Abstract
Past studies in rodent models identified the suppression of primary humoral immune responses as one of the most sensitive sequela associated with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure. Yet, the sensitivity of humoral immunity to TCDD in humans represents an important toxicological data gap. Therefore, the objectives of this investigation were two-fold. The first was to assess the induction of known aryl hydrocarbon receptor (AHR)–responsive genes in primary human B cells as a measure of early biological responses to TCDD. The second was to evaluate the direct effect of TCDD on CD40 ligand–induced immunoglobulin M (IgM) secretion by human primary B cells. The effects of TCDD on induction of AHR-responsive genes and suppression of the IgM response were also compared with B cells from a TCDD-responsive mouse strain, C57BL/6. AHR-responsive genes in human B cells exhibited slower kinetics and reduced magnitude of induction by TCDD when compared with mouse B cells. Evaluation of B-cell function from 12 donors identified two general phenotypes; the majority of donors exhibited similar sensitivity to suppression by TCDD of the IgM response as mouse B cells, which was not attributable to decreased B-cell proliferation. In a minority of donors, no suppression of the IgM response by TCDD was observed. Although donor-to-donor variation in sensitivity to TCDD was observed, human B cells from the majority of donors evaluated showed impairment of effector function by TCDD. Collectively, data presented in this series of studies demonstrate that TCDD impairs the humoral immunity of humans by directly targeting B cells.
Keywords: TCDD, immunotoxicology, antibody response, human B cell, AHR
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and dioxin-like compounds (DLCs) are ubiquitous environmental pollutants with related chemical structures. TCDD and DLCs also share the characteristic of being highly lipophilic, a property that contributes to their bioaccumulation in the food chain, making diet the primary route of animal and human exposure (Schecter et al., 2001). TCDD, the prototypic compound of this group, has been studied extensively in laboratory animals and serves as a model compound for studies of toxicities and mechanisms of action involving the aryl hydrocarbon receptor (AHR) (Rowlands and Gustafsson, 1997). Upon ligand binding, the cytosolic AHR translocates into the nucleus, where it forms a heterodimer with the AHR nuclear translocator (ARNT). The ligand-AHR-ARNT complex then binds to defined nucleotide sequences, termed dioxin-responsive element, located in promoter or enhancer regions of various “AHR-responsive” genes to regulate transcription (Hankinson, 1995). The induction of AHR-responsive genes, such as cytochrome P450 1A1 (CYP1A1), has been commonly used to identify AHR transcriptional activation.
A variety of toxicities, including immune suppression, are observed in laboratory animals treated with TCDD (Safe, 1986). In murine models, suppression of the primary antibody response by TCDD has historically been observed as a particularly sensitive endpoint (Holsapple et al., 1991). In contrast, few studies have directly assessed the toxicity of TCDD and DLCs in humans or using human-derived primary cells or tissues, including potential adverse effect on immune competence. Interestingly, epidemiological studies suggest a potential association between impairment of humoral immunity and exposure to DLCs in humans. Decreased plasma levels of IgG were reported in a study of residents living in areas contaminated by dioxin in Seveso, Italy, as well as in Korean veterans exposed to Agent Orange during the Vietnam War (Baccarelli et al., 2002; Kim et al., 2003). Likewise, in a study of Dutch preschool children, prenatal exposure to DLCs was correlated with lower antibody levels after primary vaccination and higher prevalence of recurrent middle ear infections (Weisglas-Kuperus et al., 2000). In a study of Yusho (Yu-Cheng) patients who were accidentally exposed to DLCs, decreased serum immunoglobulin M (IgM) and IgA levels were observed (Lu and Wu, 1985; Nakanishi et al., 1985).
Previous spleen cell separation-reconstitution studies identified the B cell as the cell-type most sensitive to impairment by TCDD in the primary antibody response in mice (Dooley and Holsapple, 1988; Holsapple et al., 1986; Tucker et al., 1986). The first studies that extended the immunotoxicological investigation of TCDD from mouse to humans used tonsillar lymphocytes. Results from these studies suggested that TCDD could, in fact, modulate antibody secretion by human B cells (Wood and Holsapple, 1993; Wood et al., 1992, 1993). Concordant with the above observations, AHR expression was confirmed in human and mouse B cells (Allan and Sherr, 2005; Williams et al., 1996) as was the absolute requirement of AHR in suppression of IgM response by TCDD, at least in mice (Sulentic et al., 1998; Vorderstrasse et al., 2001). In spite of these important investigations, to date, no study has comprehensively characterized TCDD-mediated induction of AHR-responsive genes or the direct effects on antibody responses in primary human B cells. Therefore, in light of these previous findings, the objectives of the present series of studies were to investigate the direct effects of TCDD on freshly isolated human peripheral blood B cells in order to characterize the time- and concentration-dependent induction of AHR-responsive genes as an early biological response to TCDD and to assess the primary IgM response as a functional outcome of exposure to TCDD in human B cells.
MATERIALS AND METHODS
Chemical and cell culture.
TCDD (99.1% pure) was obtained from Accustandard (New Haven, CT) as a solution in dimethyl sulfoxide (DMSO). Isolated human or mouse B cells were cultured in RPMI-1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated bovine calf serum (HyClone, Logan, UT), 100 U/ml of penicillin (Invitrogen), 100 μg/ml of streptomycin (Invitrogen), and 50μM 2-mercaptoethanol. The stable-transfected mouse fibroblast line expressing human CD40 ligand (CD40L; CD40L-L cell) was a generous gift from Dr David Sherr (Boston University School of Public Health). CD40L-L cells were maintained in Dulbecco's Modified Eagle Medium (Invitrogen) supplemented with 10% bovine calf serum (HyClone), 100 U/ml of penicillin, 100 μg/ml of streptomycin, 50μM of 2-mercaptoethanol, and 1× HT supplement (Invitrogen). In all cases, cells were cultured at 37°C in 5% CO2.
Mice.
Virus-free female C57BL/6 mice (6 weeks of age) were purchased from Charles River (Portage, MI). Mice were randomized, transferred to plastic cages containing sawdust bedding (five mice per cage), and quarantined for 1 week. Mice were provided food (Purina certified laboratory chow) and water ad libitum and were not used for experimentation until their body weight was 17–20 g. Animal holding rooms were kept at 21°C–24°C and 40–60% humidity with a 12-h light/dark cycle. All experiments, as described, were approved by the Michigan State University Institutional Animal Care and Use Committee.
Human leukocyte packs.
Human leukocyte packs collected from anonymous donors were purchased from the Gulf Coast Regional Blood Center (Houston, TX). All donors were screened for human immunodeficiency virus and hepatitis at the blood center.
Isolation of mouse and human B cells.
Mouse B cells were isolated from spleens that were made into single-cell suspensions by passage through a 40-μm cell strainer (BD Biosciences, San Jose, CA). Human naive (CD19+CD27−) B cells were isolated from peripheral blood mononuclear cells (PBMCs) enriched from each leukocyte pack by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ). Negative selection of human or mouse B cells was conducted using MACS Naive human B-cell or Mouse B-Cell Isolation Kits following the manufacturer's protocols (Miltenyi Biotec, Auburn, CA) and as described previously (Lu et al., 2009a). In all cases, the purity of isolated B cells was ≥ 95%.
Real-time PCR.
Total RNA was isolated using the RNeasy Kit (Qiagen, Valencia, CA) following the manufacturer's protocol. RNA was reverse transcribed into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The expression of target genes was determined by TaqMan real-time PCR using ABI PRISM 7900HT Sequence Detection System (Applied Biosystems). Relative steady-state mRNA levels of the target genes were calculated and normalized to the endogenous reference, 18S ribosomal RNA using the ΔΔCT method. All primers were purchased from Applied Biosystems: human CYP1A1 (Hs00153120_m1), mouse CYP1A1 (Mm00487217), human CYP1A2 (Hs01070374_m1), mouse CYP1A2 (Mm00487224), human CYP1B1 (Hs00164383_m1), mouse CYP1B1 (Mm00487229_m1), human AHR repressor (Hs01005075_m1), mouse AHR repressor (Mm00477443_m1), human TCDD-inducible poly (ADP-ribose) polymerase (TIPARP) (Hs00296054_m1), mouse TIPARP (Mm00724822_m1), human NAD(P)H dehydrogenase quinone 1 (NQO1) (Hs00168547_m1), mouse NQO1 (Mm00500821_m1), human aldehyde dehydrogenase 3 family, member 1 (ALDH3A1) (Hs00167469_m1), mouse ALDH3A1 (Mm00839312_m1), and human glutathione S-transferase A1 (GSTA1) (Hs00275575_m1).
CD40L-dependent in vitro IgM response.
CD40L-L cells were trypsinized, irradiated, and seeded into culture plates 1 day prior to experimentation. CD40L-L cells were routinely checked by flow cytometry to ensure high-level expression of human CD40L. To induce in vitro IgM responses, a two-phase culture system was employed. In phase I, isolated naive human or mouse B cells (1 × 106 cells/ml) were cocultured with irradiated CD40L-L cells in the presence or absence of 10 U/ml of recombinant human or mouse interleukin (IL-2) (Roche Applied Science, Indianapolis, IN), 100 U/ml of IL-6 (human IL-6 from Roche Applied Science, mouse IL-6 from Jena Bioscience, Jena, Germany), and 20 ng/ml of recombinant human or mouse IL-10 (Bender MedSystems, Burlingame, CA) for 3 (mouse) or 4 days (human). In experiments involving TCDD treatment, both human and mouse B cells were treated immediately prior to coculture with CD40L-L cells and ILs and were exposed to TCDD for the duration of the culture period. The vehicle (VH) for TCDD was DMSO. In phase II, B cells were transferred to new culture plates without CD40L-L cells on day 3 or 4 and were cultured for an additional 3 days prior to being harvested for enzyme-linked immunospot (ELISPOT) analysis. The number of viable cells was assessed with a Coulter particle counter (Beckman Coulter, Fullerton, CA) following lysis of dead cells by treatment with pronase (Calbiochem, San Diego, CA) as described previously (Schatz et al., 1993).
Enumeration of IgM-secreting cells by ELISPOT.
ELISPOT was performed as described previously (Lu et al., 2009b). Briefly, ELISPOT wells were coated with purified antihuman (BD Biosciences) or mouse (Sigma-Aldrich, St Louis, MO) IgM antibody and blocked with 5% bovine serum albumin. Harvested cells were washed and incubated in the ELISPOT wells for 16–20 h. Biotin-conjugated antihuman or mouse IgM antibody (Sigma) and streptavidin-horseradish peroxidase (Sigma) were sequentially added to the wells. The spots were developed with the Aminoethylcarbazole Staining Kit (Sigma). Data were collected and analyzed using the Cellular Technology Ltd ImmunoSpot system (Cellular Technology Ltd, Shaker Heights, OH).
Sequence analysis of exons of the human AHR.
Total DNA was isolated from frozen human leukocytes from each donor using DNeasy Blood and Tissue Kit (Qiagen) following the manufacturer's protocol. Sequence analysis was conducted using a 3730xl DNA analyzer (Applied Biosystems) by Functional Biosciences, Inc. (Madison, WI). Primers used for the sequencing reactions were predesigned for each exon of the human AHR and tested using human genomic DNA standard.
Flow cytometric analysis of B-cell proliferation activated by CD40L.
The B-cell proliferation studies were conducted as described previously (Lu et al., 2009a). In brief, isolated mouse or naive human B cells were incubated with 5μM of CellTrace Violet (Violet Cell Proliferation Kit, Invitrogen) at 5 × 106 cells/ml following manufacturer's protocol. Labeled cells were washed, adjusted to the desired cell density, cocultured with CD40L-L cells in the presence of IL-2, IL-6, and IL-10, and harvested on day 4 (mouse) or day 5 (human) for flow cytometric analysis. Live/Dead Fixable Near-Infrared Dead Cell Stain Kit (Invitrogen) was used to exclude dead cells per the manufacturer's protocol. Cells were assessed on a FACSCantoII cell analyzer (BD Biosciences) and analyzed using FlowJo (Tree Star, Ashland, OR) off-line analysis software.
Statistical analysis.
Graphpad Prism 4.00 (Graphpad Software, San Diego, CA) was used for all statistical analysis. The mean ± SE was determined for each treatment group in the individual experiments. Homogeneous data were evaluated by one-way ANOVA, and Dunnett's two-tailed t-test was used to compare treatment groups with the VH control when significant differences were observed. For data expressed as either fold change (Figs. 6 and 7) or percent (Fig. 9), logarithmic transformation was conducted prior to statistical analysis as described previously (Kaplan et al., 2010).
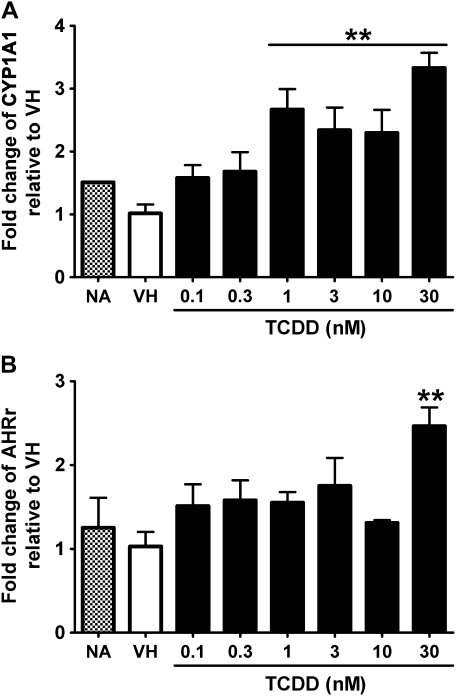
FIG. 6.
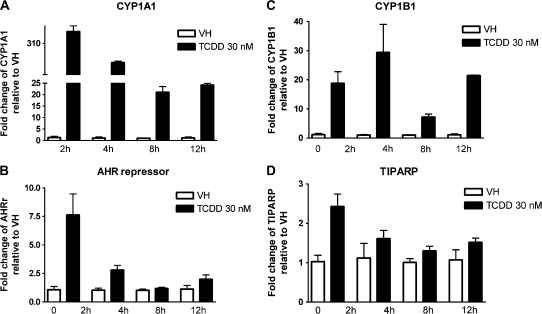
Concentration-dependent induction of CYP1A1 and AHR repressor expression by TCDD in naive human B cells. Naive human B cells (1 × 106/ml) were treated with TCDD at indicated concentrations or VH (0.05% DMSO) for 12 h. Total RNA was isolated, and steady-state mRNA levels of (A) CYP1A1 and (B) AHR repressor were measured by TaqMan real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared with the VH control group. **p < 0.01, compared with VH control group. These data are representative of three separate experiments using B cells isolated from three individual donors.
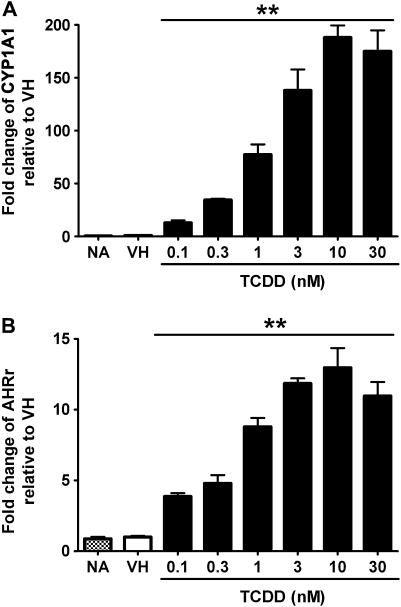
FIG. 7.
Concentration-dependent induction of CYP1A1 and AHR repressor expression by TCDD in mouse B cells. Mouse B cells (1 × 106/ml) were treated with TCDD at indicated concentrations or VH (0.05% DMSO) for 2 h. Total RNA was isolated, and steady-state mRNA levels of (A) CYP1A1 and (B) AHR repressor were measured by TaqMan real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared with the VH control group. **p < 0.01, compared with VH control group. Data are representative of two separate experiments with three experimental replicates per group.
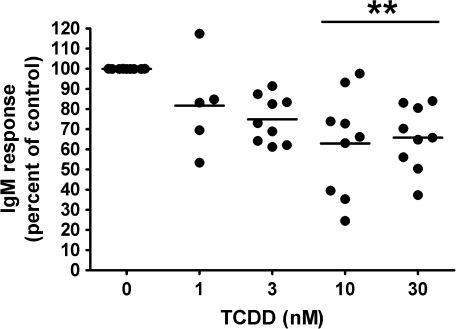
FIG. 9.
Analysis of TCDD-mediated effect on the CD40L-induced IgM response in human B cells from multiple “responsive” donors. **p < 0.01, compared with the VH control group.
RESULTS
Expression Kinetics of AHR-Responsive Genes in TCDD-Treated B Cells
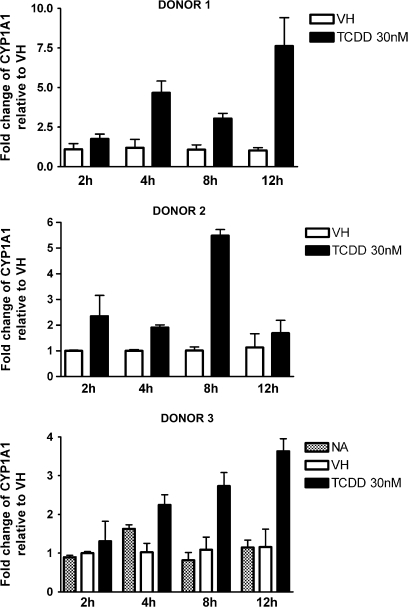
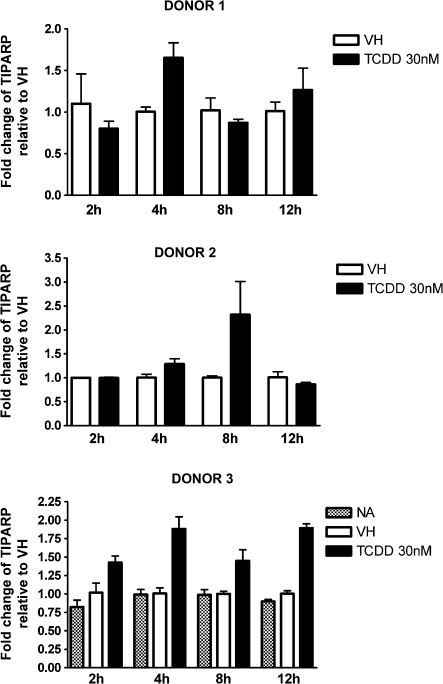
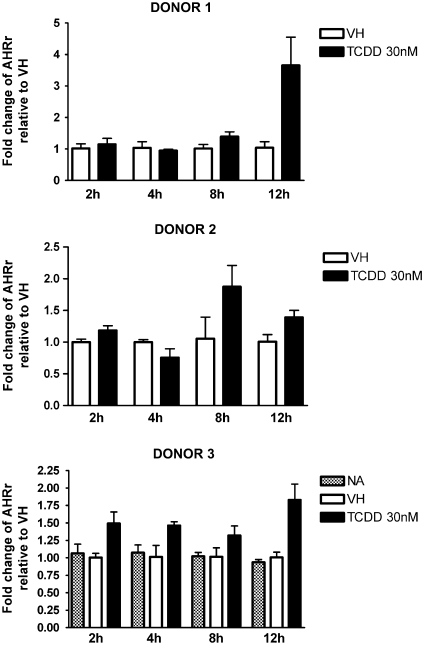
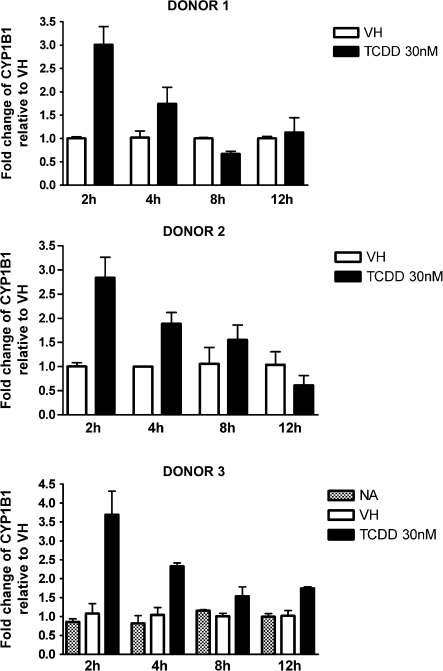
CYP1A1 is expressed in human PBMCs and can be further induced by treatment with TCDD or 3-methylcholanthrene (Nohara et al., 2006; Yamamoto et al., 2004). In the present study, naive B cells were isolated from three individual donors, treated with VH or 30nM TCDD, and incubated for various time periods to obtain the expression kinetics of AHR-responsive genes induced by TCDD. Because of the limited number of B cells recovered from each leukocyte pack, NA (no treatment) group was only included in one of three donors. In preliminary studies in which TCDD was used over a broad range of concentrations (0.3–30nM), a wide variety of well-characterized AHR-responsive genes were measured in human B cells treated with TCDD and either not detected (CYP1A2, ALDH3A1, and GSTA1) or not significantly induced by TCDD (NQO1) (data not shown). In mouse B cells, neither CYP1A2 nor ALDH3A1 was found to be expressed, and NQO1 was not significantly induced by TCDD (data not shown). Therefore, the present studies focused on CYP1A1, CYP1B1, AHR repressor, and TIPARP, which were consistently detected and induced by TCDD in B cells from multiple human donors. As shown in Figures 1–4, CYP1A1 and CYP1B1 were induced by TCDD more robustly when compared with AHR repressor and TIPARP in naive human B cells. Moreover, the four genes demonstrated distinct expression kinetics with the induction of CYP1B1 being more rapid and less sustained over the time course compared with CYP1A1 and AHR repressor. A similar time course study was conducted using mouse B cells. In contrast to the expression kinetics in naive human B cells, all four genes responded more rapidly in mouse B cells, as indicated by the peak time of induction and with greater magnitude of induction, as exhibited by the fold change compared with the VH control (Fig. 5). In mouse B cells, the induction of CYP1A1, AHR repressor, and TIPARP peaked at 2 h and declined at later time points, while CYP1B1 exhibited the greatest magnitude of induction at 2 h but also showed biphasic expression kinetics between 4 and 12 h. When the fold induction was compared among the four genes, CYP1A1 was the most highly induced gene by TCDD in both naive human and mouse B cells.
FIG. 1.
Time-dependent induction of CYP1A1 gene expression by TCDD in naive human B cells. Naive human B cells (1 × 106/ml) were treated with 30nM of TCDD or VH (0.05% DMSO) for 2, 4, 8, and 12 h. Total RNA was isolated, and steady-state mRNA levels of CYP1A1 were measured by TaqMan real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared with the VH control group at the same time point. The results are the mean ± SE as determined for each group. Data from three individual donors are presented.
FIG. 4.
Time-dependent induction of TIPARP gene expression by TCDD in naive human B cells. Naive human B cells (1 × 106/ml) were treated with 30nM of TCDD or VH (0.05% DMSO) for 2, 4, 8, and 12 h. Total RNA was isolated, and steady-state mRNA levels of TIPARP were measured by TaqMan real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared with the VH control group at the same time point. The results are the mean ± SE as determined for each group. Data from three individual donors are presented.
FIG. 5.
Time-dependent induction of (A) CYP1A1, (B) AHR repressor, (C) CYP1B1, and (D) TIPARP gene expression by TCDD in mouse B cells. Mouse B cells (1 × 106/ml) were treated with 30nM of TCDD or VH (0.05% DMSO) for the indicated time periods. Total RNA was isolated, and steady-state mRNA levels of (A) CYP1A1, (B) AHR repressor, (C) CYP1B1, and (D) TIPARP were measured by TaqMan real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared with the VH control group at the same time point. Data are representative of two separate experiments with three experimental replicates per group.
FIG. 2.
Time-dependent induction of AHR repressor gene expression by TCDD in naive human B cells. Naive human B cells (1 × 106/ml) were treated with 30nM of TCDD or VH for 2, 4, 8, and 12 h. Total RNA was isolated, and steady-state mRNA levels of AHR repressor were measured by TaqMan real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared with the VH control group at the same time point. The results are the mean ± SE as determined for each group. Data from three individual donors are presented.
FIG. 3.
Time-dependent induction of CYP1B1 gene expression by TCDD in naive human B cells. Naive human B cells (1 × 106/ml) were treated with 30nM of TCDD or VH for 2, 4, 8, and 12 h. Total RNA was isolated, and steady-state mRNA levels of CYP1B1 were measured by TaqMan real-time PCR and normalized to endogenous 18S ribosomal RNA. Data are presented as fold change compared with the VH control group at the same time point. The results are the mean ± SE as determined for each group. Data from three individual donors are presented.
Concentration-Dependent Induction of AHR-Responsive Genes in TCDD-Treated B Cells
No study to date has characterized the concentration-dependent expression of AHR-responsive genes in human B cells treated with TCDD for comparison to a TCDD-responsive mouse strain such as the C57BL/6 mouse. For these experiments, the induction of AHR-responsive genes was measured at the peak time of induction for human and mouse B cells over an extensive range of TCDD concentrations spanning from 0.1 to 30nM. CYP1A1 and AHR repressor were genes of choice because they were induced consistently in human B cells derived from multiple donors, and their expression followed similar kinetics in both human and mouse B cells. Twelve h and 2 h were selected as the peak time for CYP1A1 and AHR repressor in human and mouse B cells, respectively, based on results from kinetic studies described above. As shown in Figures 6 and 7, both genes were induced in human and mouse B cells treated with TCDD in a concentration-dependent manner. When the magnitude of induction was compared between human and mouse B cells, it appeared that both CYP1A1 and AHR repressor were induced with greater magnitude in mouse B cells than in human B cells.
TCDD Effects on CD40L-Induced IgM Responses of B Cells
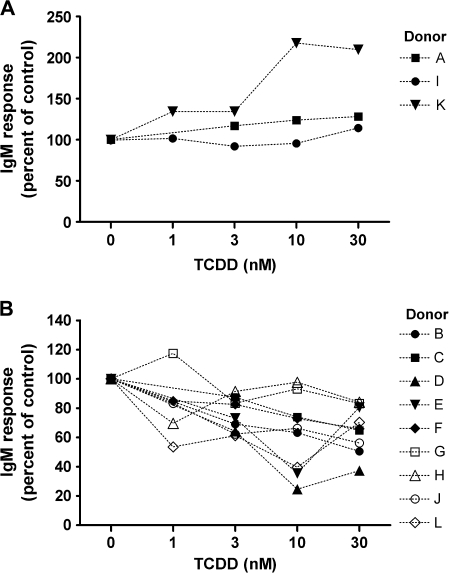
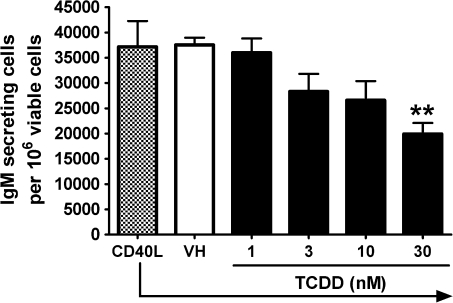
To assess the TCDD-mediated effect on the IgM antibody response in naive human B cells for comparison to primary mouse B cells, a CD40L-dependent in vitro B cell activation model was used (Lu et al., 2009a). The CD40L-dependent in vitro B cell activation model strongly induced IgM antibody responses in both human and mouse B cells using virtually identical culture conditions. For assessment of human IgM responses, B cells isolated from 12 human donors were assayed. Because of differences between donors with respect to the magnitude of each donor’s control antibody response, the IgM responses in all treatment groups were normalized to percentage of the VH-treated group (presented as 0nM of TCDD) for B cells from each donor. The results from multiple donors are presented in Figure 8. For some donors, treatment groups for only 3, 10, and 30nM TCDD were used because of the limited number of B cells recovered. Figure 8A shows data from three donors whose B cells were either not affected by TCDD at all concentrations tested or in one case, donor K, actually exhibited enhancement by TCDD treatment at higher concentrations. Interestingly, B cells from donors A, I, and K showed no suppression of the IgM response at doses as high as 100, 50, and 50nM, respectively (data not shown). In Figure 8B, data are included from nine donors whose B cells exhibited some level of suppression of the IgM response by TCDD. In Figure 9, results from the nine donors were pooled such that each of the nine “responsive” donors was considered as one biological replicate in one “experiment,” ultimately consisting of a total of nine replicates. Using this approach, 10 and 30nM of TCDD significantly suppressed the CD40L-induced IgM response to approximately 60–70% of VH control group in B cells isolated from responsive donors. The two distinct “phenotypes” observed among B cells from different donors are intriguing, and the mechanistic basis remains to be elucidated. In light of the critical role of the AHR in mediating TCDD toxicities including the immunotoxicity on B cells, the inherent differences within the AHR gene among humans may contribute to the different B cell–specific phenotypes observed in the present study. In preliminary studies that aimed at characterizing such potential differences, we sequenced the exons of the AHR from three responsive donors and three “nonresponsive” donors among all the donors we assayed thus far. Interestingly, two of the three nonresponsive donors were found to possess previously characterized polymorphisms within the exons of the AHR: one has 132 T > C in codon 44 of exon 2 encoding part of the basic helix-loop-helix domain and the other has 1661 G > A in codon 554 of exon 10 encoding the transactivation domain (Harper et al., 2002). By contrast, no polymorphism was identified in the exons of the AHR from the three responsive donors. Consistent with the responsive human donors, the CD40L-induced IgM antibody response in mouse B cells was suppressed by TCDD treatment in a concentration-dependent manner with 30nM of TCDD decreasing the response to approximately 50% of the VH control group (Fig. 10). In both human and mouse B-cell experiments, TCDD did not significantly affect cell viability as assessed at the end of the culture period for each culture; however, there was a decrease in cell number per culture in human B cells (∼15–20%).
FIG. 8.
Effect of TCDD on the CD40L-induced IgM response in human B cells: (A) “nonresponsive” donors versus (B) “responsive” donors. Naive human B cells (1 × 106/ml) were treated with TCDD at indicated concentrations or VH and then cultured with irradiated CD40L-L cells (1.5–3 × 103 cells per well) in the presence of recombinant human IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 4 days, and CD40L stimulation was removed on day 4. Cells were cultured for another 3 days prior to being harvested on day 7 to enumerate IgM-secreting cells by ELISPOT. The cell number was determined by a particle counter, and the viability was assessed by the pronase activity assay. Data were normalized to no TCDD group (100%) and presented as percentage of control. B cells from multiple human donors were assessed.
FIG. 10.
Effect of TCDD on the CD40L-induced IgM response in mouse B cells. Mouse B cells (1 × 106/ml) were treated with TCDD at concentrations indicated or VH and then cocultured with irradiated CD40L-L cells (5 × 104 cells per well) in the presence of recombinant mouse IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 3 days, and CD40L stimulation was removed on day 3. Cells were cultured for another 3 days prior to being harvested on day 6 to enumerate IgM-secreting cells by ELISPOT. The cell number was determined by a particle counter, and the viability of cells was determined by pronase activity assay. **p < 0.01, compared with the VH control group. Data are representative of three separate experiments with at least three experimental replicates per group.
TCDD Effects on CD40L-Induced Proliferation of B Cells
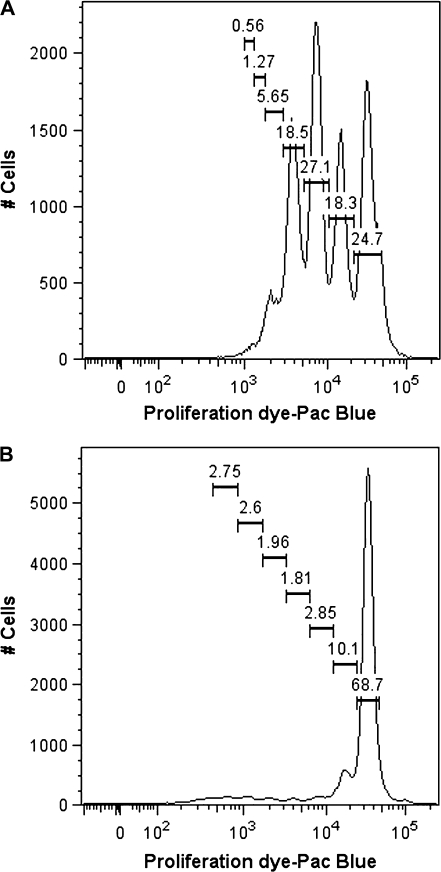
A critical function of B cells is the ability to respond to various stimuli by rapid self-expansion. B-cell proliferation not only amplifies the magnitude of the antibody response by expanding the cells that are committed to an antibody-secreting phenotype but may also serve a regulatory role (Tangye and Hodgkin, 2004). Robust proliferation of both mouse and naive human B cells was induced by the CD40L activation model employed in the present study (Lu et al., 2009a). Therefore, the effect of TCDD on CD40L-induced proliferation of mouse and naive human B cells was also characterized using flow cytometry. As shown in Figure 11 and Table 1, CD40L induced human B cells to undergo multiple divisions with TCDD treatment having no significant effect on the number of divisions. A slightly fewer number of cells progressed to the later divisions in the highest TCDD (30nM) treatment group, although the effect was very modest. Studies were conducted using B cells from three different donors; although the level of proliferation varied, the lack of significant TCDD effect was consistent. In parallel experiments, fewer mouse B cells were induced by CD40L to undergo division when compared with human B cells, although the number of divisions that the proliferating cells underwent was comparable (Table 1). Similar to human B cells, TCDD did not significantly effect CD40L-induced mouse B-cell proliferation. These results suggest that decreased proliferation is not responsible for the suppression of CD40L-induced IgM response by TCDD. These results are also consistent with previous studies in which mouse and human B-cell proliferation, as measured indirectly by [3H]-thymidine incorporation, was found to be relatively refractory to TCDD (Allan et al., 2006; Dooley and Holsapple, 1988; Holsapple et al., 1986; Wood and Holsapple, 1993).
FIG. 11.
CD40L-induced proliferation of human and mouse B cells. Human or mouse B cells (1 × 106/ml) were labeled with the proliferation dye and then cocultured with irradiated CD40L-L cells (1.5 × 103 cells per well for human and 5 × 104 cells per well for mouse) in the presence of recombinant human or mouse IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 4 or 3 days, and CD40L stimulation was removed on day 4 or 3. Cells were cultured for one additional day prior to being harvested on day 5 or 4 to flow cytometric analysis. Dead cells were excluded from the analysis using the Live/Dead Near-Infrared Dead Cell Staining Kit. Data are representative of two separate experiments (for human, two experiments using B cells derived from two separate donors) and are concatenated from three experimental replicates per group.
TABLE 1.
Effect of TCDD on CD40L-Induced Proliferation of Human and Mouse B Cells
| Percentage of total viable cells |
|||||||
| Treatment groups | Division 0 | Division 1 | Division 2 | Division 3 | Division 4 | Division 5 | Division 6 |
| Human B cells | |||||||
| CD40L | 24.7 | 18.3 | 27.1 | 18.5 | 5.65 | 1.27 | 0.56 |
| CD40L + VH | 24.7 | 16.9 | 28.1 | 19.8 | 5.09 | 1.19 | 0.4 |
| CD40L + TCDD 1nM | 23.6 | 21.1 | 32.2 | 15.4 | 3.11 | 0.59 | 0.21 |
| CD40L + TCDD 3nM | 22.9 | 18 | 31.2 | 18.6 | 3.95 | 0.93 | 0.3 |
| CD40L + TCDD 10nM | 23.6 | 18.8 | 31.4 | 17.7 | 3.59 | 0.71 | 0.22 |
| CD40L + TCDD 30nM | 22.7 | 19.7 | 32.1 | 17.1 | 3.51 | 0.72 | 0.25 |
| Mouse B cells | |||||||
| CD40L | 68.8 | 10.1 | 2.85 | 1.81 | 1.96 | 2.6 | 2.75 |
| CD40L + VH | 64.8 | 11.7 | 3.76 | 2.48 | 2.23 | 2.83 | 3.08 |
| CD40L + TCDD 1nM | 63.6 | 11.4 | 4.21 | 2.66 | 2.54 | 3.44 | 3.35 |
| CD40L + TCDD 3nM | 63.5 | 11.4 | 4.42 | 3.03 | 2.74 | 3.47 | 3.51 |
| CD40L + TCDD 10nM | 62.5 | 11.7 | 4.71 | 3.05 | 2.75 | 3.43 | 3.25 |
| CD40L + TCDD 30nM | 59.9 | 13.5 | 5.46 | 3.39 | 2.88 | 3.58 | 3.06 |
The experimental design was as described in the legend for Figure 11. Human or mouse B cells were treated with TCDD at concentrations indicated prior to activation by CD40L. The values are concatenated from three experimental replicates per treatment group. Human or mouse B cells (1 × 106/ml) were labeled with the proliferation dye, treated with TCDD at concentrations indicated or VH, and then cocultured with irradiated CD40L-L cells (1.5 × 103 cells per well for human and 5 × 104 cells per well for mouse) in the presence of recombinant human or mouse IL-2 (10 U/ml), IL-6 (100 U/ml), and IL-10 (20 ng/ml) for 4 or 3 days, and CD40L stimulation was removed on day 4 or 3. Cells were cultured for one additional day prior to being harvested on day 5 or 4 to flow cytometric analysis. Dead cells were excluded from the analysis using the Live/Dead Near-Infrared Dead Cell Staining Kit. Data are representative of two separate experiments and are concatenated from three experimental replicates per group.
DISCUSSION
Previous studies, mostly conducted in mice, have demonstrated that TCDD is a potent immunotoxicant capable of producing numerous immune perturbations including profound suppression of the primary antibody response (Holsapple et al., 1991). Because of similarities between the mouse and human immune system, TCDD-mediated immunotoxicity in mice has raised serious concerns pertaining to effects on immune competence in humans, including suppression of humoral immunity. This notion has been further supported by studies of a limited number of cohorts where an association was observed between exposure to TCDD and/or DLCs and altered circulating antibody levels (Baccarelli et al., 2002; Kim et al., 2003; Lu and Wu, 1985; Nakanishi et al., 1985; Weisglas-Kuperus et al., 2000). It is important to emphasize that this epidemiologic association has been, at best, suggestive because of numerous confounding factors; the most serious being the absence of information concerning levels of exposure to these chemicals. Because of these and numerous other confounding factors coupled with technical limitations of studying immune function in primary human leukocytes, little is known about the immunotoxicology of TCDD in humans. For the first time in this report, the direct effects of TCDD on human peripheral blood B-cell function were evaluated. Our studies show that although well-characterized genes within the “AHR-responsive gene battery” were markedly less sensitive to induction by TCDD in human B cells than in mouse B cells, suppression of the IgM antibody response by TCDD was remarkably similar in sensitivity between human “responders” and the C57BL/6 mouse. Equally noteworthy, 3 of the 12 donors evaluated in this study, termed “nonresponders,” exhibited no suppression of the IgM responses, even at high TCDD concentrations.
In studies characterizing the time- and concentration-dependent AHR-responsive gene expression profiles induced by TCDD, the expression kinetics of CYP1A1, AHR repressor, and TIPARP were slower in human B cells when compared with B cells from C57BL/6 mice. CYP1B1 was an exception, because it was induced most robustly by TCDD as early as 2 h posttreatment in both human and mouse B cells. These results were consistent with the expression kinetics of CYP1A1 in mouse and human mononuclear cells treated with TCDD (Nohara et al., 2006). Likewise, the overall magnitude of induction for AHR-responsive genes in human B cells was not as pronounced as in mouse B cells. For example, at the time of peak expression for both CYP1A1 and the AHR repressor, the fold induction by TCDD was modest in human B cells compared with mouse B cells. Importantly, a number of notable differences between the human and mouse AHR have been identified, which may contribute to the observed differential effects. For instance, because of a difference in amino acid sequence identified within the N-terminal ligand-binding domain at residue A375V, the human AHR has approximately 10-fold lower relative affinity for TCDD than the AHRb allele carried by the C57BL/6 mouse (Ema et al., 1994). Likewise, the human AHR and the C57BL/6 AHR share limited sequence homology within their transactivation domains (Flaveny et al., 2010). In addition, the mouse and human AHRs differentially recruit LXXLL coactivator motif proteins (Flaveny et al., 2008). Collectively, one or more of the aforementioned distinctions between the human and mouse AHR may account for the differences observed here in the induction kinetics and magnitude of TCDD-responsive genes in B cells. The expression profiles of the AHR-responsive genes measured here reflect the level of AHR activation and the general transcriptional activity of AHR induced by TCDD, as opposed to being directly linked to altered lymphocyte function.
To evaluate the effects of TCDD on human B-cell function, we chose to use a polyclonal B-cell activation model that mimics the cognate interaction between T cell–associated CD40L and the B cell–associated CD40. This B-cell activation model also requires direct addition of T cell–derived B-cell differentiating cytokines IL-2, IL-6, and IL-10 (Lu et al., 2009b). The approach has a number of important strengths. This B-cell activation model strongly induces the differentiation of human peripheral blood B cells into antibody-secreting cells, which in itself poses significant technical challenges when studying human B-cell function. Employing a CD40L-expressing fibroblast line (CD40L-L cells) as the source of CD40L insured high-level CD40L expression and eliminates the requirement for T helper accessory cells for studies of T cell–dependent B-cell differentiation. Moreover, the CD40L-L cells were irradiated prior to initiation of the experiment, again insuring that their only role is to serve as stimulators of B cells through CD40-CD40L interaction. Therefore, a significant strength of this approach is that it assesses the direct effects of TCDD specifically on B cells. Utilizing a polyclonal activation approach is also critical for evaluating the primary antibody response because the number of naive B-cell precursors, in peripheral blood of any given donor, possessing antigen receptors to a given defined antigen is extremely low. Because of the high level of sequence homology shared between human and mouse CD40L protein (Spriggs et al., 1992), irradiated CD40L-L cells, which express the human CD40L, are equally capable of activating mouse and human B cells, allowing for very similar culture conditions for activating B cells from both species. Last, because only B cells are used in the assay, induction of a mixed lymphocyte response because of haplotype mismatch is eliminated. It is noteworthy that since accessory cells are not required in the CD40L-dependent B-cell activation model system used, these studies do not rule out the possibility that TCDD may also produce effects on accessory cell function, which could further contribute to suppression of humoral immunity in humans in addition to the direct effects described here on B cells. However, the role of altered accessory cell function on suppression of primary antibody responses by TCDD in the mouse has been previously reported to be modest, but similar studies have yet to be conducted using human primary leukocytes, mainly because of the technical hurdles discussed above (Dooley and Holsapple, 1988; Dooley et al., 1990).
Although peripheral blood leukocytes represent one of the most accessible human tissues available for research, there have been few immunotoxicological assessments of xenobiotics using human leukocytes and likewise very few investigating the immunotoxicity of TCDD using human primary leukocytes. Significant challenges including donor-to-donor variability, limitation in the number of leukocytes that can be obtained from a given donor, and the technical obstacles in inducing specific immunological responses using human leukocytes have been the limiting factors for these types of investigations. Studies by Wood and Holsapple (1993) were the first to investigate the effects of TCDD on human primary B cells derived from tonsils (Wood et al., 1992, 1993). However, a common concern pertaining to the use of tonsils as the source of leukocytes is that they are rarely obtained from healthy donors. With this modest caveat, these studies demonstrated that when using the superantigen toxic shock syndrome toxin-1 (TSST-1) to induce IgM response in human tonsillar B cells in the presence of irradiated T cells, the IgM response was suppressed by TCDD (Wood and Holsapple, 1993). Moreover, the suppression of the TSST-1–induced antibody response was not associated with a decrease by TCDD in [3H]-thymidine incorporations, suggesting that B-cell proliferation was unaffected.
The present studies extended the initial investigations of TCDD on human B cells by Wood and Holsapple (1993) and provided a number of new and important insights. While the IgM responses in naive B cells from the 12 human donors exhibited various degrees of sensitivity to TCDD, perhaps most interesting was the observation that three of these donors showed no suppression of the IgM response. In fact, B cells from two of the three donors were subjected to exceptionally high concentrations of TCDD, up to 50 and 100nM. The mechanistic basis for this lack of responsiveness to suppression by TCDD of the IgM response in these three donors is unclear, but it is tempting to speculate that it is due, in part, to polymorphisms in the AHR. In humans, a surprisingly small number of sequence variations, or polymorphisms, have been identified within the AHR, and convincing associations between these polymorphisms and phenotypes as demonstrated by AHR-mediated responses are yet to be established (Harper et al., 2002). In the present study, polymorphisms were identified in the coding regions of the AHR in two out of the three human donors whose B cells were insensitive to TCDD. One of the two polymorphisms identified, codon 554 G > A, was shown to cause failure to induce CYP1A1 induction in vitro when combined with another two AHR polymorphisms (Wong et al., 2001). Moreover, identification of 3 out of 12 donors who were refractory to suppression of the IgM response, at least under the assay conditions used here, suggests that this phenotype is not particularly rare. When the IgM response data were pooled for the remaining nine responsive donors and expressed as percentage of control, even with the level of variability observed among human donors, TCDD treatment at 10 and 30nM significantly suppressed the IgM response in human B cells. It is important to emphasize that in this investigation, only naive human peripheral blood B cells, as assessed by CD27−, were used rather than the entire peripheral blood B-cell pool. The rationale for excluding memory B cells is that greater than 95% of splenic B cells in C57BL/6 mice possess a phenotype that is close to naive human peripheral blood B cells (CD27−), and therefore, in order to make meaningful comparisons between mouse and human B cells in this investigation, naive human B cells were used exclusively (Lu et al., 2009b). Using this strategy, we observed that the relative magnitude of suppression between donors was comparable to mouse B cells, with the exception of the nonresponsive donors. Also similar between human and mouse B cells is the insensitivity to modulation of CD40L-induced proliferation by TCDD. In fact, to our knowledge, this is the first time that the effects of TCDD on B-cell proliferation have been directly assessed, as opposed to prior indirect measurements such as DNA synthesis. One notable difference between mouse and human B cells was their overall responsiveness to CD40L-induced proliferation with a significantly greater percentage of human B cells being induced to proliferate than mouse B cells.
Interestingly, in light of the diminished magnitude of induction by TCDD of AHR battery genes in human B cells compared with mouse, the finding that there was similar sensitivity to suppression of the IgM responses by TCDD between B cells from C57BL/6 mice and responsive donors was surprising. One possible interpretation for this lack of concordance between TCDD-mediated AHR-responsive gene induction and suppression of B-cell function is that suppression of the IgM response, although dependent on the AHR, may involve events that are not solely dependent on transcriptional regulation. It is also intriguing that in a recent study using genetically engineered mice expressing the human AHR, a greater number of genes functionally clustered around cell cycle regulation and the immune response were differentially regulated after TCDD treatment than in mice expressing the mouse AHR (Flaveny et al., 2010). Conversely, in mice expressing the mouse AHR, a greater number of genes functionally clustered around metabolism and membrane transport were differentially regulated after TCDD treatment than in mice expressing the human AHR.
In summary, the present study provides novel comparisons between primary human and C57BL/6 mouse B cells. These data are especially important given the concerns raised in the context of data extrapolation from rodents to the human. In fact, it has been well recognized that immunotoxicological investigations conducted exclusively using animal models may not always be predictive of human toxicity (Selgrade, 1999; Vos and Van Loveren, 1998). This point was illustrated recently using this same CD40L-dependent B-cell activation model, in which it was observed that arsenic markedly suppressed the IgM response in mouse but not human B cells (Lu et al., 2009a). Therefore, in light of the uncertainties associated with cross-species differences in toxicity, studies are presently ongoing using this B-cell activation model for further in-depth investigations into the mechanisms by which TCDD impairs the IgM response in human B cells.
FUNDING
National Institutes of Health grants (R01 ES002520, P42 ES004911); research grant from the Dow Chemical Company to N.E.K.; research fellowship from the Syngenta AG to H.L.
Acknowledgments
We thank Dr David Sherr at Boston University School of Public Health for generously providing human CD40L-expressing mouse fibroblasts, Dr Sandra O'Reilly for cell irradiation, and Mrs Kimberly Hambleton for administrative assistance in preparing and submitting the manuscript.
References
- Allan LL, Schlezinger JJ, Shansab M, Sherr DH. CYP1A1 in polycyclic aromatic hydrocarbon-induced B lymphocyte growth suppression. Biochem. Biophys. Res. Commun. 2006;342:227–235. doi: 10.1016/j.bbrc.2006.01.131. [DOI] [PubMed] [Google Scholar]
- Allan LL, Sherr DH. Constitutive activation and environmental chemical induction of the aryl hydrocarbon receptor/transcription factor in activated human B lymphocytes. Mol. Pharmacol. 2005;67:1740–1750. doi: 10.1124/mol.104.009100. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Mocarelli P, Patterson DG, Jr, Bonzini M, Pesatori AC, Caporaso N, Landi MT. Immunologic effects of dioxin: new results from Seveso and comparison with other studies. Environ. Health Perspect. 2002;110:1169–1173. doi: 10.1289/ehp.021101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley RK, Holsapple MP. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: I. The role of the B lymphocyte. Immunopharmacology. 1988;16:167–180. doi: 10.1016/0162-3109(88)90005-7. [DOI] [PubMed] [Google Scholar]
- Dooley RK, Morris DL, Holsapple MP. Elucidation of cellular targets responsible for tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of antibody responses: II. The role of the T-lymphocyte. Immunopharmacology. 1990;19:47–58. doi: 10.1016/0162-3109(90)90026-b. [DOI] [PubMed] [Google Scholar]
- Ema M, Ohe N, Suzuki M, Mimura J, Sogawa K, Ikawa S, Fujii-Kuriyama Y. Dioxin binding activities of polymorphic forms of mouse and human arylhydrocarbon receptors. J. Biol. Chem. 1994;269:27337–27343. [PubMed] [Google Scholar]
- Flaveny C, Reen RK, Kusnadi A, Perdew GH. The mouse and human Ah receptor differ in recognition of LXXLL motifs. Arch. Biochem. Biophys. 2008;471:215–223. doi: 10.1016/j.abb.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaveny CA, Murray IA, Perdew GH. Differential gene regulation by the human and mouse aryl hydrocarbon receptor. Toxicol. Sci. 2010;114:217–225. doi: 10.1093/toxsci/kfp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- Harper PA, Wong JY, Lam MS, Okey AB. Polymorphisms in the human AH receptor. Chem. Biol. Interact. 2002;141:161–187. doi: 10.1016/s0009-2797(02)00071-6. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Dooley RK, McNerney PJ, McCay JA. Direct suppression of antibody responses by chlorinated dibenzodioxins in cultured spleen cells from (C57BL/6 x C3H)F1 and DBA/2 mice. Immunopharmacology. 1986;12:175–186. doi: 10.1016/0162-3109(86)90001-9. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Morris DL, Wood SC, Snyder NK. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced changes in immunocompetence: possible mechanisms. Annu. Rev. Pharmacol. Toxicol. 1991;31:73–100. doi: 10.1146/annurev.pa.31.040191.000445. [DOI] [PubMed] [Google Scholar]
- Kaplan BL, Lawver JE, Karmaus PW, Ngaotepprutaram T, Birmingham NP, Harkema JR, Kaminski NE. The effects of targeted deletion of cannabinoid receptors CB1 and CB2 on intranasal sensitization and challenge with adjuvant-free ovalbumin. Toxicol. Pathol. 2010;38:382–392. doi: 10.1177/0192623310362706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HA, Kim EM, Park YC, Yu JY, Hong SK, Jeon SH, Park KL, Hur SJ, Heo Y. Immunotoxicological effects of Agent Orange exposure to the Vietnam War Korean veterans. Ind. Health. 2003;41:158–166. doi: 10.2486/indhealth.41.158. [DOI] [PubMed] [Google Scholar]
- Lu H, Crawford RB, North CM, Kaplan BL, Kaminski NE. Establishment of an immunoglobulin m antibody-forming cell response model for characterizing immunotoxicity in primary human B cells. Toxicol. Sci. 2009a;112:363–373. doi: 10.1093/toxsci/kfp224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Kaplan BL, Ngaotepprutaram T, Kaminski NE. Suppression of T cell costimulator ICOS by Delta9-tetrahydrocannabinol. J. Leukoc. Biol. 2009b;85:322–329. doi: 10.1189/jlb.0608390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YC, Wu YC. Clinical findings and immunological abnormalities in Yu-Cheng patients. Environ. Health Perspect. 1985;59:17–29. doi: 10.1289/ehp.59-1568085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y, Shigematsu N, Kurita Y, Matsuba K, Kanegae H, Ishimaru S, Kawazoe Y. Respiratory involvement and immune status in Yusho patients. Environ. Health Perspect. 1985;59:31–36. doi: 10.1289/ehp.59-1568074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohara K, Ao K, Miyamoto Y, Ito T, Suzuki T, Toyoshiba H, Tohyama C. Comparison of the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced CYP1A1 gene expression profile in lymphocytes from mice, rats, and humans: most potent induction in humans. Toxicology. 2006;225:204–213. doi: 10.1016/j.tox.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit. Rev. Toxicol. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- Safe SH. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu. Rev. Pharmacol. Toxicol. 1986;26:371–399. doi: 10.1146/annurev.pa.26.040186.002103. [DOI] [PubMed] [Google Scholar]
- Schatz AR, Koh WS, Kaminski NE. Delta 9-tetrahydrocannabinol selectively inhibits T-cell dependent humoral immune responses through direct inhibition of accessory T-cell function. Immunopharmacology. 1993;26:129–137. doi: 10.1016/0162-3109(93)90005-b. [DOI] [PubMed] [Google Scholar]
- Schecter A, Cramer P, Boggess K, Stanley J, Papke O, Olson J, Silver A, Schmitz M. Intake of dioxins and related compounds from food in the U.S. population. J. Toxicol. Environ. Health A. 2001;63:1–18. doi: 10.1080/152873901750128326. [DOI] [PubMed] [Google Scholar]
- Selgrade MK. Use of immunotoxicity data in health risk assessments: uncertainties and research to improve the process. Toxicology. 1999;133:59–72. doi: 10.1016/s0300-483x(99)00006-2. [DOI] [PubMed] [Google Scholar]
- Spriggs MK, Armitage RJ, Strockbine L, Clifford KN, Macduff BM, Sato TA, Maliszewski CR, Fanslow WC. Recombinant human CD40 ligand stimulates B cell proliferation and immunoglobulin E secretion. J. Exp. Med. 1992;176:1543–1550. doi: 10.1084/jem.176.6.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulentic CE, Holsapple MP, Kaminski NE. Aryl hydrocarbon receptor-dependent suppression by 2,3,7, 8-tetrachlorodibenzo-p-dioxin of IgM secretion in activated B cells. Mol. Pharmacol. 1998;53:623–629. [PubMed] [Google Scholar]
- Tangye SG, Hodgkin PD. Divide and conquer: the importance of cell division in regulating B-cell responses. Immunology. 2004;112:509–520. doi: 10.1111/j.1365-2567.2004.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AN, Vore SJ, Luster MI. Suppression of B cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol. Pharmacol. 1986;29:372–377. [PubMed] [Google Scholar]
- Vorderstrasse BA, Steppan LB, Silverstone AE, Kerkvliet NI. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol Appl Pharmacol. 2001;171:157–164. doi: 10.1006/taap.2000.9122. [DOI] [PubMed] [Google Scholar]
- Vos JG, Van Loveren H. Experimental studies on immunosuppression: how do they predict for man? Toxicology. 1998;129:13–26. doi: 10.1016/s0300-483x(98)00059-6. [DOI] [PubMed] [Google Scholar]
- Weisglas-Kuperus N, Patandin S, Berbers GA, Sas TC, Mulder PG, Sauer PJ, Hooijkaas H. Immunologic effects of background exposure to polychlorinated biphenyls and dioxins in Dutch preschool children. Environ. Health Perspect. 2000;108:1203–1207. doi: 10.1289/ehp.001081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CE, Crawford RB, Holsapple MP, Kaminski NE. Identification of functional aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator in murine splenocytes. Biochem. Pharmacol. 1996;52:771–780. doi: 10.1016/0006-2952(96)00360-7. [DOI] [PubMed] [Google Scholar]
- Wong JM, Harper PA, Meyer UA, Bock KW, Morike K, Lagueux J, Ayotte P, Tyndale RF, Sellers EM, Manchester DK, et al. Ethnic variability in the allelic distribution of human aryl hydrocarbon receptor codon 554 and assessment of variant receptor function in vitro. Pharmacogenetics. 2001;11:85–94. doi: 10.1097/00008571-200102000-00010. [DOI] [PubMed] [Google Scholar]
- Wood SC, Holsapple MP. Direct suppression of superantigen-induced IgM secretion in human lymphocytes by 2,3,7,8-TCDD. Toxicol. Appl. Pharmacol. 1993;122:308–313. doi: 10.1006/taap.1993.1200. [DOI] [PubMed] [Google Scholar]
- Wood SC, Jeong HG, Morris DL, Holsapple MP. Direct effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on human tonsillar lymphocytes. Toxicology. 1993;81:131–143. doi: 10.1016/0300-483x(93)90005-d. [DOI] [PubMed] [Google Scholar]
- Wood SC, Karras JG, Holsapple MP. Integration of the human lymphocyte into immunotoxicological investigations. Fundam. Appl. Toxicol. 1992;18:450–459. doi: 10.1016/0272-0590(92)90143-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Ihara K, Nakayama H, Hikino S, Satoh K, Kubo N, Iida T, Fujii Y, Hara T. Characteristic expression of aryl hydrocarbon receptor repressor gene in human tissues: organ-specific distribution and variable induction patterns in mononuclear cells. Life Sci. 2004;74:1039–1049. doi: 10.1016/j.lfs.2003.07.022. [DOI] [PubMed] [Google Scholar]