Abstract
Mitochondrial compromise has been documented in infants born to women infected with the human immunodeficiency virus (HIV-1) who received nucleoside reverse transcriptase inhibitor (NRTI) therapy during pregnancy. To model these human exposures, we examined mitochondrial integrity at birth and 1 year in brain cortex and liver from offspring of retroviral-free Erythrocebus patas dams-administered human-equivalent NRTI doses for the last half (10 weeks) of gestation. Additional infants, followed for 1 year, were given the same drugs as their mothers for the first 6 weeks of life. Exposures included: no drug, Zidovudine (AZT), Lamivudine (3TC), AZT/3TC, AZT/Didanosine (ddI), and Stavudine (d4T)/3TC. In brain and liver, oxidative phosphorylation (OXPHOS) enzyme activities (complexes I, II, and IV) showed minimal differences between unexposed and NRTI-exposed offspring at both times. Brain and liver mitochondria from most NRTI-exposed patas, both at birth and 1 year of age, contained significant (p < 0.05) morphological damage observed by electron microscopy (EM), based on scoring of coded photomicrographs. Brain and liver mitochondrial DNA (mtDNA) levels in NRTI-exposed patas were depleted significantly in the 3TC and d4T/3TC groups at birth and were depleted significantly (p < 0.05) at 1 year in all NRTI-exposed groups. In 1-year-old infants exposed in utero to NRTIs, mtDNA depletion was 28.8–51.8% in brain and 37.4–56.5% in liver. These investigations suggest that some NRTI-exposed human infants may sustain similar mitochondrial compromise in brain and liver and should be followed long term for cognitive integrity and liver function.
Keywords: Zidovudine, Lamivudine, Stavudine, Didanosine, electron microscopy, mitochondrial DNA quantity, oxidative phosphorylation
The United Nations Acquired Immune Deficiency Syndrome statistics regarding worldwide spread of the human immunodeficiency virus (HIV-1) (2008 Report on the Global AIDS Epidemic, http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/) indicates that the epidemic is growing fastest in sub-Saharan Africa, where more than three young women are infected for every young man, and where one in three pregnant women may be infected. Worldwide, the percentage of pregnant women receiving antiretroviral prophylaxis more than tripled (10–35%) between 2004 and 2007 and the use of antiretroviral therapy during pregnancy will continue to increase rapidly for the foreseeable future. The use of antiretroviral drugs for inhibition of maternal-fetal HIV-1 transmission has been one of the great success stories of the war on HIV-1/AIDS, and these drugs must be given to save the lives of children (Connor et al., 1994; Mofenson and Committee on Pediatric AIDS, 2000; Sperling et al., 1996); however, there is clinical evidence that infants exposed to NRTIs in utero and after birth, in compliance with the current Centers for Disease Control guidelines (Mofenson and Munderi, 2002; Perinatal HIV Guidelines Working Group, 2006; Public Health Service Task Force Perinatal HIV Guidelines Working Group, 2002; Sperling et al., 1996), may be at risk for genotoxicity (Poirier et al., 2004) and increased cancer rates (Benhammou et al., 2008).
Long-term use of nucleoside reverse transcriptase inhibitors (NRTIs) has been associated with mitochondrial toxicity that includes skeletal muscle and cardiac wasting (Beach, 1998; Dalakas et al., 1990; Lewis and Dalakas, 1995), elevated serum lactic acid (Brinkman, 2001; Gerard et al., 2000), abnormal oxidative phosphorylation (OXPHOS) enzyme activity (Mhiri et al., 1991; Tomelleri et al., 1992), and abnormal alterations in the quantity of mitochondrial (mt) DNA (Dagan et al., 2002; Lewis and Dalakas, 1995). In HIV-1–uninfected NRTI-exposed infants, because few abnormal clinical findings were reported at birth or during the early years of life (Brogly et al., 2007; Caselli et al., 2000; Culnane et al., 1999; Hanson et al., 1999; Lipshultz et al., 2000; Mofenson and Munderi, 2002; Newschaffer et al., 2000; Tuomala et al., 2002), it was generally assumed that in utero exposures were of insufficient duration to compromise the mitochondrial integrity of the fetus. However, that premise was reconsidered when two children born to mothers receiving Zidovudine (AZT) and Lamivudine (3TC) during pregnancy (Blanche et al., 1999) died at about 1 year of age from severe mitochondrial toxicity. Follow-up of a cohort of approximately 2700 HIV-1–uninfected children born to HIV-1–infected mothers and exposed in utero to NRTIs revealed some degree of mitochondrial dysfunction in approximately 30 children under the age of 10 years, many of whom had no clinical symptoms (Barret et al., 2003). Subsequent biomarker studies showed a high frequency of mitochondrial morphological damage, by electron microscopy (EM), and mtDNA depletion in umbilical cord, cord blood, and peripheral blood, taken from NRTI-exposed but HIV-1–uninfected infants (Divi et al., 2004; Poirier et al., 2003; Shiramizu et al., 2003). In addition, depletion of leukocyte mtDNA in peripheral blood was found to persist in HIV-1–uninfected 2-year-old children born to HIV-1–infected mothers receiving NRTI therapy during pregnancy (Poirier et al., 2003). Taken together, these findings suggest that long-term follow-up of infants exposed in utero to NRTIs, and development of relevant experimental models, should be given high priority.
To this end, we have used retrovirus-free Erythrocebus patas monkeys as a model for NRTI exposure in human pregnancy. This is an appropriate species because the patas placentation and NRTI pharmacokinetics are similar to those found in humans (Divi et al., 2007). The absence of retrovirus infection allows for elucidation of drug effects, and exposure of pregnant dams to human-equivalent protocols of the NRTI combinations used in clinical practice yields information directly relevant to mothers and infants. Our particular focus has been to explore the status of patas offspring born to pregnant dams given human-equivalent NRTI exposures for the final half (10 weeks) of gestation. Infants were either taken by cesarean section at term or born naturally, exposed to NRTIs for the first 6 weeks of life, and followed for the first year of life. In the current series of experiments, the exposure groups included no drug, AZT, 3TC, AZT/3TC, AZT/didanosine (ddI), and Stavudine (d4T)/3TC. The experimental design includes analysis of mitochondrial morphological evaluation by EM, OXPHOS enzyme assays using isolated mitochondria, and mtDNA quantification by hybrid capture-chemiluminescence assay (HC-CA). Because a great deal of information has been generated by this study, we have reported the results for heart, including lactic acid levels and echocardiography (Divi et al., 2005), and skeletal muscle (Divi et al., 2007) separately, and here we present data for the brain cortex and liver from the same animals.
MATERIALS AND METHODS
Monkey maintenance, NRTI sources, and exposure protocols.
Monkey maintenance and exposure have been previously described in detail (Divi et al., 2005, 2007) and will therefore be mentioned briefly here. Monkeys were maintained and exposed to NRTIs at Bioqual, Inc. (Rockville, MD) under conditions approved by the American Association for Accreditation of Laboratory Animal Care using protocols reviewed by the Institutional Animal Care and Use Committee of Bioqual, Inc. Female patas monkeys were kept with the males until the female was assessed to be pregnant, as previously described (Lu et al., 1993). Pregnant dams were given NRTIs during the final 10 or 4 weeks (depending on the exposure protocol) of the 20-week gestation. The drugs and exposure protocols for pregnant monkeys and offspring have been described previously (Divi et al., 2005, 2007). For the assays described here, three to four patas infants were typically used per exposure group, but as this varied by assay, the actual numbers used are included in the tables and figure legends.
Zidovudine (AZT) was obtained from Sigma Chemical Co. (St Louis, MO), and Lamivudine (3TC, Epivir) was obtained as a pediatric liquid clinical formulation from Glaxo-Wellcome (Raleigh, NC). All other NRTIs were purchased as clinical formulations from the NIH Veterinary Pharmacy (Bethesda, MD). Each drug was dissolved in syrup that was placed inside of a 1/4 to 1/3 piece of banana and given to the patas as a treat. AZT was given in two 20-mg doses, for a total of 40 mg/day, 5 days/week. Stavudine (d4T, Zerit) was given in two 4.5-mg doses, for a total dose of 9 mg/day, 5 days/week. Didanosine (ddI, Videx) was given as two daily doses of 20 mg (total 40 mg/day) each, 30 min before a meal, along with an antacid (Zantac) for stomach protection. AZT, d4T, and ddI were all given for the last 10 weeks of gestation. 3TC was given in two 12-mg doses for a total of 24 mg/day, 5 days/week for the last 4 weeks of gestation. Unexposed monkeys received bananas containing syrup twice daily.
Monkey offspring were taken either near-term by cesarean section (Gerschenson et al., 2000, 2004), or born naturally, hand-raised for the first 6 weeks of life, and grown to 1 year of age. For the 1 year study, newborn monkeys were dosed twice daily per os by syringe for the first 6 weeks of life (Divi et al., 2005, 2007) and were taken at 52 weeks of age, 46 weeks after the last dose of NRTI was given.
Isolation of brain and liver mitochondria.
The procedure for isolation of brain and liver mitochondria is essentially the same as that previously described (Divi et al., 2005, 2007). The sample was minced on ice, homogenized in three steps of 30 s each in homogenization buffer (210mM mannitol, 70mM sucrose, 1mM EDTA, 20mM HEPES [pH 7.4], 2mM dithiothreitol, and 1 mg/ml leupeptin [Roche Diagnostics, Indianapolis, IN]) using a Polytron tissue processor (Sorvall, Asheville, NC) and centrifuged twice at 1000 × g for 5 min to remove cellular debris and nuclei. The mitochondria were collected by centrifugation at 20,000 × g for 20 min, gently resuspended in 50- to 100-μl aliquots of homogenization buffer, frozen in liquid nitrogen, and stored at −80°C. Mitochondrial protein was quantified by the Coomassie Brilliant Blue method using bovine serum albumin as a standard (Bradford, 1976).
OXPHOS enzyme-specific activity and protein assays.
Specific activities of the OXPHOS enzyme complexes I, II, and IV were quantified using a Hewlett Packard diode array spectrophotometer as previously described (Trounce et al., 1996). Complex I: NADH-ubiquinone oxidoreductase rotenone-sensitive activity was measured by the oxidation of NADH. Complex II: succinate-ubiquinone oxidoreductase activity was measured by the reduction of 2,6-dichloro-phenol-indophenol when coupled to complex II–catalyzed reduction of decylubiquinone. Complex IV: ferrocytochrome c:oxygen oxidoreductase activity was measured by following the oxidation of reduced cytochrome c. Mitochondrial protein concentrations were measured by the Coomassie Brilliant Blue method using bovine serum albumin as a standard (Bradford, 1976).
Transmission EM of brain and liver and scoring of EM photomicrographs.
Brain and liver were taken at birth and 1 year of age and processed as previously described (Divi et al., 2005, 2007). Briefly, tissues were cut into 1- to 2-mm slices and fixed in 4% paraformaldehyde: 2% glutaraldehyde in 100mM phosphate buffer, pH 7.4, for 24 h at 4°C. The fixative was replaced with sodium cacodylate buffer (0.1M) and later by 1% osmium tetroxide. Tissues were dehydrated in a series of graded alcohol washes, placed in 100% propylene oxide, and infiltrated and embedded in epoxy resin (EMBed-812; EMS, Hatfield, PA). Thin sections, cut with an ultramicrotome (Ultracut E; Leica, Northvale, NJ), were mounted on a 200-meshed grid and stained with uranyl acetate and lead citrate (EM Stain; Leica), stabilized by carbon evaporation (Linde, Ontario, Canada), observed, and photographed at ×10,000 magnification with an electron microscope (H7600; Hitachi, Tokyo, Japan) operated at 80 kV. Images were captured randomly while manually moving in a Z-line pattern on the grid. For each monkey, 10 EM photomicrographs, enlarged to ×30,000, were evaluated for mitochondrial morphology. The degree of mitochondrial and cellular pathology was scored by three different investigators, all of whom were given coded EM photographs to eliminate bias. The scoring scheme for each tissue is detailed in the “Results” section and in the legends to Tables 3 and 4.
TABLE 3.
Scoringa (n = number of monkeys/group) of brain cortex EM photos for mitochondrial morphological damage (scale 0 to +5) in patas monkeys taken at birth and 1 year of age.
| Damage score (mean ± SE) |
||
| NRTI exposure | At birth | At 1 year |
| None | 1.6 ± 0.33 (n = 5) | 1.6 ± 0.04 (n = 4) |
| AZT | NS | 2.4 ± 0.23 (n = 4) |
| 3TC | 2.8 ± 0.48 (n = 3) | NS |
| AZT/3TC | 3.2 ± 0.44b (n = 3) | 2.6 ± 0.15b (n = 4) |
| AZT/ddI | 3.4 ± 0.29b (n = 3) | 3.0 ± 0.27b(n = 4) |
| d4T/3TC | 2.8 ± 0.38b (n = 3) | 2.5 ± 0.47b (n = 4) |
Note. NS, no sample.
Scoring paradigm for mitochondrial status in brain cortex: (0) = nearly all mitochondria possessed an intact membrane, compact, well-defined cristae, and a dark matrix, whereas an infrequent mitochondrion showed less definitive cristae and a lighter matrix (that were considered to be within normal limits), and the rough endoplasmic reticulum (if visible) was uniform and compressed; (+1) = majority of mitochondria were intact, and although a few possessed discontinuous membranes, no loss of cristae material, matrical density, or irregularity in endoplasmic reticulum or golgi apparatus was apparent; (+2) = an increasing number of mitochondria were dissolved with membrane disruptions but minimal loss of cristae and matrical density, whereas most of the remaining mitochondria looked essentially normal but did display disruption in endoplasmic reticulum and golgi apparatus structure; (+3) = majority of mitochondria possessed membrane disruptions, partial loss of cristae material, and elevated matrical lucency along with widespread, distention, and increased lucency surrounding the mitochondria; (+4) = nearly all mitochondria possessed dissolved membranes and loss of cristae and matrical structure, along with swelling, disorganization, and increased lucency of the surrounding structures; (+5) = in many areas, the mitochondrial membrane was completely dissolved and the cristae were fragmented and disorganized; in other areas, mitochondria containing no central architecture were apparent, and all other areas looked like those in samples graded (+4).
Values (in bold) for groups exposed to the two NRTI combinations were significantly different from the corresponding unexposed control group for monkeys taken at birth (p = 0.024) and at 1 year of age (p = 0.025) by one-way ANOVA. At birth, the Holm-Sidak method for multiple comparisons showed significance (p < 0.05) for control versus AZT/3TC, control versus AZT/ddI, and control versus d4T/3TC. At 1 year of age, the Holm-Sidak method for multiple comparisons showed significance (p < 0.05) for control versus AZT/ddI, as well as marginal significance (p < 0.06) for control versus AZT/3TC and control versus d4T/3TC.
TABLE 4.
Scoringa of Liver EM Photos for Mitochondrial Morphological Damage (Scale 0 to +5) in from Patas Monkeys Taken at Birth and 1 Year of Age
| Damage score (Mean ± SE) |
||
| NRTI exposure | At birth | At 1 year |
| None | 1.5 ± 0.19 (n = 5) | 1.6 ± 0.18 (n = 4) |
| AZT | NS | 3.2 ± 0.19b (n = 4) |
| 3TC | 2.4 ± 0.22b (n = 4) | NS |
| AZT/3TC | 2.2 ± 0.12b (n = 3) | 3.1 ± 0.17b (n = 4) |
| AZT/ddI | 1.9 ± 0.14 (n = 3) | 3.2 ± 0.25b (n = 4) |
| d4T/3TC | 2.4 ± 0.22 (n = 3) | 2.9 ± 0.15b (n = 4) |
Note. NS, no sample.
Scoring paradigm for mitochondrial status in liver: (0) = nearly all mitochondria possessed an intact membrane, compact, well-defined cristae, and an intensely dark matrix, whereas an infrequent mitochondrion showed minimal swelling that was considered to be within normal limits, and the rough endoplasmic reticulum was uniform and compressed; (+1) = majority of mitochondria were intact, and although a few possessed discontinuous membranes, no loss of cristae material, matrical density, or rough endoplasmic reticulum integrity was apparent; (+2) = an increasing number of mitochondria were swollen with membrane disruptions but minimal loss of cristae and matrical density, whereas most of the remaining mitochondria looked essentially normal but did display minimal rough endoplasmic reticulum disruption or distention; (+3) = majority of mitochondria possessed membrane disruptions, partial loss of cristae material, and elevated matrical lucency along with widespread rough endoplasmic reticulum disruption, distention, and increased lucency; (+4) = nearly all mitochondria possessed dissolved membranes and loss of cristae and matrical structure, along with swelling, disorganization, and increased lucency of the rough endoplasmic reticulum; (+5) = in many areas, the mitochondrial membrane was completely dissolved and the cristae were fragmented and disorganized, in other areas, giant swollen mitochondria containing no central architecture were apparent, and all other areas looked like those in samples graded (+4).
Values (in bold) for liver at birth for groups exposed to NRTIs were significantly different from the corresponding unexposed control group (p = 0.025) by one-way ANOVA. The Holm-Sidak method for multiple comparisons showed significant differences (p < 0.05) for control versus 3TC and control versus AZT/3TC. Values (in bold) for liver at 1 year of age for groups exposed to NRTIs were significantly different from the corresponding unexposed control group for monkeys taken at birth (p < 0.001) by one-way ANOVA. The Holm-Sidak method for multiple comparisons also showed significant differences (p < 0.05) for control versus any NRTI-exposed group.
DNA preparation.
DNA was extracted from tissues using the QIAamp DNA mini kit, according to the manufacturer's protocol (Qiagen, Valencia, CA). Briefly, tissues were equilibrated to room temperature, sliced into small pieces, washed with 100mM PBS without calcium and magnesium (pH 7.4), homogenized in three volumes of PBS, lysed with Qiagen AL buffer, and digested with proteinase K and RNase A. The DNA was separated using spin columns provided with the kit and was subsequently extracted into 10mM Tris, pH 7.4, containing 1mM EDTA and stored at −80°C until analysis. The quality and quantity of DNA were determined spectrophotometrically by absorbance at 260 nm.
MtDNA quantification by HC-CA.
The protocol for HC-CA is described in detail elsewhere (Divi et al., 2005) and so will be outlined only briefly here. Sample DNA containing both nuclear and mtDNA was mixed with a 500-bp amplicon of the human cystic fibrosis (CFTR-Human) gene prepared by PCR amplification using specific primers (Ensembl gene ENSG00000001626; forward: 5′-AAGCTTCAGATCACTGTGGAAGAGG; reverse: 5′-GACTTGCACTTGCTTGAGTTCCG) and the human genomic DNA as template. The CTFR-human DNA was used as a nonspecific carrier matrix so that all samples would have a total of 1 μg DNA. Diluted samples were then mixed with 0.1μM patas monkey complex I (NADH dehydrogenase) ND4/ND5 (5′-amino-TTCTCATAATCGCCCACGGA) (GenBank ID: D85291) oligonucleotide (Invitrogen, Carlsbad, CA) in 10 μl of 2× Expand High Fidelity PCR buffer without MgCl2 (Roche Diagnostics) and denatured together at 95°C for 5 min followed by hybridization at 60°C for 30 min. The hybrid was labeled with 1.0 μl of ULS Platinum Bright (Applied Biosystems, Foster City, CA) at 55°C for 1 h. The ULS-hybridized DNA was separated from unhydrolyzed oligo and unreacted Platinum Bright using YM-10 microcon spin columns (Millipore, Billerica, MA) washed with binding buffer (10mM phosphate buffer, pH 7.4, with 1mM EDTA).
The purified hybrid was diluted to 0.5 ml in binding buffer, and an aliquot (100 μl) in four replicates was transferred to a DNA-Bind (amide-binding N-oxysuccinamide surface) 96-well plate (Corning, Acton, MA) and incubated at 37°C for 1 h to capture the hybrid. The plates were washed once with wash buffer (2× saline sodium citrate, 0.1% SDS) and three times with PBST (PBS with 0.05% Tween 20). They were blocked with 0.25% I-Block (Casein; Applied Biosystems) in PBS for 1 h at 37°C and again washed three times with PBST. Alkaline phosphatase (AP; Applied Biosystems), diluted 1:2000 in I-block buffer, was added to all the wells and incubated for 1 h at room temperature before wells were washed with PBST and rinsed with 10mM Tris buffer (pH 8.0). Following incubation with an AP substrate (CDP-Star with Emerald II; Applied Biosystems) for 30 min at room temperature, luminescence was measured using a TR 717 Luminometer (Applied Biosystems). The values for mtDNA quantity are expressed as luminescence units (LUs) per nanogram of total patas DNA, with unexposed samples compared with exposed samples.
Statistical methodology.
For comparing the NRTI-exposed groups with the unexposed group for OXPHOS values, one-way ANOVA was used followed by Student's t-test. For evaluation of EM photos and mtDNA quantity, the data were first subjected to tests for normality and equal variance and then analyzed by one-way ANOVA. Subsequently, the Holm-Sidak method for multiple comparisons was used to determine which exposed groups were significantly different from the unexposed group. Values of p ≤ 0.05 were considered significant.
RESULTS
Overall Toxicity and Response to NRTI Exposures
Typical clinical antiretroviral drug protocols, based on recommendations by the Centers for Disease Control, call for dosing of the pregnant mother for the last 6 months of pregnancy and to the infant for the first 6 weeks of life. In the patas monkey model, NRTIs were given to the pregnant dam for the last 10 weeks (approximately 50%) of gestation, with the exception of 3TC, which was given for the last 4 weeks of gestation. Though the actual NRTI doses given to HIV-1–infected women vary to some extent because of differences in body weight, for the purposes of designing the patas experiments, it was assumed that a pregnant woman weighs 70 kg and a near-term pregnant patas monkey weighs approximately 7 kg. Given these assumptions, the patas monkey daily doses, calculated on a gm/kg body weight basis for AZT, 3TC, ddI, and d4T were approximately 80, 83, 100, and 96% of the daily doses given to pregnant women, respectively. NRTI doses given to the infants after birth were adjusted upward at 4 weeks of age to account for the rapid increase in body weight. No overt, clinically apparent toxicity was observed in either the dams or their infants, either during or after drug administration.
OXPHOS Enzyme–Specific Activities at Birth and 1 Year of Age
Specific activities of OXPHOS complexes I, II, and IV were determined by enzyme assays in brain cortex mitochondria taken from patas infants at birth and 1 year of age (Table 1), and there were no significant NRTI-related alterations, compared with the unexposed controls. Statistical comparisons were not possible in groups with a single animal; however, the monkey exposed to AZT/3TC and taken at birth had a complex II value that was more than double that found in the single control.
TABLE 1.
OXPHOS Complex I, II, and IV Enzyme Activities (nmol/min/mg protein ± SE) in Brain Cortex Mitochondria from Patas Infants Taken at Birth and 1 Year of Age
| NRTI exposure | Age of monkeys | Complex I | Complex II | Complex IV |
| None | Birth (n = 1) | 31.5 | 63.0 | 153.0 |
| 1 year (n = 4) | 75.7 ± 26.74 | 78.1 ± 9.50 | 262.9 ± 92.90 | |
| 3TC | Birth (n = 3) | 24.7 ± 4.00 | 173.1 ± 60.37 | 194.3 ± 37.24 |
| AZT | 1 year (n = 4) | 81.0 ± 28.62 | 68.3 ± 15.31 | 206.4 ± 72.92 |
| AZT/3TC | Birth (n = 1) | 29.4 | 146.6 | 233.6 |
| 1 year (n = 3) | 60.0 ± 24.50 | 62.5 ± 7.67 | 200.6 ± 81.89 | |
| AZT/ddI | Birth (n = 3) | 30.5 ± 1.25 | 128.5 ± 7.02 | 184.7 ± 37.60 |
| 1 year (n = 4) | 74.1 ± 26.18 | 64.7 ± 9.24 | 202.4 ± 71.5 | |
| d4T/3TC | Birth (n = 3) | 31.3 ± 3.00 | 135.5 ± 11.44 | 184.5 ± 15.67 |
| 1 year (n = 3) | 57.6 ± 23.50 | 54.7 ± 5.01 | 227.7 ± 92.94 |
For the liver (Table 2), specific activities of OXPHOS Complexes I, II, and IV were measured at birth only, and there was a marginally (p = 0.06) significant alteration in complex I in the group exposed to d4T/3TC, compared with the unexposed controls.
TABLE 2.
OXPHOS Complex I, II, and IV Enzyme Activities (nmol/min/mg protein ± SE) in Liver Mitochondria from Patas Infants Taken at Birth
| NRTI exposure | Number of monkeys | Complex I | Complex II | Complex IV |
| None | 3 | 29.2 ± 3.18 | 120.5 ± 15.21 | 94.6 ± 6.73 |
| 3TC | 3 | 11.3 ± 6.55 | 116.5 ± 22.06 | 118.4 ± 21.34 |
| AZT/3TC | 2 | 27.2 ± 14.35 | 167.2 ± 19.00 | 191.3 ± 54.60 |
| AZT/ddI | 3 | 49.6 ± 16.77 | 132.0 ± 20.88 | 121.0 ± 29.73 |
| d4T/3TC | 3 | 48.2 ± 6.57a | 159.6 ± 17.28 | 130.5 ± 35.06 |
Note. Value in bold is marginally significantly different (p= 0.06) from that found in unexposed monkeys by t-test.
EM of Brain and Liver and Scoring of EM Photomicrographs
EM of brain cortex and liver taken from patas offspring at birth and 1 year of age showed substantial morphological damage in NRTI-exposed groups compared with the unexposed controls. Representative EM photomicrographs for brain cortex and liver are shown in Figures 1 and 2, respectively.
FIG. 1.
EM photomicrographs (×30,000 magnification) of brain cortex from infant patas taken: at birth after in utero exposure to human-equivalent protocols containing: no drug (A), AZT/ddI (B), or d4T/3TC (C); and at 1 year after in utero exposure to human-equivalent protocols containing no drug (D), AZT/ddI (E), or d4T/3TC (F) (× 30,000 magnification). The photos show swelling of mitochondria in the NRTI-exposed groups (B, C, E, and F, white arrows); disruption of mitochondrial membranes; and dissolution of some cristae with high mitochondrial luminosity (B, C, E, and F, white arrow heads).
FIG. 2.
EM photomicrographs (×30,000 magnification) of liver from infant patas taken: at birth after in utero exposure to human-equivalent protocols containing: no drug (A), AZT/ddI (B), or d4T/3TC (C); and at 1 year after in utero exposure to human-equivalent protocols containing no drug (D), AZT/ddI (E), or d4T/3TC (F) (× 30,000 magnification). The photos show swelling of mitochondria in the NRTI-exposed groups (B, C, E, and F, white arrows); disruption of mitochondrial membranes and dissolution of some cristae with high mitochondrial luminosity (B, C, E, and F, white arrow heads); and clusters (possible clonal expansions) of highly damaged, similar appearing, mitochondria (E and F).
Figures 1A and 1D show representative brain cortex EM photomicrographs from unexposed patas at birth and 1 year of age, respectively. In both photos, the mitochondria (arrows) are round or oblong and dark with dense, well-defined cristae. Figures 1B and 1C show brain taken at birth from infants exposed to AZT/ddI and d4T/3TC, respectively, and in contrast to the controls, the mitochondria (arrows) are larger and contain translucent areas where the cristae have decomposed. This damage did not resolve by 1 year of age, as can be seen in Figures 1E and 1F which show EM photomicrographs from monkeys exposed to AZT/ddI and d4T/3TC, respectively. At both time points, there were similar manifestations of NRTI-induced mitochondrial damage in NRTI-exposed brain, including mitochondrial swelling, membrane damage with loss of cristae, and abnormalities of the endoplasmic reticulum. Typically, the most highly damaged mitochondria were observed in the patas groups exposed to the two NRTI combinations.
Figure 2 shows representative EM photomicrographs of patas liver. Figures 2A and 2D show unexposed animals at birth, with normal, dense, dark, and round mitochondria (arrows) and well-defined endoplasmic reticulum. In Figure 2B, showing liver at birth after exposure to AZT/ddI, the mitochondrial damage appears minimal, but by 1 year of age (Fig. 2E) the mitochondria are markedly enlarged showing loss of cristae (arrows) and degeneration of the endoplasmic reticulum. Further degeneration of the cristae, with an increase in luminescence and an apparent increase in proliferation of damaged mitochondria (arrows), can be seen in Figure 2F, which shows the liver of a 1-year-old monkey exposed in utero to d4T/3TC. These photos demonstrate that the overall level of mitochondrial compromise in the liver may be more extensive at 1 year of age than at birth, suggesting a progressive process.
Similar to the previously published EM studies of patas heart and skeletal muscle (Divi et al., 2005, 2007) which employed these same animals, mitochondrial ultrastructural damage was scored in brain and liver on a scale of 0 to +5 by a team of investigators. Photomicrographs (10 per individual) were coded to eliminate bias, and an average score for each animal was derived based on the specific scoring schemes developed for each tissue. The schemes for brain and liver are presented in the footnotes to Tables 3 and 4, respectively, and the scoring results are presented in the Tables.
Table 3 shows brain cortex values (mean ± SE) for monkeys taken at birth and at 1 year of age. In the patas groups exposed to two NRTIs (AZT/3TC, AZT/ddI, and d4T/3TC), the levels of mitochondrial damage were significantly (p < 0.05) increased, compared with the unexposed controls, both at birth and at 1 year of age. Values for liver are shown in Table 4. At birth, the liver showed significant (p < 0.05) increases in mitochondrial morphological damage in the AZT/3TC and AZT/ddI groups. At 1 year of age, all the NRTI-exposed groups had significant (p < 0.05) increases in mitochondrial damage, compared with the unexposed control group, and the numbers suggest that, in the liver, a progression of mitochondrial damage may occur between birth and 1 year of age.
Quantification of Brain Cortex and Liver mtDNA by HC-CA
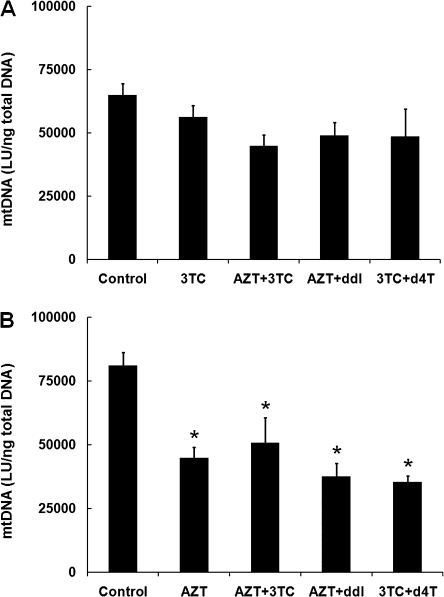
Relative mtDNA levels were quantified using the PCR-based HC-CA procedure described previously for heart and skeletal muscle (Divi et al., 2005, 2007). Values for mtDNA were expressed as LU/ng total patas DNA, and the reliability of the assay was validated by comparison to a human mitochondrial DNA (mtDNA) standard curve. The data for brain cortex are presented in Figure 3, which shows values for patas infants taken at birth (Fig. 3A), and 1 year of age (Fig. 3B). At birth, there were decreases in mtDNA quantity of 37.4, 22.0, 18.9, and 35.6% in the groups exposed to 3TC, AZT/3TC, AZT/ddI, and d4T/3TC, respectively, with statistically significant mtDNA depletion in the groups exposed to 3TC and d4T/3TC, compared with the unexposed controls (p < 0.05). At 1 year of age (Fig. 3B), there were decreases of 52.2, 43.5, and 53.3% in the groups exposed to AZT/3TC, AZT/ddI, and d4T/3TC, respectively, and these were all significantly different from the unexposed controls (p < 0.002). Therefore, the infant patas brains sustained a progressive loss of mtDNA between birth and 1 year of age, despite the absence of NRTI drug dosing during the last 46 weeks of life.
FIG. 3.
Brain cortex mtDNA (LU/ng total patas DNA, mean ± SE) determined by HC-CA. (A) Patas offspring taken at birth after in utero exposure to no drug (n = 4), 3TC (n = 4), AZT/3TC (n = 3), AZT/ddI (n = 3), or d4T/3TC (n = 3). One-way ANOVA showed significant depletion in the NRTI-treated groups compared with the controls (p = 0.01), and the Holm-Sidak method for multiple comparisons showed significant (p < 0.05) depletion in groups exposed to 3TC and d4T/3TC. (B) Patas offspring taken at 1 year of age after in utero plus 6 weeks post-birth exposure to no drug (n = 4), AZT (n = 4), AZT/3TC (n = 3), AZT/ddI (n = 4), or d4T/3TC (n = 4), followed by 46 weeks with no drug exposure. By one-way ANOVA, all the NRTI-exposed groups were significantly (p < 0.001) depleted compared with the unexposed group. Also, by the Holm-Sidak procedure for multiple comparisons, all NRTI-exposed groups were significantly (p < 0.002) depleted in mtDNA compared with the unexposed group.
Figure 4 shows values for mtDNA in liver at birth (Fig. 4A) and 1 year of age (Fig. 4B). At birth, mtDNA was decreased by 13.4, 30.9, 24.7, and 25.3% in the groups exposed to 3TC, AZT/3TC, AZT/ddI, and d4T/3TC, respectively, but none of the values was significantly different from the unexposed controls. At 1 year of age, mtDNA depletion in the groups exposed to the NRTIs was 44.7, 37.4, 53.7, and 56.5% in groups exposed to AZT, AZT/3TC, AZT/ddI, and d4T/3TC, respectively, and all these groups had significantly less mtDNA than the unexposed controls (p < 0.05). These data show that there is a progressive loss of mtDNA in livers of 1-year-old patas infants exposed in utero and post-birth, which occurs despite the absence of drug exposure between 7 and 52 weeks of age.
FIG. 4.
Liver mtDNA (LU/ng total patas DNA, mean ± SE) determined by HC-CA. (A) Patas offspring taken at birth after in utero exposure to no drug (n = 5), 3TC (n = 4), AZT/3TC (n = 3), AZT/ddI (n = 3), or d4T/3TC(n = 3). There were no significant differences between the unexposed group and any of the NRTI-exposed groups by one-way ANOVA. (B) Patas offspring taken at 1 year of age after in utero plus 6 weeks post-birth exposure to no drug (n = 4), AZT (n = 4), AZT/3TC (n = 4), AZT/ddI (n = 4), and d4T/3TC (n = 4). Differences between unexposed and NRTI-exposed groups were significant (p < 0.001) by one-way ANOVA. By the Holm-Sidak procedure for multiple comparisons, all the NRTI-exposed groups (AZT, AZT/3TC, AZT/ddI, and d4T/3TC) were significantly (p < 0.05) depleted in mtDNA compared with the unexposed group.
DISCUSSION
Here we have documented progressive mitochondrial compromise in samples of brain cortex and liver taken from newborn and 1-year-old patas monkeys exposed in utero to human-equivalent NRTI protocols. Whereas changes in mitochondrial OXPHOS enzyme-specific activities were minimal, mitochondrial morphology was significantly altered in brain both at birth and 1 year of age in most of the NRTI-exposed groups. In addition, liver mitochondria appeared to undergo progressive morphological degeneration between birth and 1 year of age. This study also showed a progressive depletion of mtDNA quantity in both brain cortex and liver of NRTI-exposed infants. Compared with unexposed patas, the mtDNA loss in brain and liver of NRTI-exposed patas was about 50% at 1 year of age and ranged from 13 to 37% at birth.
We previously published data for heart and skeletal muscle (Divi et al., 2005, 2007) from these same animals, showing evidence of mitochondrial compromise based on evaluation of EM photomicrographs and mtDNA quantitation. Both heart and skeletal muscle from NRTI-exposed patas sustained significant depletion of mtDNA at birth. In NRTI-exposed patas at 1 year of age, skeletal muscle mtDNA increased but did not reach normal levels, and heart mtDNA levels increased abnormally to about 200% of those found in hearts of unexposed patas. Viewing this study as a whole, it would appear that heart, brain, and liver of patas infants exposed in utero to human-equivalent NRTI protocols sustain a progressive mitochondrial compromise up to 1 year of age. Ongoing studies using the patas model will follow infants up to 3 years of age to search for a time at which these mitochondrial manifestations of drug toxicity may resolve.
It is important to note that the doses of drug used in these studies were not at all toxic to the patas dams. We found no adverse effects in the dams, either symptomologically or in the clinical chemistry assays, which included lactate and creatine kinase measurements and were performed several times during gestation and through the perinatal period. There is human clinical precedent for this in that the AZT doses given to pregnant women to prevent mother-to-child HIV-1 transmission are not typically toxic to the mother. Where mitochondrial toxicities, with the rare manifestation of lactic acidosis, have been observed in the mothers, there has often been long-term NRTI therapy prior to pregnancy. The pharmacokinetics of NRTIs in several species of monkey studied (Poirier et al., 1999), and in the patas (Divi et al., 2008), are almost identical to those observed in humans (O'Sullivan et al., 1993). In the patas, we have measured AZT/3TC pharmacokinetics in adult, nonpregnant animals but not in pregnant dams. However, the uniformity in NRTI metabolism, and similarity in gestational pharmacodynamics among all the primate species studied (Patterson et al., 1997) suggests that the pregnant patas will process NRTIs in a fashion very similar to pregnant women and the other primate counterparts.
Persistence of mitochondrial compromise as a consequence of in utero NRTI exposures has been observed in AZT-exposed HIV-1–uninfected infants followed at birth, 1 year, and 2 years of age (Poirier et al., 2003). The study compared infants born to: HIV-1–uninfected mothers who received no drug; HIV-1–infected mothers who received no drug; and HIV-1–infected mothers who received AZT during pregnancy. Peripheral blood mononuclear cells (PBMCs) of children born to HIV-1–infected mothers who did not receive antiretroviral therapy had depleted levels of mtDNA compared with unexposed controls born to uninfected mothers. The addition of AZT significantly depleted mtDNA further. In addition, for all groups the mtDNA levels did not change between birth and 2 years of age, demonstrating a persistent loss of mtDNA levels at 1 and 2 years of age. Whereas the human study showed a persistent mtDNA depletion in PBMC, the current patas study shows a progressive depletion of mtDNA within brain and liver by 1 year of age. Therefore, long-term monitoring of cognitive function and liver status may be advantageous in infants exposed transplacentally to antiretroviral NRTIs.
The progressive depletion of mtDNA observed in these studies in the patas brain raises concern for the cognitive functioning of children born to HIV-1–infected mothers receiving highly active antiretroviral therapy (HAART). Fortunately, almost all current studies designed to examine neurodevelopment in infants born to HIV-1–infected mothers have not reported neurodevelopmental/cognitive compromise that is due only to HAART exposure. Alimenti et al. (2006) studied infants (n = 63) at 18–36 months of age and found all scores lower in the HAART-exposed group. However, these differences disappeared upon correction for maternal substance abuse during pregnancy. In a second study, Williams et al. (2010) examined children (n = 1840) enrolled in the Pediatric AIDS Clinical Trials Group 219/219C at 2 years of age. In this cohort where all children were born to HIV-1–infected mothers, 92% were exposed to HAART and 8% to no antiretroviral drugs, and both groups demonstrated similar overall mental and psychomotor development. In a retrospective study, Lindsey et al. (2007) compiled neurodevelopment data in HIV-1–infected (n = 145) and HIV-1–uninfected (n = 1959) children aged 7 weeks to 3.5 years, who were exposed to HAART with and without protease inhibitors. Overall the HIV-1–infected children scored significantly lower than the HIV-1–uninfected children for both mental and motor development, though it was unclear which of many factors, including HIV-1 status, long-term HAART therapy, maternal lifestyle, and/or socioeconomics, might have contributed to the lower scores, there was no change when protease inhibitors were added. There is some evidence for mitochondrial compromise in various types of psychiatric disorders (Shao et al., 2008), and further evidence for clinical mitochondrial dysfunction in HIV-1-uninfected infants born to HIV-1–infected mothers receiving antiretroviral drugs during pregnancy (Benhammou et al., 2007). However, the relationship between transplacental HAART exposure and neurodevelopment requires further study, and we plan to explore this with the patas model.
In contrast to the highly significant abnormalities in mitochondrial morphology and mtDNA levels found in the NRTI-exposed patas infants, mitochondrial functional compromise, indicated by alterations in OXPHOS-specific activities, was minimal and observed only in groups exposed to the two NRTI combinations. We analyzed brain at birth and 1 year of age for complex I, II, and IV activities, and there were no significant OXPHOS changes at 1 year of age, so for liver we performed OXPHOS enzyme assays on the samples obtained at birth only. The lack of any substantial OXPHOS alterations in these organs is consistent with the functional status of the patas infants, who appear normal in their rate of growth and development. There is, however, some level of concern that the molecular and morphological mitochondrial abnormalities seen at birth and 1 year of age may progress to clinical mitochondrial dysfunction as a result of the normal stressful activities of daily adult life.
In response to external suggestions, we elected to examine 3TC alone in patas taken at birth, despite the fact that 3TC is never used alone in the clinic. 3TC is generally considered to be nontoxic and that assumption was supported by the present studies. It appears that most of the mitochondrial compromise seen in the NRTI two-drug combinations was because of either AZT or d4T. A further indication of this was found in the group of monkeys exposed in utero to AZT alone and taken at 1 year of age. Because two previous studies from this laboratory (Gerschenson et al., 2000, 2004) had examined the question of AZT-exposed patas at birth, we did not repeat those experiments. However, in the monkeys exposed in utero to AZT and taken at 1 year of age, there was significant mitochondrial morphological damage in liver and significant depletion of mtDNA in both brain and liver, again reaffirming the previously observed AZT-induced mitochondrial compromise, and additionally showing the persistence of mitochondrial compromise at 1 year of age.
FUNDING
Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research; federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN2612008001E.
Acknowledgments
We wish to thank Dr Mariana Gerschenson for assistance in establishing the early monkey studies. The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- Alimenti A, Forbes JC, Oberlander TF, Money DM, Grunau RE, Papsdorf MP, Maan E, Cole LJ, Burdge DR. A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics. 2006;118:e1139–e1145. doi: 10.1542/peds.2006-0525. [DOI] [PubMed] [Google Scholar]
- Barret B, Tardieu M, Rustin P, Lacroix C, Chabrol B, Desguerre I, Dollfus C, Mayaux MJ, Blanche S. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–1785. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- Beach JW. Chemotherapeutic agents for human immunodeficiency virus infection: Mechanism of action, pharmacokinetics, metabolism, and adverse reactions. Clin. Ther. 1998;20:2–25. doi: 10.1016/s0149-2918(98)80031-3. [DOI] [PubMed] [Google Scholar]
- Benhammou V, Tardieu M, Warszawski J, Rustin P, Blanche S. Clinical mitochondrial dysfunction in uninfected children born to HIV-infected mothers following perinatal exposure to nucleoside analogues. Environ. Mol. Mutagen. 2007;48:173–178. doi: 10.1002/em.20279. [DOI] [PubMed] [Google Scholar]
- Benhammou V, Warszawski J, Bellec S, Doz F, Andre N, Lacour B, Levine M, Bavoux F, Tubiana R, Mandelbrot L, et al. Incidence of cancer in children perinatally exposed to nucleoside reverse transcriptase inhibitors. AIDS. 2008;22:2165–2177. doi: 10.1097/QAD.0b013e328311d18b. [DOI] [PubMed] [Google Scholar]
- Blanche S, Tardieu M, Rustin P, Slama A, Barret B, Firtion G, Ciraru-Vigneron N, Lacroix C, Rouzioux C, Mandelbrot L, et al. Persistent mitochondrial dysfunction and perinatal exposure to antiretroviral nucleoside analogues. Lancet. 1999;354:1084–1089. doi: 10.1016/S0140-6736(99)07219-0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brinkman K. Management of hyperlactatemia: no need for routine lactate measurements. AIDS. 2001;15:795–797. doi: 10.1097/00002030-200104130-00016. [DOI] [PubMed] [Google Scholar]
- Brogly SB, Ylitalo N, Mofenson LM, Oleske J, van Dyke R, Crain MJ, Abzug MJ, Brady M, Jean-Philippe P, Hughes MD, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–938. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- Caselli D, Klersy C, de Martino M, Gabiano C, Galli L, Tovo PA, Arico M. Human immunodeficiency virus-related cancer in children: incidence and treatment outcome–report of the Italian register. J. Clin. Oncol. 2000;18:3854–3861. doi: 10.1200/JCO.2000.18.22.3854. [DOI] [PubMed] [Google Scholar]
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'Sullivan MJ, VanDyke R, Bey M, Shearer W, Jacobson RL, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N. Engl. J. Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- Culnane M, Fowler M, Lee SS, McSherry G, Brady M, O'Donnell K, Mofenson L, Gortmaker SL, Shapiro DE, Scott G, et al. Lack of long-term effects of in utero exposure to zidovudine among uninfected children born to HIV-infected women. Pediatric AIDS Clinical Trials Group Protocol 219/076 Teams. JAMA. 1999;281:151–157. doi: 10.1001/jama.281.2.151. [DOI] [PubMed] [Google Scholar]
- Dagan T, Sable C, Bray J, Gerschenson M. Mitochondrial dysfunction and antiretroviral nucleoside analog toxicities: what is the evidence? Mitochondrion. 2002;1:397–412. doi: 10.1016/s1567-7249(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL. Mitochondrial myopathy caused by long-term zidovudine therapy. N. Engl. J. Med. 1990;322:1098–1105. doi: 10.1056/NEJM199004193221602. [DOI] [PubMed] [Google Scholar]
- Divi RL, Doerge DR, Twaddle NC, Shockley ME, St Claire MC, Harbaugh JW, Harbaugh SW, Poirier MC. Metabolism and pharmacokinetics of the combination Zidovudine plus Lamivudine in the adult Erythrocebus patas monkey determined by liquid chromatography-tandem mass spectrometric analysis. Toxicol. Appl. Pharmacol. 2008;226:206–211. doi: 10.1016/j.taap.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Divi RL, Leonard SL, Kuo MM, Walker BL, Orozco CC, St Claire MC, Nagashima K, Harbaugh SW, Harbaugh JW, Thamire C, et al. Cardiac mitochondrial compromise in 1-yr-old Erythrocebus patas monkeys perinatally-exposed to nucleoside reverse transcriptase inhibitors. Cardiovasc. Toxicol. 2005;5:333–346. doi: 10.1385/ct:5:3:333. [DOI] [PubMed] [Google Scholar]
- Divi RL, Leonard SL, Walker BL, Kuo MM, Shockley ME, St Claire MC, Nagashima K, Harbaugh SW, Harbaugh JW, Poirier MC. Erythrocebus patas monkey offspring exposed perinatally to NRTIs sustain skeletal muscle mitochondrial compromise at birth and at 1 year of age. Toxicol Sci. 2007;99:203–213. doi: 10.1093/toxsci/kfm143. [DOI] [PubMed] [Google Scholar]
- Divi RL, Walker VE, Wade NA, Nagashima K, Seilkop SK, Adams ME, Nesel CJ, O'Neill JP, Abrams EJ, Poirier MC. Mitochondrial damage and DNA depletion in cord blood and umbilical cord from infants exposed in utero to Combivir. AIDS. 2004;18:1013–1021. doi: 10.1097/00002030-200404300-00009. [DOI] [PubMed] [Google Scholar]
- Gerard Y, Maulin L, Yazdanpanah Y, De, L. T X, Amiel C, Maurage CA, Robin S, Sablonniere B, Dhennain C, et al. Symptomatic hyperlactataemia: an emerging complication of antiretroviral therapy. AIDS. 2000;14:2723–2730. doi: 10.1097/00002030-200012010-00012. [DOI] [PubMed] [Google Scholar]
- Gerschenson M, Erhart SW, Paik CY, St.Claire MC, Nagashima K, Skopets B, Harbaugh SW, Harbaugh JW, Quan W, Poirier MC. Fetal mitochondrial heart and skeletal muscle damage in Erythrocebus patas monkeys exposed in utero to 3'-azido-3'-deoxythymidine (AZT) AIDS Res. Hum. Retroviruses. 2000;16:635–644. doi: 10.1089/088922200308864. [DOI] [PubMed] [Google Scholar]
- Gerschenson M, Nguyen V, Ewings EL, Ceresa A, Shaw JA, St Claire MC, Nagashima K, Harbaugh SW, Harbaugh JW, Olivero OA, et al. Mitochondrial toxicity in fetal Erythrocebus patas monkeys exposed transplacentally to zidovudine plus lamivudine. AIDS Res. Hum. Retroviruses. 2004;20:91–100. doi: 10.1089/088922204322749530. [DOI] [PubMed] [Google Scholar]
- Hanson IC, Antonelli TA, Sperling RS, Oleske JM, Cooper E, Culnane M, Fowler MG, Kalish LA, Lee SS, McSherry G, et al. Lack of tumors in infants with perinatal HIV-1 exposure and fetal/neonatal exposure to zidovudine. J. Acquir. Immune. Defic. Syndr. Hum. Retrovirol. 1999;20:463–467. doi: 10.1097/00042560-199904150-00008. [DOI] [PubMed] [Google Scholar]
- Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat. Med. 1995;1:417–422. doi: 10.1038/nm0595-417. [DOI] [PubMed] [Google Scholar]
- Lindsey JC, Malee KM, Brouwers P, Hughes MD. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119:e681–e693. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, Lai WW, Moodie DS, Sopko G, McIntosh K, et al. Absence of cardiac toxicity of zidovudine in infants. Pediatric Pulmonary and Cardiac Complications of Vertically Transmitted HIV Infection Study Group. N. Engl. J. Med. 2000;343:759–766. doi: 10.1056/NEJM200009143431102. [DOI] [PubMed] [Google Scholar]
- Lu LJ, Anderson LM, Jones AB, Moskal TJ, Salazar JJ, Hokanson JA, Rice JM. Persistence, gestation stage-dependent formation and interrelationship of benzo[a]pyrene-induced DNA adducts in mothers, placentae and fetuses of Erythrocebus patas monkeys. Carcinogenesis. 1993;14:1805–1813. doi: 10.1093/carcin/14.9.1805. [DOI] [PubMed] [Google Scholar]
- Mhiri C, Baudrimont M, Bonne G, Geny C, Degoul F, Marsac C, Roullet E, Gherardi R. Zidovudine myopathy: a distinctive disorder associated with mitochondrial dysfunction. Ann. Neurol. 1991;29:606–614. doi: 10.1002/ana.410290607. [DOI] [PubMed] [Google Scholar]
- Mofenson L. M., and Committee on Pediatric AIDS. Technical report: Perinatal human immunodeficiency virus testing and prevention of transmission. Pediatrics. 2000;106:1–12. doi: 10.1542/peds.106.6.e88. [DOI] [PubMed] [Google Scholar]
- Mofenson LM, Munderi P. Safety of antiretroviral prophylaxis of perinatal transmission for HIV-infected pregnant women and their infants. J. Acquir. Immune. Defic. Syndr. 2002;30:200–215. doi: 10.1097/00042560-200206010-00010. [DOI] [PubMed] [Google Scholar]
- Newschaffer CJ, Cocroft J, Anderson CE, Hauck WW, Turner BJ. Prenatal zidovudine use and congenital anomalies in a medicaid population. J. Acquir. Immune. Defic. Syndr. 2000;24:249–256. doi: 10.1097/00126334-200007010-00009. [DOI] [PubMed] [Google Scholar]
- O'Sullivan MJ, Boyer PJ, Scott GB, Parks WP, Weller S, Blum MR, Balsley J, Bryson YJ. The pharmacokinetics and safety of zidovudine in the third trimester of pregnancy for women infected with human immunodeficiency virus and their infants: phase I acquired immunodeficiency syndrome clinical trials group study (protocol 082). Zidovudine Collaborative Working Group. Am. J. Obstet. Gynecol. 1993;168:1510–1516. doi: 10.1016/s0002-9378(11)90791-1. [DOI] [PubMed] [Google Scholar]
- Patterson TA, Binienda ZK, Lipe GW, Gillam MP, Slikker W, Jr, Sandberg JA. Transplacental pharmacokinetics and fetal distribution of azidothymidine, its glucuronide, and phosphorylated metabolites in late-term rhesus macaques after maternal infusion. Drug Metab. Dispos. 1997;25:453–459. [PubMed] [Google Scholar]
- Perinatal HIV Guidelines Working Group. Public health service task force recommendations for use of antiretroviral drugs in pregnant HIV-1 infected women for maternal health and interventions to reduce perinatal HIV-1 transmission in the United States. 2006. 1–65. Available at: http://aidsinfo.nih.gov/ContentFiles/PerinatalGL.pdf. Accessed October 12, 2006. [PubMed] [Google Scholar]
- Poirier MC, Divi RL, Al-Harthi L, Olivero OA, Nguyen V, Walker B, Landay AL, Walker VE, Charurat M, Blattner WA. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J. Acquir. Immune. Defic. Syndr. 2003;33:175–183. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- Poirier MC, Olivero OA, Walker DM, Walker VE. Perinatal genotoxicity and carcinogenicity of anti-retroviral nucleoside analog drugs. Toxicol. Appl. Pharmacol. 2004;199:151–161. doi: 10.1016/j.taap.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Poirier MC, Patterson TA, Slikker W, Jr, Olivero OA. Incorporation of 3'-azido-3'-deoxythymidine (AZT) into fetal DNA and fetal tissue distribution of drug after infusion of pregnant late-term rhesus macaques with a human-equivalent AZT dose. J Acquir. Immune. Defic. Syndr. 1999;22:477–483. doi: 10.1097/00126334-199912150-00008. [DOI] [PubMed] [Google Scholar]
- Public Health Service Task Force Perinatal HIV Guidelines Working Group. Summary of the updated recommendations from the Public Health Service Task force to reduce perinatal human immunodeficiency virus-1 transmission in the United States. Obstet. Gynecol. 2002;99:1117–1126. doi: 10.1016/s0029-7844(02)01985-3. [DOI] [PubMed] [Google Scholar]
- Shao L, Martin MV, Watson SJ, Schatzberg A, Akil H, Myers RM, Jones EG, Bunney WE, Vawter MP. Mitochondrial involvement in psychiatric disorders. Ann. Med. 2008;40:281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Shikuma KM, Kamemoto L, Gerschenson M, Erdem G, Pinti M, Cossarizza A, Shikuma C. Placenta and cord blood mitochondrial DNA toxicity in HIV-infected women receiving nucleoside reverse transcriptase inhibitors during pregnancy. J. Acquir. Immune. Defic. Syndr. 2003;32:370–374. doi: 10.1097/00126334-200304010-00004. [DOI] [PubMed] [Google Scholar]
- Sperling RS, Shapiro DE, Coombs RW, Todd JA, Herman SA, McSherry GD, O'Sullivan MJ, Van Dyke RB, Jimenez E, Rouzioux C, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N. Engl. J. Med. 1996;335:1621–1629. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- Tomelleri G, Tonin P, Spadaro M, Tilia G, Orrico D, Barelli A, Bonetti B, Monaco S, Salviati A, Morocutti C. AZT-induced mitochondrial myopathy. Ital. J. Neurol. Sci. 1992;13:723–728. doi: 10.1007/BF02229156. [DOI] [PubMed] [Google Scholar]
- Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- Tuomala RE, Shapiro DE, Mofenson LM, Bryson Y, Culnane M, Hughes MD, O'Sullivan MJ, Scott G, Stek AM, Wara D, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N. Engl. J. Med. 2002;346:1863–1870. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- Williams PL, Marino M, Malee K, Brogly S, Hughes MD, Mofenson LM. Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics. 2010;125:e250–e260. doi: 10.1542/peds.2009-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]