine, PCBs, brominated flame retardants, polybrominated diphenyl ethers, interactions, additivity
Halogenated aromatic hydrocarbons, including polychlorinated biphenyls (PCBs), dioxins, dibenzofurans, and brominated flame retardants (BFRs), are widespread environmental contaminants shown to alter the behavior and/or neurochemistry of laboratory animals, particularly during development (Branchi et al., 2003, 2005; Lilienthal et al., 1990; Roegge et al., 2000). In addition, there is significant epidemiological evidence from studies of children exposed in utero and during lactation demonstrating that exposure to PCBs, and to a lesser extent BFRs, are associated with alterations in behavior, including reductions in measures of cognition and attention and elevations in impulsivity (Gray et al., 2005; Jacobson and Jacobson, 2003; Jacobson et al., 1992; Stewart et al., 2005). The mechanisms responsible for these changes are still not completely understood but may include toxicant-induced changes in regulation of dopamine (DA) neurotransmission because small changes in catecholaminergic modulation can have profound effects on behavior (Arnsten and Li, 2005; Berridge et al., 2006; Oades et al., 2005).
Although environmental and body burden levels of PCBs have declined dramatically since their industrial use in the United States was banned in the mid 1970’s (Erickson, 1997), detectable levels of PCBs are still found in the environment and in the human body. In contrast, BFR levels increased dramatically from the 1970’s until 2004 (Hites, 2004; Schecter et al., 2005) when two important BFRs (penta- and octa-brominated diphenyl ethers) were banned in Europe and production ceased in the United States (Costa and Giordano, 2007). The high concentrations of BFRs in human serum and adipose tissue raise concerns about their possible health effects (Birnbaum and Staskal, 2004), and several recent studies (Branchi et al., 2003; Eriksson et al., 2006; Viberg et al., 2003) have demonstrated that BFRs alter behavior and neurochemical function in laboratory rodents and affect neurochemistry in vitro (Dingemans et al., 2007; Giordano et al., 2008; Kodavanti et al., 2005; Mariussen and Fonnum, 2003; Reistad et al., 2006). Few studies have, however, directly compared the effects of these two structurally related neurotoxicants, either alone or in combination, on alterations in DA neurochemistry at different developmental ages.
Polybrominated diphenyl ethers (PBDEs) and PCBs are chemically similar and inhibit plasma membrane dopamine transporters (DAT) and vesicular monoamine transporters (VMAT) (Bemis and Seegal, 2004; Mariussen and Fonnum, 2001, 2003). Previous environmental contaminant combination studies showed synergistic effects of PCBs and BFRs on behavior and cytotoxicity (Eriksson et al., 2006; Gao et al., 2009; He et al., 2009), whereas others showed mainly additive effects of methylmercury, PCBs, and BFRs on glutamate uptake into rat brain synaptosomes and of methylmercury and PCB on viability of rat pheochromocytoma cells (Andersen et al., 2009; Vettori et al., 2006). We hypothesized that coexposure to PCB and PBDE would result in greater effects on DA neurochemistry than exposure to either contaminant alone. We examined the effects of exposure of isolated nerve terminals (synaptosomes) from the striatum of developing rats to FRM, DE-71, or a combination thereof, on changes in synaptosomal and medium DA and 3,4-dihydroxyphenylacetic acid (DOPAC) concentrations. Our results demonstrate a greater potency of FRM, relative to DE-71, to reduce synaptosomal DA levels and increase medium DA concentrations, as well as an age dependency for these effects only for FRM. The effects of the FRM/DE-71 mixture were also greater in synaptosomes from the younger animals compared with synaptosomes from older postnatal animals, however, the combined effects were additive at all ages.
MATERIALS AND METHODS
Animals.
Timed-pregnant Long-Evans rats were obtained from Taconic Farms (Germantown, NY). Rat pups at 7, 14, and 21 days of age, from litters born in our facility, were used for the preparation of striatal synaptosomes. All procedures involving the use of animals were performed according to protocols approved by the Wadsworth Center Institutional Animal Care and Use Committee.
Source and selection of PCB and BFR mixtures.
A PCB mixture, referred to here as the Fox River Mix (FRM), was prepared by and obtained from the University of Illinois for collaborative studies. This PCB mixture is a formulation of Aroclors 1242, 1248, 1254, and 1260 in a 35:35:15:15, respectively, ratio that closely approximates the PCB congener pattern found in contaminated fish consumed by residents near the Fox River in Wisconsin (Kostyniak et al., 2005). FRM is slightly more toxic than Aroclor 1254 (A1254) in vivo, has low dioxin toxic equivalence, and the congener composition is reported in Kostyniak et al. (2005). The polybrominated diphenyl ether mixture DE-71 (also referred to as pentabromodiphenyl ether or PBDE), consisting of a mixture of tri-, tetra-, penta-, and hexabromodiphenyl ethers, was kindly provided by Dr Kevin Crofton of the U.S. Environmental Protection Agency from a sample originally obtained from the Great Lakes Chemical Company (lot number 755O0K20A; West Lafayette, IN). FRM and DE-71 were chosen for this study because they are environmentally relevant mixtures (Kostyniak et al., 2005; Law et al., 2006; Mariussen et al., 2008) and coexposure of humans to PCB and PBDEs is much more likely to involve several PCB and PBDE congeners and diastereomers rather than single congeners. FRM and DE-71 were regarded as single chemicals for the interaction study.
Stock solutions of FRM and DE-71 were prepared in dimethylformamide (DMF), using a molecular weight of 296 for FRM (calculated on the basis of the average weight of each Aroclor that makes up the mixture; Kostyniak et al., 2005) and a molecular weight of 564.7 for DE-71 (from the Great Lakes Chemical Company’s Material Safety Data Sheet for DE-71). As FRM and DE-71 are mixtures of individual PCB congeners and PBDE congeners, respectively, these molecular weights are approximations.
Synaptosomal preparation for contaminant exposure.
The isolation of striatal synaptosomes is based on a modification of procedures described by Löscher et al. (1985). Briefly, preweaning male rat pups at 7, 14, or 21 days of age were anesthetized with CO2, decapitated, and their brains rapidly removed. A forebrain block, made by a coronal cut at the optic chiasm, was isolated and the striata dissected free hand. Striata from 5–12 rats (depending on age) were pooled to yield ∼390 mg of striatal tissue at each age. The tissue was homogenized in ice-cold 0.32M sucrose using a Potter-Elvehjem glass-Teflon tissue grinder, centrifuged at 1000 × g for 10 min, and the supernatant collected and layered onto 1.2M sucrose. Centrifugation at 50,000 × g with a ω2t setting of 1.6 × 1010 yielded an interface layer which was collected, diluted, layered onto 0.8M sucrose, and centrifuged using the same conditions described above to yield a purified synaptosomal pellet (P-3 fraction). Synaptosomal pellets were resuspended in a volume of oxygenated 4-[2-hydroxyethyl]-1-piperazineethanesulfonic acid (HEPES)-buffered Hank’s solution (HBHS) equivalent to the starting wet weight of the striatal tissue and kept on ice until use.
Exposure of synaptosomes to FRM and DE-71.
Eighteen-microliter aliquots of synaptosomes were suspended in 450 μl of HBHS containing 1% horse serum and either 0.2% DMF or DMF containing FRM or DE-71 to yield medium concentrations of 10, 20, or 40μM of the contaminants. The final protein concentrations in the incubation mixtures were (mean ± SE) 153 ± 3.9 μg/ml (postnatal days [PND] 7), 264 ± 7.5 μg/ml (PND14), and 312 ± 12.6 μg/ml (PND21), with the differences in protein concentrations reflecting the lower total protein levels in brain tissue at PND7 and PND14 compared with PND21. Mixtures of FRM and DE-71 were prepared similarly to yield 10μM FRM + 10μM DE-71, 20μM FRM + 20μM DE-71, or 40μM FRM + 40μM DE-71. The final DMF concentration for all exposures was 0.2%. This synaptosomal suspension was distributed into 96-well plates (130 μl/well) and incubated for 30 min in a humidified shaking water bath under an atmosphere of 95% O2/5% CO2 at 37°C. After exposure, the samples were transferred to microcentrifuge tubes and centrifuged for 2 min at 8740 × g to separate the synaptosomes from the medium. Hundred microliters of the resulting supernatant was removed and acidified by the addition of an equal volume of 0.4N HClO4 while the synaptosomal pellet was sonicated in 100 μl of 0.2N HClO4. All samples were frozen at −80°C until analysis. Experiments were repeated four times, each time with freshly prepared synaptosomes using three wells per experimental exposure condition. This design allowed all exposure conditions to be tested in the same plate at the same time within an experiment.
Analysis of DA and DOPAC concentrations in synaptosomes and medium.
Quantification of DA and DOPAC concentrations in both synaptosomes and medium was performed by high-performance liquid chromatography with electrochemical detection as described previously (Bemis and Seegal, 1999; Chishti et al., 1996). Neurotransmitter and metabolite concentrations were corrected for synaptosomal protein content (determined by the bicinchoninic acid method [Pierce, Rockford, IL]). Total DOPAC concentrations (medium + synaptosomes) are reported since DOPAC is transported across the plasma membrane (Lamensdorf et al., 2000), and summation of the changes in both compartments provides the best estimate of the ability of FRM or DE-71 to alter DA storage/handling that results in the metabolism of DA to DOPAC.
Statistical analysis.
Data were analyzed using three-way ANOVA to determine significant main effects of age, treatment (FRM or DE-71) and dose, as well as significant interactions. FRM-DE-71 mixture dose-response data were analyzed by two-way ANOVA. Post hoc analysis with Dunnett’s test was used to test for significant differences between individual doses and the control group. All exposure data were normalized to vehicle control and is presented as mean ± SE (unless otherwise is stated). Actual concentrations (nanogram per milligram protein) are reported in Supplementary table 1.
Bliss' model of independence (Bliss, 1939), also known as independent joint action, was used to calculate the predicted additive effect of the FRM/DE-71 mixture from the results obtained with FRM alone and DE-71 alone. This model is based upon the assumption that the substances in a mixture act independently from each other, such that each chemical acts as if it was the only chemical present (Borgert et al., 2005). All effects (f) are first transformed to fractions of 1 (0 ≤ f ≤ 1) (for medium DA, the maximal observed effect was used as the maximal effect). fA is the fractional effect of chemical A alone at a particular dose, and fB is the fractional effect of chemical B alone. However, if chemical A is already present, the additional possible effect of chemical B is the fraction fB multiplied by the remaining possible effect, which is 1 − fA. Thus, the additional effect of chemical B, in the presence of chemical A, equals fB (1 − fA). Therefore, the total effect of a mixture of the two chemicals is fA + fB (1 − fA) which equals fA + fB − fA × fB. Thus, for binary mixtures, Bliss independence is defined by Equation 1:
| (1) |
The experimental data were obtained as described above and plotted, with 95% confidence intervals, together with the predicted additive data (calculated using Equation 1). If the confidence intervals of the observed data did not overlap with the predicted additive data, the toxicants were considered to interact (Kortenkamp and Altenburger, 1998).
RESULTS
Effects of PCB (FRM) or DE-71 on Synaptosomal DA Function
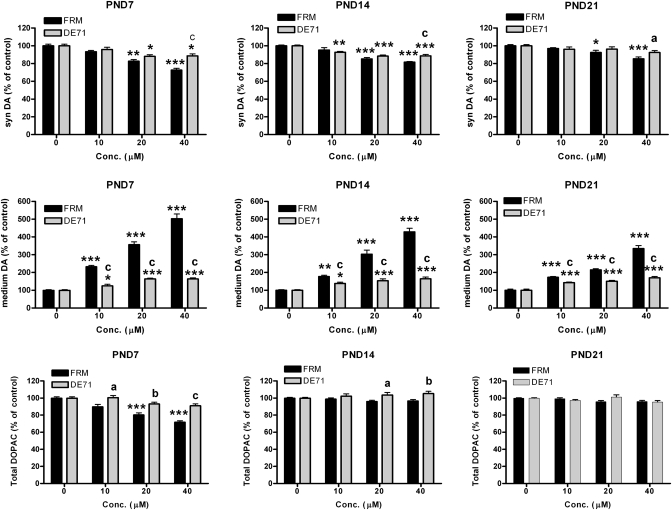
FRM (20 and 40μM) significantly reduced DA concentrations in synaptosomes from animals of all ages (Fig. 1, upper panels). The magnitude of the effects were age dependent, demonstrated by a significant interaction between FRM and age (p ≤ 0.01), with decreases in synaptosomal DA of 23.3 ± 3.8%, 14.9 ± 3.9%, and 13.9 ± 3.3% in PND7, PND14, and PND21 animals, respectively, at 40μM. DE-71 (20 and 40μM) significantly reduced synaptosomal DA concentrations in synaptosomes from PND7 and PND14 animals (p ≤ 0.001), although the decreases following exposure were smaller in magnitude than following exposure to equimolar concentrations of FRM. Unlike FRM, the effects of DE-71 on synaptosomal DA concentrations were not significantly different between synaptosomes derived from animals of different ages. Statistically significant differences between the effects of equimolar concentrations of FRM and DE-71 were found only at 40μM (p ≤ 0.001), where FRM caused approximately twofold greater effects than DE-71.
FIG. 1.
Dose-dependent effects of 0, 10, 20, and 40μM of PCBs (FRM) or the pentabromodiphenyl ether DE-71 on concentrations of synaptosomal DA (syn DA), medium DA, and total DOPAC, expressed as percentage of age-appropriate control, in P3 synaptosomes derived from PND7, PND14, and PND21 rat pups. Data are mean ± SEM from 10–12 wells combined from four independent experiments; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, significantly different from the respective control for each compound at each age. a, p ≤ 0.05; b, p ≤ 0.01; c, p ≤ 0.001, significantly different from FRM at the same concentration.
Effects of PCB (FRM) or DE-71 on Medium DA Concentrations
FRM exposure significantly increased media DA concentrations in a dose-dependent manner (p ≤ 0.001) (Fig. 1, middle panel). The magnitude of the elevations were age dependent, ranging (at 40μM FRM) from fivefold to fourfold and threefold compared with control levels at PND7, PND14 and PND21, respectively. As with synaptosomal DA levels, there was a significant interaction between FRM exposure and the age of the animals from which the synaptosomes were generated (p ≤ 0.001). Thus, the effects of FRM on medium DA concentrations were strongly influenced by the age of the animals from which the synaptosomes were prepared.
DE-71 also significantly increased medium DA in a dose-dependent manner (p ≤ 0.001); however, the elevations were significantly smaller than those seen following exposure to equimolar concentrations of FRM for all doses at all ages (p ≤ 0.001). There was no interaction between DE-71 exposure and the age of the tissue from which the synaptosomes were generated—i.e., the elevations in medium DA concentrations following exposure to DE-71 did not differ significantly between synaptosomes derived from rats of different preweaning ages.
Effects of PCB (FRM) or DE-71 on Total DOPAC Concentrations
FRM significantly and dose dependently reduced total DOPAC concentrations at PND7 (p ≤ 0.001), with reductions of 20.0 ± 3.3% and 26.4 ± 3.0% compared with control at 20 and 40μM, respectively (Fig. 1, lower panel). However, there were no reductions in total DOPAC levels in synaptosomes derived from PND14 and PND21 animals, as demonstrated by a significant interaction between FRM and age (p ≤ 0.001). DE-71 did not significantly alter total DOPAC concentrations at any of the ages or concentrations examined. The sum of total (synaptosomal and media levels) DA and total DOPAC (total DA + total DOPAC), which indicates changes in synthesis or degradation of DA and its primary metabolite, was not changed by FRM or DE-71 at any age (not shown).
Effect of a FRM/DE-71 Mixture
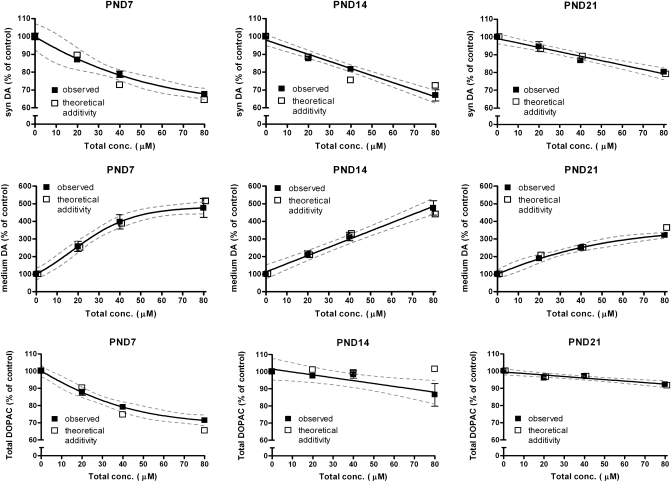
Exposure to a 1:1 mixture of FRM and DE-71 significantly and dose dependently reduced synaptosomal DA at all concentrations on PND7 (p ≤ 0.001) and PND14 (p ≤ 0.001) and at 20μM FRM + 20μM DE-71 and 40μM FRM + 40μM DE-71 on PND21 (p ≤ 0.001) (Fig. 2). The mixture effects were similar in PND7 and PND14 synaptosomes, causing, respectively, 10 ± 3.9% and 11.2 ± 4.3% reductions at total concentration of 20μM, 19.6 ± 3.7% and 14.9 ± 4.0% reductions at total concentration of 40μM, and 29.8 ± 3.6% and 31.6 ± 6.0% reductions at total concentration of 80μM, respectively. In contrast, the mixture effects on synaptosomal DA levels in PND21 synaptosomes were smaller, and the FRM/DE-71 mixture only reduced DA levels by 21.4 ± 1.6% at the highest concentration.
FIG. 2.
Experimental and predicted effects of a 1:1 mixture of FRM and DE71 on synaptosomal DA (syn DA), medium DA, and total DOPAC levels in synaptosomes from PND7, PND14, and PND21 rat pups. Predicted additivity was calculated using Bliss’ model of independent action. The experimental data are mean ± SEM of 10–12 wells combined from four independent experiments, shown with 95% confidence intervals (dotted lines).
Medium DA was increased in a dose-dependent fashion by all concentrations of the mixture at all ages (p ≤ 0.01). As for synaptosomal DA, the mixture effects were greater at PND7 and PND14 than at PND21, with the highest mixture concentration increasing medium DA fourfold to fivefold relative to controls in PND7 and PND14. DOPAC levels were reduced by all mixture concentrations on PND7 (p ≤ 0.001) and by 40μM FRM + 40μM DE-71 on PND14 (p ≤ 0.05) and PND21 (p ≤ 0.001). The mixture reduced DOPAC levels to a greater extent in synaptosomes from PND7 rats compared with both PND14 and PND21, with reductions of 11.2 ± 4.2%, 19.8 ± 3.3%, and 26.1 ± 4.2% at PND7 at total mixture concentrations of 20, 40, and 80μM, respectively, compared with no significant reductions at 20 and 40μM and 13.7 ± 7.1% and 10.7 ± 2.3% reductions at 80μM in synaptosomes from PND14 and PND21 rats, respectively. Two-way ANOVA showed significant effects of age (p ≤ 0.001), mixture concentration (p ≤ 0.001), and a significant interaction between age and concentration (p ≤ 0.01) for synaptosomal DA, medium DA, and DOPAC.
Predicted theoretical additive effects of the FRM/DE-71 mixture were calculated according to Bliss’ independence model. In general, the additive model predicted the experimental data very well (Fig. 2). We observed tendencies toward antagonism or synergism between FRM and DE-71 on DOPAC levels at PND7 and PND14 and on medium DA levels at PND21; however, these effects were only present at the highest concentrations, and the observed effects did not differ by more than 14% from the predicted additive data (Fig. 2).
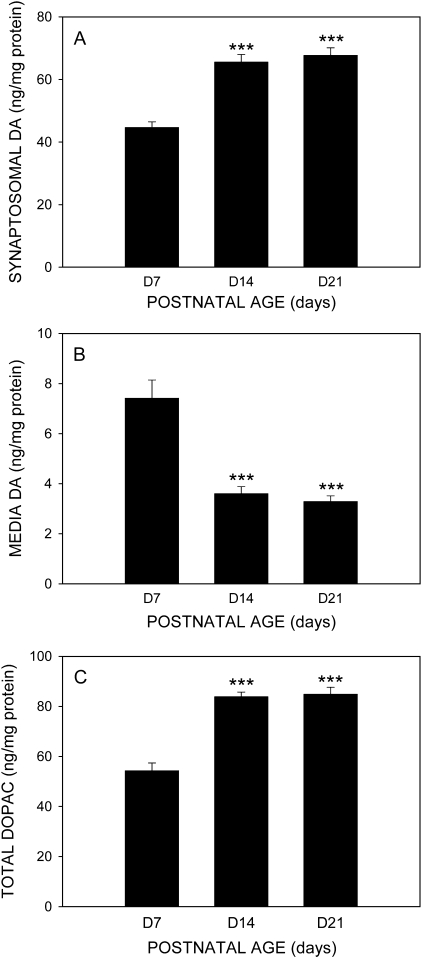
Age-Dependent Changes in Synaptosomal and Medium DA and Total DOPAC Levels
DA concentrations in synaptosomes from PND14 and PND21 animals were more than 50% higher than levels seen in PND7 synaptosomes (Fig. 3A) (p ≤ 0.001). Conversely, medium DA concentrations decreased significantly from 7.4 ± 0.73 ng/mg protein in PND7 synaptosomes to 3.6 ± 0.28 ng/mg protein in PND14 synaptosomes and 3.3 ± 0.2 ng/mg protein in PND21 synaptosomes (Fig. 3B) (p ≤ 0.001). There were no significant differences in DA concentrations between PND14 and PND21 synaptosomes. Concentrations of DA in PND21 synaptosomes were not significantly different from adult (PND70) synaptosomes (Dreiem and Seegal, unpublished observation). Total DOPAC concentrations (the sum of synaptosomal and medium DOPAC) mirrored age-related changes in synaptosomal DA, increasing significantly from levels seen in PND7 synaptosomes (54.3 ± 3.1 ng/mg protein) to 83.9 ± 1.8 ng/mg protein in PND14 and 84.9 ± 2.7 ng/mg protein in PND21 synaptosomes (Fig. 3C) (p ≤ 0.001).
FIG. 3.
Concentrations of synaptosomal DA (A), medium DA (B), and total DOPAC (C), expressed as nanogram per milligram protein, in P3 synaptosomes derived from PND7, PND14, and PND21 rat pups. Columns represent means and bars represent SEM of 10–12 wells combined from four independent experiments; ***p ≤ 0.001, significantly different with respect to PND7 animals.
DISCUSSION
FRM, and to a lesser extent DE-71, reduced synaptosomal DA levels in striatal synaptosomes from 7-, 14-, and 21-day-old rat pups. FRM was generally twofold to threefold more potent than DE-71. This observation is similar to the findings of Mariussen and Fonnum (2001, 2003), who report approximately threefold greater inhibition of [3H]-DA uptake into adult rat synaptosomes by the commercial PCB mixtures A1254 and Aroclor 1242 (A1242) compared with DE-71. The FRM-induced reductions in synaptosomal DA reported here were accompanied by large increases in medium DA levels, similar to the effects observed after exposure to PCB congeners that inhibit the plasma membrane DAT (Bemis and Seegal, 2004; Garris et al., 2003; Mariussen and Fonnum, 2001) and the DAT blockers nomifensine and GBR12935 (Bemis and Seegal, 2004; Dreiem and Seegal, unpublished results). These findings suggest that FRM acts via DAT inhibition to reduce synaptosomal DA levels and increase medium DA levels. In vivo, activation of presynaptic DA autoreceptors by extrasynaptosomal DA can downregulate DA synthesis (Wolf and Roth, 1990), and this mechanism has been suggested to be partly responsible for PCB-induced DA reductions in vivo (Seegal et al., 2002). In the present study, however, the sum of total DA and total DOPAC was not altered by PCB or PBDE exposure, whereas a reduction would have been expected if DA synthesis was reduced. Therefore, feedback regulation of DA synthesis is not a likely mechanism for the observed results. Another possible mechanism for reductions in synaptosomal DA is inhibition of the vesicular DA transporter, VMAT, and several PCB congeners act via this mechanism (Bemis and Seegal, 2004; Mariussen et al., 1999). However, VMAT inhibition decreases medium DA and increases DOPAC levels, as seen after exposure to the VMAT inhibitor RO4-1284 (Bemis and Seegal, 2004). Thus, the large medium DA increases and DOPAC reductions that were observed after FRM exposure (Fig. 1) cannot be caused by VMAT inhibition. Therefore, we conclude that the observed effects of FRM are mediated by DAT inhibition, leading to reduced reuptake of released DA.
DE-71 caused much smaller reductions in synaptosomal DA than FRM at all ages. The reductions in synaptosomal DA were accompanied by twofold increases in medium DA; however, DOPAC levels were not altered (Fig. 1). Thus, DE-71 cannot be classified as only inhibiting DAT or only inhibiting VMAT (which manifest as elevations in total DOPAC levels), and the effects of DE-71 probably involve low-level inhibition of both transporters, as was reported for PCB congeners 153 (2,2′,4,4′,5,5′-hexachlorobiphenyl) and 103 (2,2′,4,5′,6-pentachlorobiphenyl) (Bemis and Seegal, 2004). In contrast to the observations in the present study, DE-71 is a potent inhibitor of VMAT in isolated vesicles (Mariussen and Fonnum, 2003). This discrepancy is likely to be due to the difference between isolated vesicles and synaptosomes. In a synaptosomal preparation, DE-71 is added to the medium and must diffuse through the synaptic membrane to the vesicular membrane before it can exert its effect on VMAT. This process may lower its access and thereby its potency as a VMAT inhibitor compared with its effects in isolated vesicles. Thus, it is likely that the effects of DE-71 observed here arise from low-level inhibition of both VMAT and DAT.
We used Bliss’ model of independence to evaluate the extent to which there were interactions between FRM and DE-71 on dopaminergic neurochemistry in striatal synaptosomes. The experimental data agreed with the model (Fig. 2), indicating that there is no interaction between FRM and DE-71 on DA function. We therefore conclude that the effects of FRM and DE-71 are additive. FRM contains a large proportion of the potent DAT inhibitors A1254 and A1242 (Bemis and Seegal, 2004; Mariussen and Fonnum, 2001) and was found in our experiments to alter DA levels in a manner consistent with DAT inhibition (Fig. 1). DE-71 has previously been shown to be a weak DAT inhibitor and a potent VMAT inhibitor in isolated synaptic vesicles. However, although our results confirmed the weak DAT inhibition by DE-71, we have shown that DE-71 is a much less potent VMAT inhibitor in synaptosomes (Fig. 1) than in isolated vesicles (Mariussen and Fonnum, 2003). Thus, the combination of the potent DAT inhibitor FRM and the weak DAT inhibitor DE-71 causes additive effects on DA levels in isolated synaptosomes, whereas the weak VMAT inhibition by DE-71 appears to play little or no role in the combined effect of FRM and DE-71. Although kinetic studies of the effects of FRM and DE-71 on DAT were not undertaken in the present study, it is possible that FRM and DE-71 act in a noncompetitive manner to inhibit DAT function, e.g., by binding to different binding sites on the transporter. This could be considered to satisfy the underlying assumption of Bliss independence, namely that compounds with different molecular mechanisms act in an additive manner. However, in many cases, Bliss’ independence model and “concentration addition” (Loewe and Muischnek, 1926), the accepted additivity model when assuming the same mechanism for the individual compounds in a mixture, yield similar results and both accurately predict additivity (Andersen et al., 2009; Payne et al., 2000). Thus, the finding that FRM and DE-71 combine in accordance with Bliss’ additivity cannot be interpreted as a confirmation of their mode of action.
The observed additive effect is in agreement with results of previous environmental contaminant combination studies, which found mainly additive effects of methylmercury, PCBs, and BFRs on glutamate uptake into rat brain synaptosomes and of methylmercury and PCB on viability of rat pheochromocytoma cells (Andersen et al., 2009; Vettori et al., 2006). In contrast, other studies have described synergistic effects of PCBs and BFRs on behavior and cytotoxicity (Eriksson et al., 2006; Gao et al., 2009; He et al., 2009), and it is possible that PCBs and BFRs have synergistic effects on these endpoints. However, these studies did not use recognized models for prediction of mixture effects and the dose-response relationships of both toxicants were not taken into consideration. A common way to find an expected additive effect is known as “effect addition,” where the expected effect of the mixture is assumed to be equal to the sum of the individual effects of the compounds in a mixture. However, several studies have shown that effect addition frequently underestimates effects at low doses and overestimates effects at high doses, resulting in erroneous interpretations of synergy and/or antagonism (Andersen et al., 2009; Rajapakse et al., 2002; Silva et al., 2002). Our present results, obtained using an accepted method for interpretation of interactions, demonstrate that a mixture of PCBs and PBDEs has additive effects on dopaminergic neurochemistry during postnatal development.
The effects of FRM and the FRM/DE-71 mixture were greater in synaptosomes from PND7 animals than in synaptosomes from PND14 and PND 21 animals, demonstrating that the dopaminergic system is more sensitive to PCBs 1 week after birth than later in postnatal development. In contrast, DE-71 effects on DA and DOPAC levels were not dependent on the age of the animals from which the synaptosomes were derived. Because DAT inhibition appears to be mainly responsible for the effects of FRM and the FRM/DE-71 mixture, age differences in DAT activity may be responsible for the different effects at PND7, PND14, and PND21. We and others have previously reported that striatal DAT activity and ligand binding are much lower at birth and during the first postnatal week compared with adults and then increase rapidly during the following 3 weeks (Dreiem et al., 2009; Tarazi et al., 1998). Thus, because DAT activity is low at PND7 compared with PND14 and PND21, the effects of DAT inhibitors such as FRM may cause greater effects at PND7 than at PND14 and PND21. Developmental differences in D2 receptor activation or DA release could further contribute to the observed age differences in the effects of FRM. D2 receptor messenger RNA is present in striatum of PND1 rats and increases steadily until PND28 (Srivastava et al., 1992); however, [3H]spiroperidol binding and apomorphine or quinpirole inhibition was much lower during early developmental periods compared with adult levels (Srivastava et al., 1992; Wang and Pitts, 1995), suggesting that D2 receptor binding or activation is less efficient during the first 2 postnatal weeks. Less efficient D2-mediated inhibition of DA release at PND7 compared with PND14 and PND21 could further contribute to the observed differences in the effect of FRM and the FRM/DE-71 mixture at the different developmental ages. Gazzara and Andersen (1994) assessed striatal DA release from PND5 and adult rats by microdialysis and suggested that a greater proportion of synthesized DA was released from nerve terminals in 5-day-old pups compared with adults. Greater DA release from PND7 synaptosomes compared with PND14 and PND21 synaptosomes could potentiate the effects of FRM at PND7, in particular in combination with the less efficient DA reuptake in PND7 preparations. Higher DA release and lower DA reuptake at PND7 can also explain the finding that medium DA was much higher in control preparations from PND7 animals compared with synaptosomes from PND14 and PND21 animals (Fig. 3B). We propose that the low DAT activity (possibly in combination with higher DA release and less efficient D2 autoreceptor activity) at PND7 compared with PND14 and PND21 exacerbates the effect of FRM and the FRM/DE-71 mixture at this age. This causes greater effects of DAT inhibitors such as FRM and FRM/DE-71 at PND7 than at PND14 and PND21. In contrast, DE-71 alone is a much less potent DAT inhibitor than FRM, and no age dependency of DE-71 effects was observed in the present study.
Recent publications have demonstrated that PCBs and/or DE-71 induce significant neurotoxicity in SH-SY5Y cells (He et al., 2009), cerebellar granule cells (Reistad et al., 2006), and in primary cultures of mouse neurons and glia (Giordano et al., 2008); however, in all instances, exposure to these contaminants was for a period of time between 3 and 24 h. In contrast, we exposed synaptosomes to the above contaminants for only 30 min—a period of time insufficient to induce loss of synaptosomal viability analogous to cellular/neuronal apoptosis. Furthermore, even when synaptosomes were exposed for 30 min to DE-71, significant reductions in membrane potential were seen only at concentrations greater than 50μM (Mariussen and Fonnum, 2003). Thus, changes in compartmentalization of DA observed in the present study, particularly following exposure to FRM, are unlikely to reflect reductions in synaptosomal function/viability but instead reflect the consequences of DAT inhibition on DA compartmentalization.
The concentrations of FRM and DE-71 used in the present study are relatively high compared with the concentrations found in the environment, particularly for DE-71. However, until recently, the BFR levels in the environment have been increasing (Alaee and Wenning, 2002), and subpopulations who ingest large amounts of contaminated seafood may be exposed to much higher levels than the general population (Thomsen et al., 2008). Both PCBs and PBDEs can cross the blood-placenta barrier and enter the fetal brain during development (Bergonzi et al., 2009; Bi et al., 2006; Mazdai et al., 2003). The first 3 weeks of postnatal development in rats correspond to the last trimester of human development (Rice and Barone, 2000); therefore, the greater effects observed in PND7 preparations compared with PND14 and PND21 preparations suggest that the early period of the last trimester is a critical window for the effects of PCBs and PBDEs. However, human brain development continues for several years after birth (Rice and Barone, 2000; Weickert et al., 2007), and thus, the sensitive window in humans may be of long duration. Both PCBs and BFRs are secreted into breast milk (Noren and Meironyte, 2000), and it has been estimated that a breastfed infant in the United States would be exposed to 1525 ng PBDEs per day (Schecter et al., 2005). Infants and toddlers also frequently engage in mouthing behavior and ingest dust and particles that may contain high levels of BFRs from household sources, and Allen et al. (2007) estimated that children under 5 years would be exposed to three to four times higher PBDE levels compared with adults and that house dust accounts for >80% of exposure in children. Thus, the exposure levels for small children may be significant. Although it is necessary to extrapolate effects from in vitro experiments to the intact organism and from experimental animals to humans, the results of the present study demonstrate that organohalogen contaminants such as PCBs and PBDEs cause additive effects on dopaminergic neurochemistry. Thus, for risk assessment, it is necessary to consider the total exposure to such chemicals in order to be able to more accurately determine risk.
In summary, we have shown that (1) FRM elevates medium DA and reduces synaptosomal DA concentrations to a greater extent than DE-71, (2) the effects of FRM, but not DE-71, are dependent on the age of the animal from which the synaptosomes were derived, (3) the effects of the combination of the environmentally relevant PCB mixture FRM and DE-71 is greater at PND7 and PND14 than at PND21, and (4) that FRM and DE-71 have additive effects on the developmental dopaminergic system in vitro at all ages. The results of the present study demonstrate that it is necessary to take into account the cumulative exposure to organohalogen contaminants such as PCBs and PBDEs during risk assessment.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (1R01ES015688-01) to R.F.S.
Supplementary Material
Acknowledgments
We wish to thank Leslie Eisele of the Wadsworth Center Biochemistry Shared Instrumentation Core for her assistance. The authors declare that there are no conflicting interests.
References
- Alaee M, Wenning RJ. The significance of brominated flame retardants in the environment: current understanding, issues and challenges. Chemosphere. 2002;46:579–582. doi: 10.1016/s0045-6535(01)00224-7. [DOI] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Nelson JW, Webster TF. Personal exposure to polybrominated diphenyl ethers (PBDEs) in residential indoor air. Environ. Sci. Technol. 2007;41:4574–4579. doi: 10.1021/es0703170. [DOI] [PubMed] [Google Scholar]
- Andersen IS, Voie OA, Fonnum F, Mariussen E. Effects of methyl mercury in combination with polychlorinated biphenyls and brominated flame retardants on the uptake of glutamate in rat brain synaptosomes: a mathematical approach for the study of mixtures. Toxicol. Sci. 2009;112:175–184. doi: 10.1093/toxsci/kfp178. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. Polychlorinated biphenyls and methylmercury act synergistically to reduce rat brain dopamine content in vitro. Environ. Health Perspect. 1999;107:879–885. doi: 10.1289/ehp.99107879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol. Sci. 2004;80:288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Bergonzi R, Specchia C, Dinolfo M, Tomasi C, De PG, Frusca T, Apostoli P. Distribution of persistent organochlorine pollutants in maternal and foetal tissues: data from an Italian polluted urban area. Chemosphere. 2009;76:747–754. doi: 10.1016/j.chemosphere.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol. Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Bi X, Qu W, Sheng G, Zhang W, Mai B, Chen D, Yu L, Fu J. Polybrominated diphenyl ethers in South China maternal and fetal blood and breast milk. Environ. Pollut. 2006;144:1024–1030. doi: 10.1016/j.envpol.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environ. Health Perspect. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss CI. The toxicity of poisons applied jointly. Ann. Appl. Biol. 1939;26:585–615. [Google Scholar]
- Borgert CJ, Borgert SA, Findley KC. Synergism, antagonism, or addtivity of dietary supplements: application of theory to case studies. Thromb. Res. 2005;117:123–132. doi: 10.1016/j.thromres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Alleva E, Costa LG. Polybrominated diphenyl ethers: neurobehavioral effects following developmental exposure. Neurotoxicology. 2003;24:449–462. doi: 10.1016/S0161-813X(03)00020-2. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Vitalone A, Madia F, Santucci D, Alleva E, Costa LG. Early developmental exposure to BDE 99 or Aroclor 1254 affects neurobehavioural profile: interference from the administration route. Neurotoxicology. 2005;26:183–192. doi: 10.1016/j.neuro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Chishti MA, Fisher JP, Seegal RF. Aroclors 1254 and 1260 reduce dopamine concentrations in rat striatal slices. Neurotoxicology. 1996;17:653–660. [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–1067. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, Ramakers GM, Gardoni F, van Kleef RG, Bergman A, Di LM, van den BM, Westerink RH, Vijverberg HP. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ. Health Perspect. 2007;115:865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreiem A, Shan MT, Okoniewski RJ, Sanchez-Morrissey S, Seegal RF. Methylmercury inhibits dopaminergic function in rat pup synaptosomes in an age-dependent manner. Neurotoxicol. Teratol. 2009;31:312–317. doi: 10.1016/j.ntt.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Erickson MD. Analytical Chemistry of PCBs. Boca Raton, FL: CRC Press; 1997. [Google Scholar]
- Eriksson P, Fischer C, Fredriksson A. Polybrominated diphenyl ethers, a group of brominated flame retardants, can interact with polychlorinated biphenyls in enhancing developmental neurobehavioral defects. Toxicol. Sci. 2006;94:302–309. doi: 10.1093/toxsci/kfl109. [DOI] [PubMed] [Google Scholar]
- Gao P, He P, Wang AG, Xia T, Xu BY, Xu ZX, Niu Q, Guo LJ, Chen XM. Influence of PCB153 on oxidative DNA damage and DNA repair-related gene expression induced by PBDE-47 in human neuroblastoma cells in vitro. Toxicol. Sci. 2009;107:165–170. doi: 10.1093/toxsci/kfn224. [DOI] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PE, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Gazzara RA, Andersen SL. Calcium dependency and tetrodotoxin sensitivity of neostriatal dopamine release in 5-day-old and adult rats as measured by in vivo microdialysis. J. Neurochem. 1994;62:1741–1749. doi: 10.1046/j.1471-4159.1994.62051741.x. [DOI] [PubMed] [Google Scholar]
- Giordano G, Kavanagh TJ, Costa LG. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol. Appl. Pharmacol. 2008;232:161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KA, Klebanoff MA, Brock JW, Zhou H, Darden R, Needham L, Longnecker MP. In utero exposure to background levels of polychlorinated biphenyls and cognitive functioning among school-age children. Am. J. Epidemiol. 2005;162:17–26. doi: 10.1093/aje/kwi158. [DOI] [PubMed] [Google Scholar]
- He P, Wang AG, Xia T, Gao P, Niu Q, Guo LJ, Xu BY, Chen XM. Mechanism of the neurotoxic effect of PBDE-47 and interaction of PBDE-47 and PCB153 in enhancing toxicity in SH-SY5Y cells. Neurotoxicology. 2009;30:10–15. doi: 10.1016/j.neuro.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ. Sci. Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J. Pediatr. 2003;143:780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Padgett RJ, Brumitt GA, Billings RL. Effects of prenatal PCB exposure on cognitive processing efficiency and sustained attention. Dev. Psychol. 1992;28:297–306. [Google Scholar]
- Kodavanti PR, Ward TR, Ludewig G, Robertson LW, Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships. Toxicol. Sci. 2005;88:181–192. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Altenburger R. Synergisms with mixtures of xenoestrogens: a reevaluation using the method of isoboles. Sci. Total Environ. 1998;221:59–73. doi: 10.1016/s0048-9697(98)00261-7. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Hansen LG, Widholm JJ, Fitzpatrick RD, Olson JR, Helferich JL, Kim KH, Sable HJ, Seegal RF, Pessah IN, et al. Formulation and characterization of an experimental PCB mixture designed to mimic human exposure from contaminated fish. Toxicol. Sci. 2005;88:400–411. doi: 10.1093/toxsci/kfi338. [DOI] [PubMed] [Google Scholar]
- Lamensdorf I, Eisenhofer G, Harvey-White J, Nechustan A, Kirk K, Kopin IJ. 3,4-Dihydroxyphenylacetaldehyde potentiates the toxic effects of metabolic stress in PC12 cells. Brain Res. 2000;868:191–201. doi: 10.1016/s0006-8993(00)02309-x. [DOI] [PubMed] [Google Scholar]
- Law RJ, Allchin CR, De BJ, Covaci A, Herzke D, Lepom P, Morris S, Tronczynski J, de Wit CA. Levels and trends of brominated flame retardants in the European environment. Chemosphere. 2006;64:187–208. doi: 10.1016/j.chemosphere.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Lilienthal H, Neuf M, Munoz C, Winneke G. Behavioral effects of pre- and postnatal exposure to a mixture of low chlorinated PCBs in rats. Fundam. Appl. Toxicol. 1990;15:457–467. doi: 10.1016/0272-0590(90)90032-f. [DOI] [PubMed] [Google Scholar]
- Loewe S, Muischnek H. Uber Kombinationswirkungen: 1. Mittelteilung: Hilfsmittel der Fragestellung. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmacol. 1926;114:313–326. [Google Scholar]
- Löscher W, Bohme G, Muller F, Pagliusi S. Improved method for isolating synaptosomes from 11 regions of one rat brain: electron microscopic and biochemical characterization and use in the study of drug effects on nerve terminal gamma-aminobutyric acid in vivo. J. Neurochem. 1985;45:879–889. doi: 10.1111/j.1471-4159.1985.tb04076.x. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fjeld E, Breivik K, Steinnes E, Borgen A, Kjellberg G, Schlabach M. Elevated levels of polybrominated diphenyl ethers (PBDEs) in fish from Lake Mjosa, Norway. Sci. Total Environ. 2008;390:132–141. doi: 10.1016/j.scitotenv.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem. Int. 2003;43:533–542. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Morch AJ, Fonnum F. The effect of polychlorinated biphenyls on the uptake of dopamine and other neurotransmitters into rat brain synaptic vesicles. Toxicol. Appl. Pharmacol. 1999;161:274–282. doi: 10.1006/taap.1999.8806. [DOI] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren K, Meironyte D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40:1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- Oades RD, Sadile AG, Sagvolden T, Viggiano D, Zuddas A, Devoto P, Aase H, Johansen EB, Ruocco LA, Russell VA. The control of responsiveness in ADHD by catecholamines: evidence for dopaminergic, noradrenergic and interactive roles. Dev. Sci. 2005;8:122–131. doi: 10.1111/j.1467-7687.2005.00399.x. [DOI] [PubMed] [Google Scholar]
- Payne J, Rajapakse N, Wilkins M, Kortenkamp A. Prediction and assessment of the effects of mixtures of four xenoestrogens. Environ. Health Perspect. 2000;108:983–987. doi: 10.1289/ehp.00108983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ. Health Perspect. 2002;110:917–921. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reistad T, Fonnum F, Mariussen E. Neurotoxicity of the pentabrominated diphenyl ether mixture, DE-71, and hexabromocyclododecane (HBCD) in rat cerebellar granule cells in vitro. Arch. Toxicol. 2006;80:785–796. doi: 10.1007/s00204-006-0099-8. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone SJ. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108(Suppl. 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roegge CS, Seo BW, Crofton KM, Schantz SL. Gestational-lactational exposure to Aroclor 1254 impairs radial-arm maze performance in male rats. Toxicol. Sci. 2000;57:121–130. doi: 10.1093/toxsci/57.1.121. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J. Occup. Environ. Med. 2005;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Seegal RF, Okoniewski RJ, Brosch KO, Bemis JC. Polychlorinated biphenyls alter extraneuronal but not tissue dopamine concentrations in adult rat striatum: an in vivo microdialysis study. Environ. Health Perspect. 2002;110:1113–1117. doi: 10.1289/ehp.021101113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”–eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol. 2002;36:1751–1756. doi: 10.1021/es0101227. [DOI] [PubMed] [Google Scholar]
- Srivastava LK, Morency MA, Mishra RK. Ontogeny of dopamine D2 receptor mRNA in rat brain. Eur. J. Pharmacol. 1992;225:143–150. doi: 10.1016/0922-4106(92)90094-c. [DOI] [PubMed] [Google Scholar]
- Stewart P, Reihman J, Gump B, Lonky E, Darvill T, Pagano J. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol. Teratol. 2005;27:771–780. doi: 10.1016/j.ntt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci. Lett. 1998;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Knutsen HK, Liane VH, Froshaug M, Kvalem HE, Haugen M, Meltzer HM, Alexander J, Becher G. Consumption of fish from a contaminated lake strongly affects the concentrations of polybrominated diphenyl ethers and hexabromocyclododecane in serum. Mol. Nutr. Food Res. 2008;52:228–237. doi: 10.1002/mnfr.200700123. [DOI] [PubMed] [Google Scholar]
- Vettori MV, Goldoni M, Caglieri A, Poli D, Folesani G, Ceccatelli S, Mutti A. Antagonistic effects of methyl-mercury and PCB153 on PC12 cells after a combined and simultaneous exposure. Food Chem. Toxicol. 2006;44:1505–1512. doi: 10.1016/j.fct.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol. Appl. Pharmacol. 2003;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Wang L, Pitts DK. Ontogeny of nigrostriatal dopamine neuron autoreceptors: iontophoretic studies. J. Pharmacol. Exp. Ther. 1995;272:164–176. [PubMed] [Google Scholar]
- Weickert CS, Webster MJ, Gondipalli P, Rothmond D, Fatula RJ, Herman MM, Kleinman JE, Akil M. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2007;144:1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Roth RH. Autoreceptor regulation of dopamine synthesis. Ann. N. Y. Acad. Sci. 1990;604:323–343. doi: 10.1111/j.1749-6632.1990.tb32003.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.