Abstract
This study evaluated the hypothesis that smoke from harm reduction cigarettes impedes attachment and proliferation of H9 human embryonic stem cells (hESCs). Smoke from three harm reduction brands was compared with smoke from a conventional brand. Doses of smoke were measured in puff equivalents (PE) (1 PE = the amount of smoke in one puff that dissolves in 1 ml of medium). Cytotoxic doses were determined using morphological criteria and trypan blue staining, and apoptosis was confirmed using Magic Red staining. Attachment and proliferation of hESC were followed at a noncytotoxic dose in time-lapse videos collected using BioStation technology. Data were mined from videos either manually or using video bioinformatics subroutines developed with CL-Quant software. Mainstream (MS) and sidestream (SS) smoke from conventional and harm reduction cigarettes induced apoptosis in hESC colonies at 1 PE. At 0.1 PE (noncytotoxic), SS smoke from all brands inhibited attachment of hESC colonies to Matrigel with the strongest inhibition occurring in harm reduction brands. At 0.1 PE, SS smoke, but not MS smoke, from all brands inhibited hESC growth, and two harm reduction brands were more potent than the conventional brand. In general, hESC appeared more sensitive to smoke than their mouse ESC counterparts. Although harm reduction cigarettes are often marketed as safer than conventional brands, our assays show that SS smoke from harm reduction cigarettes was at least as potent or in some cases more potent than smoke from a conventional brand and that SS smoke was more inhibitory than MS smoke in all assays.
Keywords: cigarette smoke, harm reduction, embryos, humans, toxicology, tobacco, bioinformatics
Tobacco smoke is comprised of both mainstream (MS) smoke, which is actively inhaled by smokers, and sidestream (SS) smoke, which burns off the tip of a cigarette (EPA, 1992). SS smoke is the major component of secondhand smoke, also called environmental tobacco smoke, and is inhaled by passive smokers. Both MS and SS smoke adversely affect many reproductive processes. For example, cigarette smoke exposure decreases birth weight, while increasing the length of time to conceive, spontaneous abortions, perinatal mortality, and congenital defects (Andres and Day, 2000; Berthiller and Sasco, 2005; Higgins, 2002; Rogers, 2008; Shiverick and Salafia, 1999). In addition, in vitro assays have consistently shown interaction of cigarette smoke with female reproductive organs (Mlynarcikova et al., 2005; Shiverick and Salafia, 1999; Talbot, 2008; Talbot and Riveles, 2005). For example, both MS and SS cigarette smoke solutions significantly impaired oviductal functioning by decreasing ciliary beat frequency, oocyte pick-up by the oviduct, and muscle contraction rates, while increasing adhesion of the oocyte cumulus complex to the oviduct (Gieseke and Talbot, 2005; Riveles et al., 2003). A similar delay in oocyte and embryo transport and muscle contraction was seen in vivo in a hamster model exposed to smoke (DiCarlantonio and Talbot, 1999). Although these epidemiological studies and in vitro assays confirm that the female reproductive organs and fetuses are targets of cigarette smoke, relatively little is known about the effects of cigarette smoke on young embryos including preimplantation stages.
In an attempt to reduce the toxicity of cigarette smoke, tobacco companies have introduced various types of harm reduction products, including harm reduction cigarettes (Warner, 2005). Harm reduction cigarettes, which are often claimed to have fewer toxins and to be less harmful than conventional brands, are made using complex filters (Marlboro Lights, Advance Lights) or by genetically altering tobacco plants to reduce nicotine concentration (Quest). In 2009, our laboratory developed assays based on mouse embryonic stem cells (mESCs) to compare the effects of MS and SS smoke from conventional (Marlboro Red) and harm reduction (Marlboro Lights, Advance Lights, and Quest) cigarettes on attachment, survival, proliferation, and apoptosis (Lin et al., 2009). We found that SS smoke was generally more toxic to mESC in these assays than MS smoke. We also showed, unexpectedly, that SS smoke from harm reduction cigarettes was generally more inhibitory than smoke from the conventional brand.
To fully understand the effects of smoke on human embryonic development and to avoid possible species differences in response, it is necessary to perform toxicological assays using human models. Because it is clearly not possible to directly determine chemical toxicity on actual human embryos, we have developed assays with human embryonic stem cell (hESC), which model young embryos, to measure and compare the toxicity of MS and SS smoke from conventional and harm reduction products. Because hESC are more difficult to work with than mESC, we could not directly apply the methods used in our prior mouse study to hESC. We circumvented technical problems that hESC present by using BioStation technology to create time-lapse videos of cells during treatment in various in vitro assays (Lin et al., 2010). By combining BioStation technology with video bioinformatics analysis (automated processing and data mining of biological spatiotemporal data), we were able to obtain quantitative data for attachment, colony growth, and survival end points. Our data show that (1) hESC can be used to measure toxicity of environmental chemicals such as tobacco smoke, (2) BioStation technology coupled with video bioinformatics analysis facilitates assays with hESC, (3) SS smoke was more potent than MS smoke in all assays, and (4) SS smoke from harm reduction cigarettes was generally more potent than smoke from a conventional brand.
MATERIALS AND METHODS
Chemicals, media, and reagents.
Four commercial brands of cigarettes were purchased from retail dealers and used in this study. These included Marlboro Red (filter cigarettes, tar = 15 mg, nicotine = 1.1 mg) and Marlboro Lights (filter cigarettes, tar = 10 mg, nicotine = 0.8 mg) from Philip Morris, Inc. (Richmond, VA), Advance Premium Lights 100 s (filter cigarettes, tar = 10 mg, nicotine = 0.8 mg) from Brown and Williamson Tobacco (Louisville, KY), and Quest (filter cigarettes, tar = 10 mg, nicotine = 0.05 mg) from Vector Tobacco, Inc. (Mebane, NC). Marlboro Red cigarettes were chosen as they represent one of the top selling conventional brands, whereas the other brands are all harm reduction products.
mTeSR basal medium and mTeSR supplement (Stem Cell Technologies, Vancouver, Canada) were used to maintain hESC cultures and for experimentation; 6-well, 12-well, and 24-well plates (Falcon, Fisher Scientific, Chino, CA) were coated with Matrigel (BD Biosciences, Fisher Scientific, Chino, CA) for at least 2–3 h at room temperature or overnight at 2°C–4°C. PBS was made using deionized water, autoclaved, and stored at 2°C–4°C. Accutase Enzyme Cell Detachment Medium was purchased from eBioscience (San Diego, CA) and stored at −20°C.
Trypan blue (Sigma-Aldrich, St Louis, MO) solutions were made by dissolving 0.4 g of trypan blue in 80 ml of PBS at a low boil, cooling to room temperature, and sterilizing using 0.2-μm filters (Acrodisc Syringe Filters, Pall Corporation, Ann Arbor, MI). For the detection of apoptotic activity, the Fluorescent-Labeled Inhibitor of Caspases (FLICA) Poly Caspases Kit and Magic Red Caspase Detection Kit for caspases 3 and 7 (Immunochemistry Technologies, LLC Bloomington, MN) were used. The FLICA and Magic Red powder were reconstituted in dimethyl sulfoxide (DMSO; ATCC, Manassas, VA), aliquoted (5 μl) in Eppendorf tubes, and frozen at −20°C as instructed in the manual.
Preparation of smoke solutions.
Both MS and SS smoke solutions were prepared using an University of Kentucky smoking machine that was set up and operated as described previously (Lin et al., 2009; Knoll and Talbot, 1998). Smoke solutions were made by drawing either MS or SS smoke through basal mTeSR medium without mTeSR supplement and stored at −80°C. For all experiments, different concentrations of smoke solutions were diluted in complete mTeSR medium and used immediately. One cigarette was used to achieve a concentration of 10 puffs of smoke dissolved in 5 ml of medium (10 puffs/cigarette, 2 puffs/1 ml of medium). Concentrations of smoke solution were measured in puff equivalents (PE) (1 PE = the amount of smoke in one puff that dissolves in 1 ml of medium). Serial dilutions of smoke solution were made to achieve the PE concentrations used for testing, which were 0.0, 0.01, 0.1, and 1 PE. Smoke solutions were made with conventional (Marlboro Red) and harm reduction (Marlboro Lights, Advance Lights, and Quest) cigarettes.
Cell cultures.
H9 hESC, purchased from WiCell Stem Cell Institute, were grown using feeder-free conditions. Cells were cultured and maintained on Matrigel-coated six-well tissue culture plates in complete mTeSR medium. All cell cultures were observed daily for cell density and pluripotency, and the medium was changed everyday. Once cultures reached 60–70% confluency, hESC were removed from the plates using glass beads and Accutase without dilution (1 min at 37°C). Once hESC began to detach, 10–12 sterile glass beads were placed into the well and rolled gently in all directions until colonies completely detached into small clumps. The cell suspension was then neutralized using mTeSR medium and centrifuged at 200 × g. Colonies were resuspended into clumps of four to five cells for passaging and experimentation.
Morphological cytotoxicity assay and trypan blue staining.
Colony morphology and trypan blue staining were used to evaluate the cytotoxicity of cigarette smoke on H9 hESC. Colonies of four to five cells were plated on Matrigel-coated 24-well plates with 500 μl of fresh medium for 48 h prior to smoke treatment. After attachment, hESC colonies were treated with different concentrations of MS or SS smoke (0.01, 0.1, and 1 PE), whereas a well of untreated colonies served as the control. Colony morphology was observed with a Nikon inverted light microscope at 6 and 24 h of treatment, and comparisons were made between control and treated groups. After 24 h, 50 μl of 0.4% trypan blue solution was added to each well, which contained 500 μl of medium. After 5 min of staining, images were collected from each well. Dark blue cells were interpreted to be dying because of increased permeability of the cell membrane.
Apoptosis detection using poly-caspases Detection FLICA Kit and Magic Red Caspases 3 & 7 Detection Kit.
The FLICA Poly Caspases Kit and Magic Red Caspases 3 & 7 Detection Kit were used to identify apoptosis activity in control and smoke solution–treated hESC colonies. The FLICA probes contain an inhibitor sequence of caspases, called valine, alanine, aspartic acid (VAD), linked to a green or red fluorescent probe. VAD penetrates the plasma membrane and reacts with all caspases. Active caspases inside cells covalently bind to the FLICA probe and thus cells appear to be fluorescent. Excess FLICA probe not bound to active caspases is washed out of the cells. The Magic Red Caspase Detection Kit contained the MR-(DEVD)2 reagent, which was reconstituted in DMSO, stored at −20°C, and protected from light. This kit contains a cell-permeant substrate (DEVD) that is linked to cresyl violet. DEVD is specifically targeted by active caspases 3 and 7, which cause cresyl violet to fluoresce red upon cleavage of DEVD. Reagents were thawed and diluted with fresh culture media prior to usage. In our experiments, hESC colonies were preattached for 48 h on Matrigel, then treated for 5 h with smoke solution, followed by 20–25 min of incubation in Magic Red. Colonies staining red were interpreted to have apoptotic enzymes (caspases 3 and 7) activated by treatment.
Use of the BioStation IM to compare the effect of smoke from conventional and harm reduction cigarettes on hESC colony attachment.
The BioStation IM is a small incubation unit equipped with a microscope and camera that enables time-lapse video collection. This unit is extremely useful and efficient for studying dynamic cellular events such as cell attachment and spreading. For our experiments, adherent colonies were disassociated using Accutase and sterile glass beads. Colonies were broken up into clumps of three to five cells and replated onto Matrigel-coated 35-mm dishes containing SS smoke at 0.1 PE. All four brands of cigarettes were tested. The control contained mTeSR hESC culture medium without cigarette smoke. The dishes containing suspended cells were placed into the BioStation IM immediately, and images were taken at 1- to 2-min intervals over 180 min. Data were analyzed at 20-min intervals to determine which colonies were attached. Colonies were considered attached when clear spreading of the cells was observed. Videos were viewed with QuickTime, and the percentage of attached colonies was counted manually at each time point; 8–10 colonies were analyzed for each treatment and control group.
Use of BioStation CT technology to compare the effect of smoke from conventional and harm reduction cigarettes on hESC colony growth.
The BioStation CT is a high-content incubation unit equipped with a microscope and camera. This innovative technology enables time-lapse video data to be collected while cells are growing in controlled incubation conditions. Videos can then be analyzed using CL-Quant software (DR Vision, Seattle, WA) to quantify dynamic cell processes (e.g., colony growth). To perform experiments in the BioStation CT, hESC colonies containing five to eight cells were plated on Matrigel-coated 12-well plates in mTeSR medium. After 48 h of incubation, colonies were completely attached and spread, and the density of each well was around 30–40%. To follow colonies over time, they must be evenly distributed in the well to prevent merging. In treatment groups, medium was replaced with 500 μl of mTeSR medium containing 0.1 PE of MS or SS cigarette smoke, whereas control wells received 500 μl of complete mTeSR medium without smoke solution. Once medium was added, the 12-well plates were placed in a Nikon BioStation CT incubator and multiple colonies in each group were imaged every 140 min. For each experimental and control group, 5–10 colonies of similar size were selected and followed for 48 h. After the experiments were completed, images of hESC colonies were downloaded from the BioStation CT, and the growth of colonies was analyzed using CL-Quant software.
Analysis of video data using video bioinformatics tools developed with CL-Quant software.
The video bioinformatics tools that were used in this study have been described in detail previously (Lin et al., 2010). All video bioinformatics processing was carried out using CL-Quant Software with a custom-developed algorithm including three subset routines (recipes): (1) an initial segmentation code which utilized a channel mask to separate the colony and background in each image, (2) an enhancement code that filtered out any remaining image noise (e.g., cellular debris or foreign particulates), and (3) a measurement code that quantified the number of pixels in each colony. Data obtained with the CL-Quant analysis were plotted and analyzed to determine growth rates and percent increase in size of colonies over 48 h. The accuracy of the CL-Quant recipes was verified by periodically checking the fit of masks and by rerunning data to assure reproducibility.
Statistics.
Attachment data for each brand of cigarette were analyzed using a t-test in which means of the three time points showing maximum colony attachment (80, 100, and 120 min) for the control and treated groups were compared for significant differences. In the colony growth assay, the means of the percentage increase in colony size at the final sampling point were compared using ANOVA. When p < 0.05 in the ANOVA, a Dunnet's post hoc test was run in which each treatment group was compared with the control. Statistical analyses were run using InStat (GraphPad, San Diego, CA.). For both the t-test and the ANOVA, p ≤ 0.05 was considered significant.
RESULTS
Evaluation of Cytotoxicity of MS and SS Smoke from Conventional and Harm Reduction Cigarettes
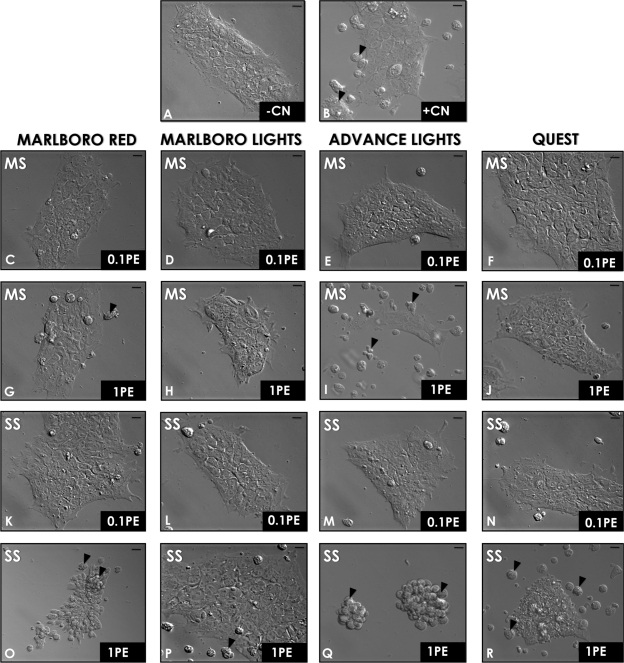
MS and SS smoke solutions from conventional and harm reduction cigarettes were evaluated for their cytotoxic effects on hESC colony morphology (loss of cells from colonies, colony elevation, and granularity in colonies) after 6 h of treatment with doses of 0.01, 0.1, and 1 PE (Fig. 1 and Table 1). Negative controls (−CN) were incubated in complete mTeSR medium (0.0 PE), whereas positive controls (+CN) were treated with 0.5% ethanol, which induces apoptosis. Colonies in the negative control group were spread, healthy, and cobblestone like (Fig. 1A). In contrast, colonies treated with ethanol showed detachment of cells and cell debris (Fig. 1B, arrows). MS smoke at 0.01 PE (data not shown) and 0.1 PE did not affect hESC colony morphology or survival (Figs. 1C–F). However, 1 PE of both Marlboro Red and Advance Lights MS smoke caused some cells to detach from the plate after 6 h of treatment (Figs. 1G and 1I, black arrows). Although colonies did not detach in 1 PE of Marlboro Lights MS smoke, cells were elevated and granular, and colonies had irregular edges (Fig. 1H), unlike the negative control. Quest MS smoke did not affect hESC colony morphology at 1 PE (Fig. 1J).
FIG. 1.
Morphological assay for cytotoxicity. Colonies were incubated for 6 h, and then their morphology was evaluated. (A) The negative control (no treatment) and (B) the positive control (0.5% ethanol). Other colonies were treated with 0.1 PE of MS smoke (C–F), 1 PE of MS smoke (G–J), 0.1 PE of SS smoke (K–N), or 1 PE of SS smoke (O–R). Scale bars = 10 μm. Arrows indicate detached cells. Representative images from three different experiments are shown. PE = amount of smoke in one puff that dissolves in 1 ml of medium).
TABLE 1.
Relative Cytotoxicity of MS and SS Cigarette Smoke on Attached hESCs Colonies
| 6 ha | 24 hb | Magic Red apoptosis detection (2 h) | |
| Marlboro Red | |||
| MS (1 PE) | + | + | + |
| SS (1 PE) | ++ | +++ | ++ |
| Marlboro Lights | |||
| MS (1 PE) | − | − | + |
| SS (1 PE) | + | +++ | ++ |
| Advance Lights | |||
| MS (1 PE) | ++ | ++ | + |
| SS (1 PE) | +++ | +++ | +++ |
| Quest (no nicotine) | |||
| MS (1 PE) | − | − | + |
| SS (1 PE) | ++ | +++ | ++ |
Note. “−,” attached, healthy, cobblestone-like colonies; “+,”elevated, granular morphology; “++,” elevated, granular morphology, and some detached cells from the colonies; “+++,” colonies detached completely.
Based on morphology of colonies at 6 h.
Based on trypan blue data at 24 h.
Like MS smoke, SS smoke solutions at 0.01 PE (data not shown) and 0.1 PE did not affect hESC colony morphology after 6 h of treatment (Fig. 1K–N). However, at 1PE, SS smoke from all four brands caused loss of cells from colonies and in some cases complete rounding up of colonies (Figs. 1O–R). SS smoke from Advance Lights was the most potent followed by Marlboro Red, Quest, and Marlboro Lights. Cells treated with Advance Lights SS smoke solutions completely detached from the plate and formed small round clumps (Fig. 1Q, black arrows). Colonies treated with Marlboro Red and Quest SS smoke solutions were partly detached (Figs. 1O and 1R, black arrows). Cells that remained attached were elevated and granular. With the morphological assay, cytotoxic effects were observed with both MS and SS smoke at 1 PE, but not at lower doses, and the effects were stronger with SS smoke than with MS smoke (Table 1).
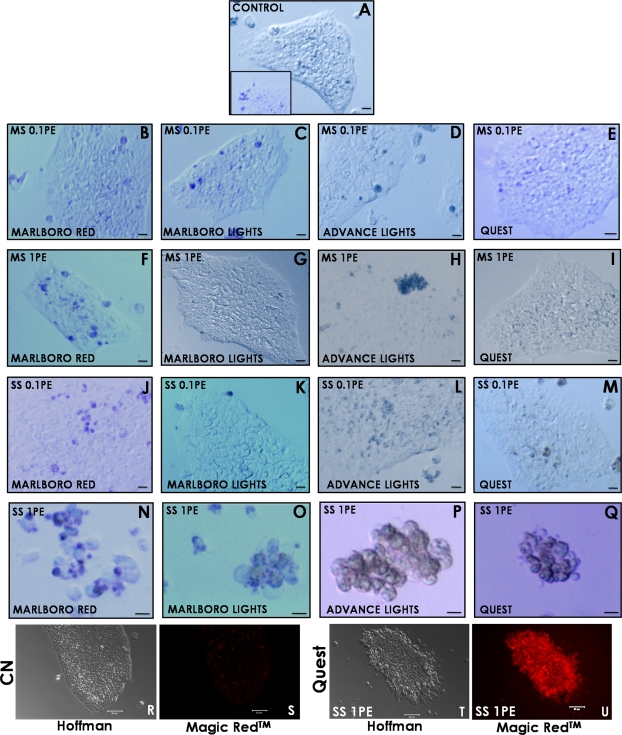
Cytotoxicity was further evaluated after 24 h of treatment with 0.01, 0.1, or 1 PE by staining colonies with trypan blue (Fig. 2 and Table 1). Although a few control colonies had a small number of blue cells, most controls had little to no staining, indicating that cells were generally healthy and viable (Fig. 2A including inset). Colonies treated with MS smoke at doses of 0.01 PE (data not shown) and 0.1 PE (Figs. 2B–E) were generally similar to the controls. However, colonies treated with 1 PE of Marlboro Red or Advance Light MS smoke solutions appeared to have more blue cells than controls (Figs. 2F and 2H). SS smoke solutions at 0.01 PE (data not shown) and 0.1 PE (Figs. 2J–M) generally produced results similar to the control. In contrast, 1 PE of SS smoke solution from all brands caused colonies to detach, round up, and stain heavily with trypan blue (Figs. 2N–Q), indicating the cells had died during treatment. The relative cytotoxicity of MS and SS smoke at 1 PE based on the morphological and trypan blue data is summarized in Table 1. For all brands except Quest, MS smoke was slightly cytotoxic at 1 PE (morphological assay), whereas SS smoke was far more cytotoxic in both assays with Advance harm reduction cigarettes being the most cytotoxic. The morphological and trypan blue data for MS and SS smoke further showed that 0.1 PE was not cytotoxic for any brand.
FIG. 2.
Trypan blue staining of hESC colonies treated with smoke solution for 24 h exhibited cell death at 1 PE but not at 0.1 PE. (A) Typical control colony with little trypan blue staining, whereas the insert shows a less frequently observed control with slightly more staining. Other colonies were treated with 0.1 PE of MS smoke (B–E), 1 PE of MS smoke (F–I), 0.1 PE of SS smoke (J–M), or 1 PE of SS smoke (N–Q). Images were taken using brightfield microscopy. Scale bars for 1 PE SS smoke treatments = 10 μm, and all other scale bars = 20 μm. (R) and (S) are Hoffman and fluorescent images of control colonies stained with Magic Red Caspase 3 & 7 Detection Kit. (T) and (U) are Hoffman and fluorescent images of colonies treated with 1 PE of Quest SS smoke solutions and then stained with Magic Red. For Magic Red groups, scale bars = 50 μm. For both trypan blue and caspase detection, images are representatives from two separate experiments. PE = amount of smoke in one puff that dissolves in 1 ml of medium.
In the cytotoxicity assays, cells in the 1 PE–treated colonies often exhibited blebs on their surfaces, leading us to the hypothesis that smoke-induced cell death occurred by apoptosis. This hypothesis was tested by staining for apoptosis with the Magic Red-Caspases 3 & 7 Detection Kit. Control groups treated with Magic Red did not show fluorescence, indicating that the colonies were healthy and caspases 3 and 7 were not activated (Figs. 2R and 2S). In contrast, all groups treated with 1 PE of MS smoke (data not shown) showed some fluorescence, whereas all groups treated with 1 PE of SS smoke solutions were markedly fluorescent (shown for Quest in Figs. 2T and 2U), indicating that at this dose, cell death was induced apoptotically.
All subsequent experiments were done using 0.1 PE of MS or SS smoke solutions because this dose was shown in the above assays to be noncytotoxic.
Conventional and Harm Reduction SS Cigarette Smoke Inhibited hESC Colony Attachment at a Noncytotoxic Dose (0.1 PE)
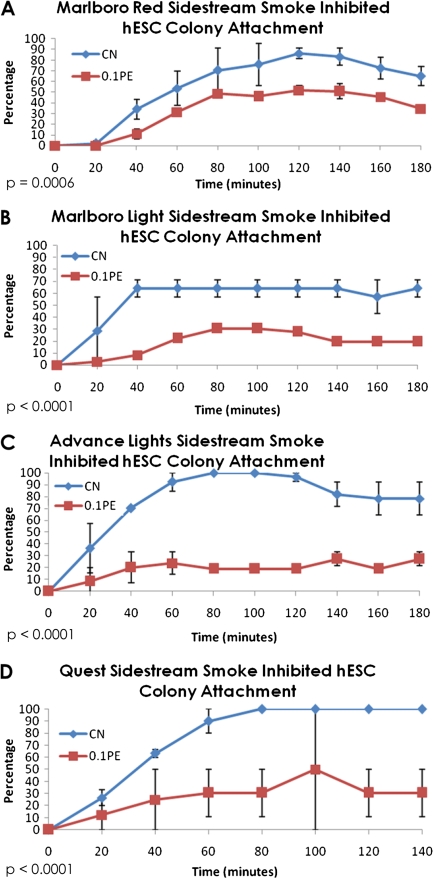
Our previous studies showed that cigarette smoke significantly inhibited attachment of mESC, and SS smoke was more inhibitory than MS smoke (Lin et al., 2009). To determine if SS smoke affects attachment of hESC in a similar manner, time-lapse videos were taken of nonattached colonies using a Nikon BioStation IM, and the kinetics of attachment (percentage of attached colonies) were determined for each control and treated group. For all brands tested, SS smoke from both conventional and harm reduction cigarettes significantly inhibited colony attachment to Matrigel when compared with the untreated control (Figs. 3A–D). Although control and treated colonies reached their maximum attachment by 2 h, the proportion of colonies that attached was significantly lower in all treatment groups (20–60%) than in the control (70–90%). The percentages of inhibition for each type of SS smoke are shown in Table 2. As was observed in our earlier work with mESC (Lin et al., 2009), the harm reduction brands were more inhibitory in this assay than Marlboro Red, the conventional brand. Table 2 further shows that the hESC were more sensitive to SS smoke exposure than the mESC.
FIG. 3.
Effect of 0.1 PE SS smoke solutions on colony attachment in the BioStation IM. (A) Control versus Marlboro Red SS smoke, (B) control versus Marlboro Lights SS smoke, (C) control versus Advance Lights SS smoke, and (D) control versus Quest SS smoke. To determine if the treatments had a significant effect, means of the control and treatment group data collected at 80, 100, and 120 min were compared by a t-test. The p values were all highly significant and are given on each graph. PE = amount of smoke in one puff that dissolves in 1 ml of medium.
TABLE 2.
Percent Inhibition of mESC and hESC Attachment at 0.1 PE of SS Smoke
| hESC | mESCa | |
| Marlboro Red (1.1 mg/cigarette) | ||
| SS (0.1 PE) (%) | 39 | 27 |
| Marlboro Lights (0.8 mg/cigarette) | ||
| SS (0.1 PE) (%) | 60 | 42 |
| Advance Lights (0.8 mg/cigarette) | ||
| SS (0.1 PE) (%) | 76 | 32 |
| Quest (no nicotine) | ||
| SS (0.1 PE) (%) | 69 | 42 |
Data taken from Lin et al. (2009).
MS and SS Smoke from Conventional and Harm Reduction Cigarettes Inhibited hESC Colony Growth at a Noncytotoxic Dose (0.1 PE)
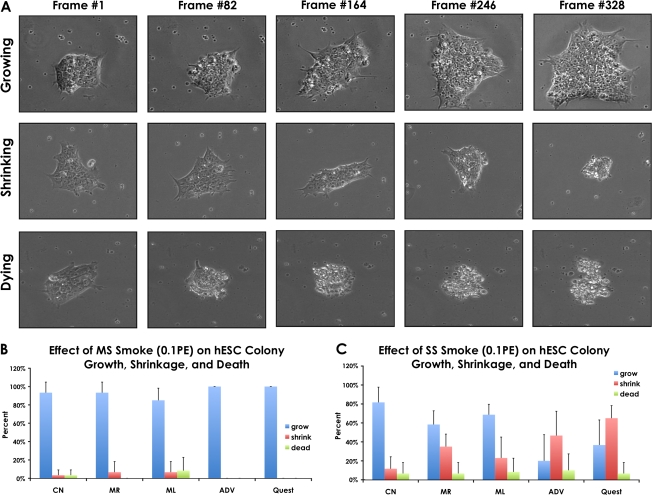
To determine how conventional and harm reduction smoke solutions affect proliferation of hESC, colonies were attached to Matrigel-coated plates, allowed to spread for 48 h, then treated with control or smoke solution for an additional 48 h during which time video data were collected on colonies in each group using the BioStation CT. All videos were first analyzed to determine if colonies grew, shrunk, or died during incubation (Fig. 4A). Although a few colonies died in most groups (Figs. 4B and 4C), including the control, this percentage was very low in all groups, consistent with our prior data showing that 0.1 PE is a noncytotoxic dose. The percentage of colonies that underwent shrinkage during exposure was very low in the groups treated with MS smoke (Fig. 4B). However, the percentage of shrinking colonies increased considerably in the SS-treated groups (Fig. 4C), indicating that these colonies were able to survive at 0.1 PE but may have been losing some cells during the exposure interval. In the time-lapse videos, some cells underwent apoptosis in colonies treated with SS smoke (Supplementary fig. 1), which may have contributed to shrinkage. Apoptosis was further confirmed in SS smoke–treated colonies using the FLICA Poly Caspases Detection Kit (Fig. 5).
FIG. 4.
Effect of 0.1 PE MS and SS smoke solutions on colony growth during 48 h of incubation in the BioStation CT. (A) Time-lapse images of growing, shrinking, and dying colonies. (B) Percentage of growing, shrinking, and dying colonies in control and MS smoke–treated groups. (C) Percentage of growing, shrinking, and dying colonies in control and SS smoke–treated groups. PE = amount of smoke in one puff that dissolves in 1 ml of medium.
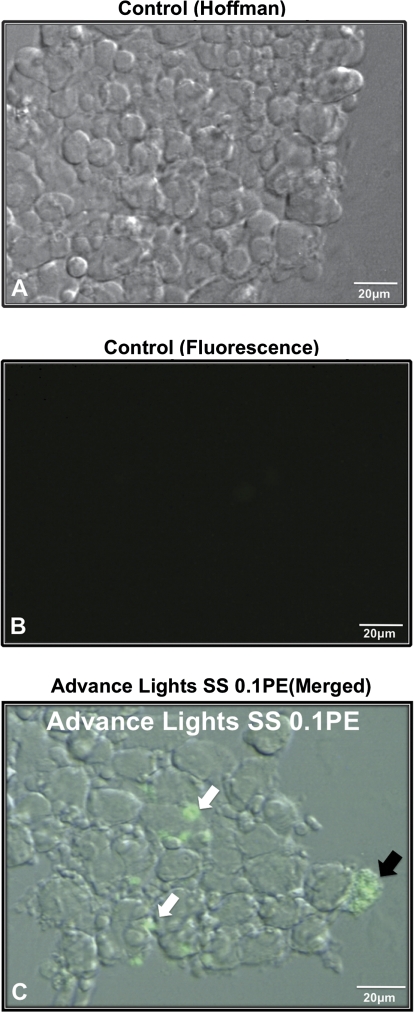
FIG. 5.
Detection of activated caspases in apoptotic cells and in apoptotic debris on smoke solution–treated hESC colonies. (A) Hoffman image of a control hESC colony. (B) Fluorescent image of the same control colony showing no caspase activity. (C) Merged Hoffman and fluorescent image of a colony treated with 0.1 PE Advance Lights SS smoke solution. In (C), black arrow indicates an intact apoptotic cell, whereas white arrows show apoptotic debris positive for activated caspases. Scale bar = 20 μm. PE = amount of smoke in one puff that dissolves in 1 ml of medium.
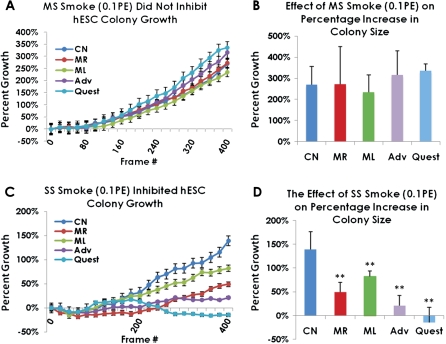
Video data were next analyzed using a video bioinformatics method to determine how smoke treatment affected colony growth (Fig. 6). Kinetic analysis of colony growth using the video bioinformatics software showed that treatment of hESC colonies with 0.1 PE of MS smoke did not alter the rate of growth when compared with the untreated control (Fig. 6A). Moreover, when analyzed by ANOVA, the mean percentage increase in colony size at the final frame was not significantly different among groups (p = 0.79) (Fig. 6B). In contrast, hESC colonies treated with SS smoke solutions had slower growth rates than the untreated control (Fig. 6C). Quest and Advance, two of the harm reduction brands, produced the greatest inhibition in rate of growth of the four brands tested. When the mean percentage increase in colony size was compared for the final frame at 48 h, all four types of SS smoke solution significantly inhibited colony growth when compared with the untreated control (Fig. 6D). Again, both the Advance and Quest brands were the most inhibitory by this criterion. When growth characteristics were compared with our earlier data with mESC (Table 3), MS smoke was generally more inhibitory in the mouse system than in the human. However, Marlboro Red and Advance Lights SS smoke solutions were far more inhibitory in the hESC system than in the mouse (Table 3).
FIG. 6.
Growth of hESC colonies in control and treated groups (0.1 PE of MS or SS smoke solution) during 48 h of incubation in the BioStation CT. (A) Growth rates of control and MS-treated colonies. (B) Percentage increase in colony size at 48 h for control and MS-treated colonies. (C) Growth rates of control and SS-treated colonies. (D) Percentage increase in colony size at 48 h for control and SS-treated colonies. For (A) and (C), values are plotted as means ± SEM. For (B) and (D), values are plotted as means ± SD. All means are based on four separate experiments, except for Quest which was based on three experiments. In each experiment, 4 to 10 colonies were analyzed. Analyses were done using CL-Quant software. PE = amount of smoke in one puff that dissolves in 1 ml of medium.
TABLE 3.
Percent Inhibition of mESC versus hESC Growth When Exposed to 0.1 PE of MS or SS Smoke
| hESCa | mESCb | |
| Marlboro Red | ||
| MS (0.1 PE) (%) | −2 | 13 |
| SS (0.1 PE) (%) | 75 | 39 |
| Marlboro Lights | ||
| MS (0.1 PE) (%) | 4 | 17 |
| SS (0.1 PE) (%) | 46 | 84 |
| Advance Lights | ||
| MS (0.1PE) (%) | −29 | 20 |
| SS (0.1PE) (%) | 88 | 39 |
| Quest (no nicotine) | ||
| MS (0.1 PE) (%) | −20 | 32 |
| SS (0.1 PE) (%) | 35 | 76 |
Negative numbers indicate growth was not inhibited relative to the control.
Data taken from Lin et al. (2009).
DISCUSSION
Our study accomplished three goals: (1) to develop an hESC-based toxicological assay, (2) to compare the toxicity of smoke from harm reduction and conventional cigarettes using the hESC assay, and (3) to compare the response of human and mouse ESC to smoke from conventional and harm reduction cigarettes. Because embryos are generally more sensitive to chemicals than adults, the risk that environmental chemicals present to human health is best evaluated using prenatal stages of development when possible (Grandjean et al., 2007). Although it is not feasible to directly study human prenatal stages experimentally, embryonic stem cells can be used to model pre- and postimplantation embryos. Although assays with mESC have been developed and validated (Genschow et al., 2004; Spielmann, 2005), hESC have not been easy to adapt to toxicological testing. Although attachment and proliferation end points worked well when evaluating the effects of cigarette smoke on mESC (Lin et al., 2009), these assays were difficult to directly adapt to the hESC model. New approaches for using hESC in toxicological testing are being explored (Adler et al., 2008), and quantitative analysis of microscopic video data is a powerful technology for characterizing the effects of toxicants on dynamic cell processes (Cervinka et al., 2008). We have introduced a method for evaluating chemical toxicity with hESC by collecting time-lapse video data using BioStation technology and then mining quantitative information from the videos either manually (attachment assay) or with video bioinformatics tools (colony growth). Quantitative analysis of video data allows dynamic cellular processes to be explored in response to chemical exposure and opens the possibility of developing new powerful in vitro assays. The cellular assays introduced in this study are relatively rapid and inexpensive, help minimize animal usage, and provide data directly on a human model.
To determine how PE relate to doses inhaled by smokers, the concentration of nicotine was compared in smoke solutions and human smokers. Nicotine in tissues, fluids, and matrices can be 2.9 (breast milk) to 87 (saliva) times higher than in plasma (Benowitz et al., 2009; Dahlstrom et al., 1990). Nicotine in plasma of active smokers ranges from 0.004 to 0.100 μg/ml (Russell et al., 1980; Dhar, 2004). Based on nicotine concentration at the approximate midpoint in this range (0.05 μg/ml), tissue nicotine concentrations in active smokers can be estimated to be from 0.145 to 4.35 μg/ml. At 0.1 PE, the nicotine concentration in Marlboro Red MS smoke was 0.3–0.6 μg/ml, which is well within the estimated nicotine concentration in tissues of active smokers.
Nicotine concentration in the serum of passive smokers varies with the amount of exposure/day and ranges from 0.006 to 0.023 μg/ml (Dhar, 2004; Russell and Feyerabend, 1975). Based on a 2.9- to 87-fold increase of nicotine in fluid/tissue versus plasma (Luck and Nau, 1984; Dahlstrom et al., 1990; Benowitz et al., 2009), nicotine in tissues of passive smokers at the midpoint of the serum range (0.015 μg/ml) would be approximately 0.044 to 1.3 μg/ml. At 0.1 PE, nicotine concentration in SS smoke solutions made on our smoking machine was estimated from a previous study (Wong et al., 2004) to be 0.2 μg/ml, which is within the estimated tissue range of passive smokers. Because 0.1 PE was noncytotoxic and reasonably simulated doses to which active and passive smokers are exposed, we used this concentration in our attachment and colony growth assays.
Attachment of cells to a substrate is essential in embryological development and prevents apoptosis of cultured hESC. Data, in general, support the conclusion that smoke and/or nicotine inhibits attachment of embryonic and differentiated cells to substrates. In our study, 0.1 PE of SS smoke from all brands inhibited attachment of hESC cells to Matrigel. Similar inhibition of hESC attachment occurred with 1.8–3.7μM of nicotine alone, and this inhibition was reversible by the nicotine antagonist tubocurarine, suggesting that action was through a nicotine receptor (Zdravkovic et al., 2008). Nicotine caused similar inhibition of periodontal fibroblast attachment in culture (James et al., 1999). However, other factors in smoke must also impair attachment because, in our study, Quest smoke solutions did not contain nicotine, yet significantly inhibited attachment. Of particular interest in our study was the finding that SS smoke from harm reduction brands was a more potent inhibitor of attachment than SS smoke from a conventional brand.
Attachment of differentiated cultured cells was also impaired by cigarette smoke. Bovine bronchial epithelial cells were inhibited from attaching to fibronectin by smoke condensate (Cantral et al., 1995), and periodontal ligament fibroblast attachment to tooth roots was significantly decreased when cells and roots were isolated from smokers versus nonsmokers (Gamal and Bayomy, 2002). Similar effects may occur in vivo during development as in utero exposure to smoke appears to increase the spacing between lung alveolar attachments in infants that died from sudden infant death syndrome (Elliot et al., 2003).
Cell proliferation and growth are also essential events during prenatal development. The noncytotoxic dose (0.1 PE) of MS smoke did not significantly affect growth; however, 0.1 PE of SS smoke from all brands slowed colony growth rate and significantly inhibited the size of colonies at 48 h. The strongest effects in this assay were produced by SS smoke from Advance and Quest cigarettes, two harm reduction brands. The reason these harm reduction brands are more potent than Marlboro Reds in the proliferation and attachment assays could be related to the source of the tobacco in these products or the methods used to cure the tobacco.
Other studies also support the conclusion that growth is negatively impacted by cigarette smoke. Condensate from Quest low-nicotine and nicotine-free cigarettes inhibited human bronchial epithelial cell growth and was more potent than condensate from a research cigarette (Chen et al., 2008). Because significant apoptosis was observed in the bronchial epithelium, doses of Quest smoke condensate may have been in the cytotoxic range. In the study by Chen et al., condensate from nicotine-free Quest-inhibited proliferation more strongly than condensate from nicotine-containing Quest, an effect that was partially reversed by adding nicotine to condensate from nicotine-free Quest. In contrast to the Quest data (Chen et al., 2008), addition of nicotine alone to culture medium increased the percentage of hESC undergoing apoptosis (Zdravkovic et al., 2008).
Few studies have investigated harm reduction smoke, in particular SS smoke, in cellular based assays. In one such study with explants of hamster oviducts, smoke from harm reduction cigarettes (Advance Lights and Omni Lights) significantly inhibited oocyte pick-up rate and smooth muscle contraction rate with SS smoke generally being more potent than MS smoke (Riveles et al., 2007). In a study using light cigarettes, mouse lungs exhibited a decrease in tissue inhibitor of metalloprotease-2 and an increase in matrix metalloprotease-2 expression, suggesting an imbalance in extracellular matrix formation (Valenca et al., 2006). These mice developed emphysema and increased levels of NFκB which increased inflammatory response in the lungs. When taken together with our studies on embryonic stem cells, work with cell and animal models supports the idea that harm reduction cigarette smoke impairs a spectrum of biological processes and that SS smoke from harm reduction brands is usually more toxic than MS smoke from the same cigarette or than SS smoke from conventional cigarettes.
Because animal models may not accurately predict toxicity for humans, we compared the effects of cigarette smoke on mESC from our previous study (Lin et al., 2009) to the results of this study and found that, in general, cells from both species responded similarly to cigarette smoke. However, the sensitivity of the two species appears to be different. Although SS smoke from both harm reduction (0.1 PE) and the conventional brand inhibited cell attachment, the negative impact was greater for hESC (60–76%) than mESC (30–42%). In the proliferation assay, mESC were more sensitive than hESC colonies to MS smoke. However, SS smoke had a greater negative impact on hESC colony growth (41–100%) than on mESC proliferation (39–84%). Although these data were collected at different times and therefore not compared statistically, they do demonstrate the desirability of using hESC for toxicological testing to obtain the best estimate of the risk posed directly to humans.
In conclusion, we have introduced a rapid hESC-based assay that provides quantitative data on dynamic cellular processes by combining time-lapse video data with video bioinformatics tools. In the future, this assay could easily be expanded to provide more end points and to evaluate other chemicals. Our assay was used to show that a noncytotoxic dose of SS smoke inhibited cell attachment to Matrigel and growth of hESC colonies. SS smoke was consistently more potent than MS smoke, and SS smoke from harm reduction brands was generally more potent than SS smoke from the conventional brand. These data demonstrate that reduction of carcinogens in harm reduction MS smoke does not necessarily reduce the toxicity of unfiltered SS smoke and that harm reduction products are not necessarily safer than their conventional counterparts. This information should be valuable to potential users of harm reduction cigarettes and should be taken into account when establishing policies regarding the sale, advertising, and use of harm reduction products.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
California Tobacco-Related Disease Research Program (XT-0167); the California Institute for Regenerative Medicine (NE-A0005A-IE); UCR Academic Senate; a National Science Foundation Integrative Graduate Education and Research Traineeship (#0903667); Graduate Division (Dissertation Fellowship to S.L.); a National Institutes of Health Minority Access to Research Careers Fellowship (to S.F.).
Supplementary Material
Acknowledgments
We are grateful to Sam Alworth, Ned Jastromb, and Randy Myers from Nikon, Inc. for teaching us how to use the BioStation CT, BioStation IM, and the CL-Quant software to analyze our video data. We also thank Dr Paul Clark for providing measurements on nicotine concentration in smoke solutions and Crystal Hua and Jiradar Tammy Choonchuersup for their help with data analysis.
References
- Adler S, Pellizzer C, Hareng L, Hartung T, Bremer S. First steps in establishing a developmental toxicity test method based on human embryonic stem cells. Toxicol. In Vitro. 2008;22:200–211. doi: 10.1016/j.tiv.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Andres RL, Day MC. Perinatal complications associated with maternal tobacco use. Semin. Neonatol. 2000;5:231–241. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., III Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009;192:29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiller J, Sasco AJ. [Smoking (active or passive) in relation to fertility, medically assisted procreation and pregnancy] J. Gynecol. Obstet. Biol. Reprod. (Paris) 2005;34(Spec no. 1):3S47–3S54. [PubMed] [Google Scholar]
- Cantral DE, Sisson JH, Veys T, Rennard SI, Spurzem JR. Effects of cigarette smoke extract on bovine bronchial epithelial cell attachment and migration. Am. J. Physiol. 1995;268:L723–L728. doi: 10.1152/ajplung.1995.268.5.L723. [DOI] [PubMed] [Google Scholar]
- Cervinka M, Cervinkova Z, Rudolf E. The role of time-lapse fluorescent microscopy in the characterization of toxic effects in cell populations cultivated in vitro. Toxicol. In Vitro. 2008;22:1382–1386. doi: 10.1016/j.tiv.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Chen J, Higby R, Tian D, Tan D, Johnson MD, Xiao Y, Kellar KJ, Feng S, Shields PG. Toxicological analysis of low-nicotine and nicotine-free cigarettes. Toxicology. 2008;249:194–203. doi: 10.1016/j.tox.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Lundell B, Curvall M, Thapper L. Nicotine and cotinine concentrations in the nursing mother and her infant. Acta Paediatr. Scand. 1990;79:142–147. doi: 10.1111/j.1651-2227.1990.tb11430.x. [DOI] [PubMed] [Google Scholar]
- Dhar P. Measuring tobacco smoke exposure: quantifying nicotine/cotinine concentration in biological samples by colorimetry, chromatography and immunoassay methods. J. Pharm. Biomed. Anal. 2004;35:155–168. doi: 10.1016/j.jpba.2004.01.009. [DOI] [PubMed] [Google Scholar]
- DiCarlantonio G, Talbot P. Inhalation of mainstream and sidestream cigarette smoke retards embryo transport and slows muscle contraction in oviducts of hamsters (Mesocricetus auratus) Biol. Reprod. 1999;61:651–656. doi: 10.1095/biolreprod61.3.651. [DOI] [PubMed] [Google Scholar]
- Elliot JG, Carroll NG, James AL, Robinson PJ. Airway alveolar attachment points and exposure to cigarette smoke in utero. Am. J. Respir. Crit. Care Med. 2003;167:45–49. doi: 10.1164/rccm.2110005. [DOI] [PubMed] [Google Scholar]
- EPA. EPA Report/600/6-90/006F: Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders. Washington, DC: U.S. EPA; 1992. [Google Scholar]
- Gamal AY, Bayomy MM. Effect of cigarette smoking on human PDL fibroblasts attachment to periodontally involved root surfaces in vitro. J. Clin. Periodontol. 2002;29:763–770. doi: 10.1034/j.1600-051x.2002.290814.x. [DOI] [PubMed] [Google Scholar]
- Genschow E, Spielmann H, Scholz G, Pohl I, Seiler A, Clemann N, Bremer S, Becker K. Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests. Altern. Lab. Anim. 2004;32:209–244. doi: 10.1177/026119290403200305. [DOI] [PubMed] [Google Scholar]
- Gieseke C, Talbot P. Cigarette smoke inhibits hamster oocyte pickup by increasing adhesion between the oocyte cumulus complex and oviductal cilia. Biol. Reprod. 2005;73:443–451. doi: 10.1095/biolreprod.105.041152. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Bellinger D, Bergman A, Cordier S, et al. The Faroes statement: Human health effects of developmental exposure to chemicals in our environment. Basic Clin. Pharmacol. Toxicol. 2007;102:73–75. doi: 10.1111/j.1742-7843.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- Higgins S. Smoking in pregnancy. Curr. Opin. Obstet. Gynecol. 2002;14:145–151. doi: 10.1097/00001703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- James JA, Sayers NM, Drucker DB, Hull PS. Effects of tobacco products on the attachment and growth of periodontal ligament fibroblasts. J. Periodontol. 1999;70:518–525. doi: 10.1902/jop.1999.70.5.518. [DOI] [PubMed] [Google Scholar]
- Knoll M, Talbot P. Cigarette smoke inhibits oocyte cumulus complex pick-up by the oviduct independent of ciliary beat frequency. Reprod. Toxicol. 1998;12:57–68. doi: 10.1016/s0890-6238(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Lin S, Fonteno S, Satish S, Bhanu B, Talbot P. Video bioinformatics analysis of human embryonic stem cell colony growth. J. Vis. Exp. 2010;39 doi: 10.3791/1933. doi:10.3791/1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Tran V, Talbot P. Comparison of toxicity of smoke from traditional and harm reduction cigarettes using embryonic stem cells as a novel model for pre-implantation development. Hum. Reprod. 2009;24:386–397. doi: 10.1093/humrep/den419. [DOI] [PubMed] [Google Scholar]
- Luck W, Nau H. Nicotine and cotinine concentrations in serum and milk of nursing smokers. Br. J. Clin. Pharmac. 1984;18:9–15. doi: 10.1111/j.1365-2125.1984.tb05014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlynarcikova A, Fickova M, Scsukova S. Ovarian intrafollicular processes as a target for cigarette smoke components and selected environmental reproductive disruptors. Endocr. Regul. 2005;39:21–32. [PubMed] [Google Scholar]
- Riveles K, Iv M, Arey J, Talbot P. Pyridines in cigarette smoke inhibit hamster oviductal functioning in picomolar doses. Reprod. Toxicol. 2003;17:191–202. doi: 10.1016/s0890-6238(02)00150-8. [DOI] [PubMed] [Google Scholar]
- Riveles K, Tran V, Roza R, Kwan D, Talbot P. Smoke from traditional commercial, harm reduction and research brand cigarettes impairs oviductal functioning in hamsters (Mesocricetus auratus) in vitro. Hum. Reprod. 2007;22:346–355. doi: 10.1093/humrep/del380. [DOI] [PubMed] [Google Scholar]
- Rogers JM. Tobacco and pregnancy: Overview of exposures and effects. Birth Defects Res. C Embryo Today. 2008;84:1–15. doi: 10.1002/bdrc.20119. [DOI] [PubMed] [Google Scholar]
- Russel MAH, Jarvis M, Feyerbend C. Relation of nicotine yield of cigarettes to blood nicotine concentration in smokers. Br. Med. J. 1980;280:972–976. doi: 10.1136/bmj.280.6219.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MA, Feyerabend C. Blood and urinary nicotine in non-smokers. Lancet. 1975;1:179–181. doi: 10.1016/s0140-6736(75)91355-0. [DOI] [PubMed] [Google Scholar]
- Shiverick KT, Salafia C. Cigarette smoking and pregnancy I: Ovarian, uterine and placental effects. Placenta. 1999;20:265–272. doi: 10.1053/plac.1998.0377. [DOI] [PubMed] [Google Scholar]
- Spielmann H. Predicting the risk of developmental toxicity from in vitro assays. Toxicol. Appl. Pharmacol. 2005;207:375–380. doi: 10.1016/j.taap.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Talbot P. In vitro assessment of reproductive toxicity of tobacco smoke and its constituents. Birth Defects Res. C Embryo Today. 2008;84:61–72. doi: 10.1002/bdrc.20120. [DOI] [PubMed] [Google Scholar]
- Talbot P, Riveles K. Smoking and reproduction: the oviduct as a target of cigarette smoke. Reprod. Biol. Endocrinol. 2005;3:52. doi: 10.1186/1477-7827-3-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenca SS, Castro P, Pimenta WA, Lanzetti M, Silva SV, Barja-Fidalgo C, Koatz VL, Porto LC. Light cigarette smoke-induced emphysema and NFkappaB activation in mouse lung. Int. J. Exp. Pathol. 2006;87:373–381. doi: 10.1111/j.1365-2613.2006.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner KE. Will the next generation of “safer” cigarettes be safer? J. Pediatr. Hematol. Oncol. 2005;27:543–550. doi: 10.1097/01.mph.0000184574.00717.6c. [DOI] [PubMed] [Google Scholar]
- Wong LS, Green H, Feugate JE, Yadav M, Nothnagel EA, Martins-Green M. Effects of “second-hand” smoke on structure and function of fibroblasts, cells that are critical for tissue repair and remodeling. BMC Cell Biol. 2004;5:13. doi: 10.1186/1471-2121-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdravkovic T, Genbacev O, LaRocque N, McMaster M, Fisher S. Human embryonic stem cells as a model system for studying the effects of smoke exposure on the embryo. Reprod. Toxicol. 2008;26:86–93. doi: 10.1016/j.reprotox.2008.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.