Abstract
Exposure to dioxin and other aryl hydrocarbon receptor (AhR) ligands results in multiple, specific developmental cardiovascular phenotypes including pericardial edema and circulatory failure in small aquarium fish models. Although phenotypes are well described, mechanistic underpinnings for such toxicities remain elusive. Here we suggest that AhR activation results in stimulation of inflammation and “eicosanoid” pathways, which contribute to the observed developmental, cardiovascular phenotypes. We demonstrate that medaka embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (0.05–1 ppb) during early development result in a dose-related increase in the prevalence of pericardial edema and that this phenotype correlates with an increase in cyclooxygenase-2 (COX-2) gene expression. Those individuals exhibiting the edema phenotype had significantly greater COX-2 mRNA than their nonedematous cohort. Selective pharmacological inhibition of COX-2, with NS-398, and genetic knock down of COX-2 with a translation initiation morpholino significantly attenuated prevalence and severity of edema phenotype. Subsequently, exposures of medaka embryos to arachidonic acid (AA) resulted in recapitulation of the pericardial edema phenotype and significantly increased COX-2 expression only in those individuals exhibiting the edema phenotype compared with their nonedematous cohort. AA exposure does not result in significant induction of cytochrome P450 1A expression, suggesting that pericardial edema can be induced independent of AhR/aryl hydrocarbon receptor nuclear translocator/dioxin response element interactions. Results from this study demonstrate that developmental exposure to TCDD results in an induction of inflammatory mediators including COX-2, which contribute to the onset, and progression of heart dysmorphogenesis in the medaka model.
Keywords: COX-2, TCDD, medaka, pericardial edema
Specific aryl hydrocarbon receptor (AhR) ligands are ubiquitous environmental contaminants and potent disruptors of cardiovascular development in vertebrates. In mammalian, avian and fish developmental models, embryonic exposure to AhR ligands result in hallmark toxicities including dilated cardiomyopathy, reduced cardiomyocyte proliferation (Aragon et al., 2008; Ivnitski et al., 2001; Kopf and Walker, 2009; Sedmera et al., 2002), altered looping, elongated atrium, ventricular hypertrophy, and impairment of atrioventricular and ventricular outflow valve formation (Grimes et al., 2008; Mehta et al., 2008). Associated reduction of cardiac efficiency eventually leads to congestive heart failure and, in fish and chick models, to edema and hemorrhage (Ivnitski et al., 2001; Walker and Catron, 2000). These phenotypes are generally conserved across vertebrate species (Carney et al., 2008; Chen and Cooper, 1999; Hornung et al., 1999); however, there are noticeable differences between piscine, avian, and mammalian models (Kopf and Walker, 2009). Certain of these cardiovascular alterations are similar to human heart pathologies including hypoplastic left heart syndrome, a severe congenital malformation previously linked to environmental exposures of dioxins and polychlorinated biphenyls (PCBs) (Grimes et al., 2008; Hinton et al., 2007). Using the occurrence and severity of these well-described developmental phenotypes, we have pursued an understanding of the mechanistic underpinning causally associated with AhR-induced cardiovascular toxicities.
Initial investigations with the small aquarium fish model, zebrafish, established the role of AhR and necessary coregulators of AhR signaling in onset and progression of developmental cardiovascular phenotypes (Carney and Prash et al., 2006). These keystone studies followed by oligonucleotide knockdown procedures demonstrated the role of zebrafish AhR2 and zfARNT1 in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)–induced developmental toxicities (Antkiewicz et al., 2006; Antkiewicz et al., 2005; Prasch et al., 2006, 2003). Because duplicate AhR and aryl hydrocarbon receptor nuclear translocator (ARNT) genes occur in teleosts, it was first essential to identify relevant gene paralogs for mediation of TCDD toxicity. These and other mechanistic investigations pointed to a “classical” nuclear mechanism including AhR/ARNT/dioxin response element (DRE) interactions as prerequisite for cardiovascular toxicities.
Evidence for an alternative nonclassical (nonnuclear) pathway of AhR activation was evolving concurrently with the above. In a seminal paper, Dong and Matsumura (2008) described AhR-mediated induction of inflammatory responses in MCF10A cells. This and subsequent studies demonstrated that cellular inflammatory mediators are highly responsive to AhR activation in vitro (Dong and Matsumura, 2008, 2009; Dong et al., 2010; Li and Matsumura, 2008). Induction of inflammatory response was described as independent of nuclear AhR/ARNT/DRE interaction, suggesting a deviation from the classical AhR mechanism. In refining this model, Matsumura (2009), proposed that AhR activation triggers a nonnuclear signaling pathway that results in a rapid increase in intracellular calcium concentration, activation of cytosolic phospholipases (PLA2), subsequent induction of COX-2, and resultant inflammatory response. This response to AhR activation occurs in multiple cell types including foam cells, macrophages, adipocytes, epithelial cells, and others (Dong and Matsumura, 2008; Li and Matsumura, 2008; Sciullo et al., 2009; Vogel et al., 2004). Although effects of this signaling pathway are long lasting and ascribed to manifestation of chronic hallmark toxicities of TCDD-AhR activation such as wasting syndrome and hydronephrosis (Matsumura, 2009; Nishimura et al., 2008), limited work suggests that this pathway may be of importance in developmental phenotypes observed in small aquarium fish models.
Cyclooxygenases-2 (COX-2) also known as prostaglandin (PG) endoperoxide G/H synthases are known targets of AhR activation. These enzymes catalyze rate-limiting steps in production and secretion of the entire family of prostanoids (Vane et al., 1998). COX-2 is rapidly upregulated in response to a variety of inflammatory signals and contributes to acute inflammatory progression through production of pro- and anti-inflammatory “eicosanoids” (Smith et al., 2000; Vane et al., 1998). Studies using small aquarium fish models such as zebrafish demonstrate a linkage between COX-2 and TCDD-induced developmental-cardiovascular phenotypes. Teraoka et al., (2009) demonstrated that COX-2 inhibition rescues TCDD-induced circulatory failure in zebrafish mesencephalic vein. In a companion study, a reduction in incidence of TCDD-induced pericardial edema was additionally observed following COX-2 inhibition (Teraoka et al., 2008). Linkage between pericardial edema phenotype and COX-2 induction, however, is not restricted to AhR ligands. Zebrafish embryos exposed to aristolochic acid a Chinese herbal medicine similar to arachidonic acid (AA), and not an AhR ligand, developed significant cardiovascular deformations including pericardial edema (PE), ventricular hypertrophy, and apparent focal destruction of endocardium (Huang et al., 2007). Inflammation markers including both COX-2 and interleukin (IL)-1β were induced, and both onset and severity of PE phenotype were attenuated by addition of certain COX-2 inhibitors. It thus appears that COX-2 plays a significant role in both normal heart development and onset of aberrant cardiovascular phenotypes. Herein, we demonstrate that AhR activation results in stimulation of the COX-2 inflammatory pathway following TCDD exposure in a small aquarium model of human disease. We demonstrate that medaka embryos exposed to TCDD during early development result in a dose-related increase in the prevalence of PE and that this phenotype correlates with modification of COX-2 gene expression. Further, studies with AA exposures demonstrate that both onset and progression of PE can be produced independently of AhR/ARNT/DRE signaling. We thus propose that both TCDD- and AA-induced developmental cardiovascular toxicities are at least partially mediated through dysregulation of COX-2 during heart development.
MATERIALS AND METHODS
Medaka culture and embryo collection.
Fish care and maintenance was provided daily in accordance with North Carolina State University IACUC approved animal protocol (NCSU# 07-183-B). Brood stock were housed in a charcoal-filtrated and UV-treated recirculating aquatic system. Water temperature and pH were monitored daily and maintained at ∼25°C ± 2°C and ∼7.4, respectively, and broodstock were maintained under a strict light: dark cycle of 16:8 h. Dry food (Otohime B1; Reed Mariculture, Campbell, CA) was fed several times per day via automated feeders with once daily supplementation of newly hatched Artemia nauplii. Under the care and culture conditions described above, medaka spawned daily producing 20–30 embryonated eggs per mass. Eggs were collected and separated (disruption of attachment filaments joining individual eggs) by gentle rolling on a moistened surface. Groups of individual embryonated eggs of specified collection date and developmental stage (Iwamatsu, 2004) were suspended in a 2% saline solution made using Instant Ocean (Aquatic Ecosystems, Apopka, FL) in a 100- × 20-mm tissue culture dish (BD Biosciences, Franklin Lanes, NJ) and housed at 26°C under gentle movement. After 5days, marine water was replaced with 1× embryo rearing medium (ERM) containing: 17.1mM NaCl and, in μMs, 272 CaCl2·2H2O, 402 KCl, and 661 MgSO4·7H2O until hatching usually 9 days postfertilization (dpf). Embryos were examined under a dissecting microscope and selected (structurally normal) embryos within a 4–5 hours postfertilization (hpf) (stages 8–9) of development were assigned to each experiment.
Chemicals.
TCDD (or dioxin) (99% purity) was purchased from Cambridge Isotope Laboratories (Andover, MA). AA (99% purity), and NS-398 (98% purity), was purchased from Calbiochem (North American affiliate of Merck KGaA, Darmstadt, Germany). All chemicals were dissolved in 100% high-performance liquid chromatography–grade dimethyl sulfoxide (DMSO).
Exposures.
Medaka embryos 4–5 hpf (developmental stages 8–9; Iwamatsu, 2004) were collected and processed as described above. All exposures were conducted in six-well tissue culture plates (Corning, Corning, NY). For each exposure, embryos were treated in three replicate experimental wells containing 10 embryos per well and each experiment replicated at least three times. Embryos were exposed to TCDD between 0.01 and 1 ppb for 1 h followed by three replicate rinses with 1× ERM. For coexposures of TCDD and NS398, embryos were first treated with TCDD, then washed with 1× ERM followed by addition of 20μM NS398. NS398 was renewed daily with media changes. Embryos were exposed to AA between 100 and 450μM, and AA-spiked media was renewed daily. To achieve coexposure of AA and NS398, embryos were first treated with AA followed by addition of NS398 1 h latter. A COX-2 morpholino (MO) antisense oligonucleotide 5′-GAGTTCATGCTCCTGAGTTTGATCT-3′ was purchased from GeneTools, LLC (Corvalis, OR, http://www.gene-tools.com). Injections were made in one- to two-cell–stage medaka embryos using beveled glass injection pipettes, a Medical Systems Cooperation PL-1000 microinjector, and Narishigie, Inc., micromanipulators, under a dissecting scope with transmitted illumination. Embryos were maintained at 26°C and monitored daily for phenotypic characterization.
Total RNA isolation.
RNA extractions for vehicle, NS-398, TCDD, TCDD + NS-398, AA, and AA + NS-398–exposed embryos were conducted 6 days postexposure (dpe). Embryos were homogenized with 1 ml of RNA Bee (TelTest, Friendswoood, TX) using a stainless steel Polytron homogenizer (Kinematica, Newark, NJ) cleaned with RNaseZAP (Sigma), diethylpyrocarbonate-treated water, and sterile deionized water. Following homogenization, total RNA was isolated as described in Volz et al. (2005). Prior to sample elution, each sample was on-column–digested with DNase to eliminate DNA contamination using an RNase-free DNase Set according to manufacturer's instructions (Qiagen, Germantown, MD) and then eluted with 30 μl of warmed (52°C) RNase-free water. RNA quantity and 260/280 ratios were determined using a NanoDrop ND-1000 spectrophotometer.
Real-time PCR.
Synthesis of cDNA was conducted with 250 ng total RNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems): random hexamers, MultiScribe Reverse Transcriptase (RT), RNase inhibitor, deoxynucleotide triphosphate mix, and 10× RT buffer in a 20-μl reaction. Relative levels of cytochrome P450 1A (CYP1A), COX-2, and 18s transcripts in DMSO, NS-398, AA, TCDD, TCDD + NS-398, and AA + NS398-treated embryos were measured using real-time PCR. The following medaka-specific real-time PCR primers were designed using PrimerQuest (Integrated DNA Technologies): COX-2, forward primer 5′-CCCTATGCGTCTTTTGAGGA-3′, reverse primer 5′-AACAATGTCGAAGCCTACGCT-3′; and 18s, forward primer 5′-CCTGCGGCTTAATTTGACTC-3′, reverse primer 5′-GACAAATCGCTCCACCAACT-3′. COX-2 and 18s cDNAs were PCR amplified separately in triplicate using a 96-well PCR plate and an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). For each 25-μl real-time PCR reaction, cDNAs were amplified using 1 μl (0.1–1 ng/μl) first-strand cDNA, 9.5 μl RNase-free water, 1 μl of 5μM forward primer, 1 μl of 5μM reverse primer, and 12.5 μl of 2× QuantiTect SYBR Green PCR Master Mix (AB applied biosystems). Real-time PCR reaction conditions were 95°C for 15 min followed by 41 cycles of 94°C for 15 s, 55°C for 30 s, and 72°C for 1 min. Relative quantitation of gene expression within each reaction was calculated following manufacturer's instructions (User Bulletin #2, ABI PRISM 7700 Sequence Detection System; Applied Biosystems). For each sample, the threshold cycle for reference (18s) amplification (Ct,18s) was subtracted from the threshold cycle for target amplification (Ct, COx-2) to yield a Ct. The threshold cycle represents the cycle number at which the fluorescence signal is significantly above the baseline. The mean or SD of Ct for DMSO-treated samples was subtracted from the mean or SD of Ct for TCDD-treated samples to yield a mean and SD of Ct for each target. Fold induction relative to DMSO-treated individuals and 95% confidence intervals were calculated using 2−Ct (Livak and Schmittgen, 2001).
Histology and imaging.
All medaka embryos were fixed in 2% paraformaldehyde/PBS (pH 7.4) for 72 h at 4°C and stored in 6% sucrose/PBS (pH 7.4) at 4°C until decorionating, mounting, and sectioning. Fish were oriented in lateral recumbency and embedded in water-soluble monomer glycol methacrylate (GMA) in Technovit 7100 glycol methacrylate (GMA Kit from EB Sciences East Granby, CT) to increase resolution of morphologic assessment. Dehydration, infiltration, and hardening followed the manufacturer's recommendations. A Leica 2065 Supercut Microtome (Leica Microsystems, Inc., Bannockburn, IL) with a knife holder for triangular glass knives was used for sectioning (2.5 μm thickness). Mounted sections were stained by H&E.
Medaka embryos were surveyed for presence of PE and imaged with a Nikon SMZ1500 camera equipped with a Nikon camera (digital sight DS-Fi1, Kanagawa, Japan) and Nikon NIS-Elements imaging software (Nikon, Melville, NY). Morphometric analyses of images were performed using Image J (National Institutes of Health, Bethesda, MD) and identical orientation (right lateral recumbency). Severity and incidence of PE were determined based on the original image scale and planar analysis yielded areal estimates for pericardial cavity. GMA-embedded H&E stained sections were surveyed for presence of heart alterations and imaged with a Nikon Eclipse E600 light microscope, a Nikon DXM 1200 digital camera, and EclipseNet imaging software (Nikon).
Statistical analysis.
Real-time PCR data and TCDD-induced PE were tested for significant differences within treatment using a Prism4 software package (GraphPad Software, Inc., San Diego, CA). Data were logarithmically transformed as needed to improve equality of variances (ANOVA, p value < 0.05), followed by Newman-Keuls Multiple Comparison test. Each experiment in which real-time RT-PCR was performed consisted of three replicates of 10 pooled embryos. The statistical analysis was consistent between replicated experiments in all cases.
RESULTS
TCDD-Induced Edema and COX-2 Expression in Medaka Embryos at 6 dpf
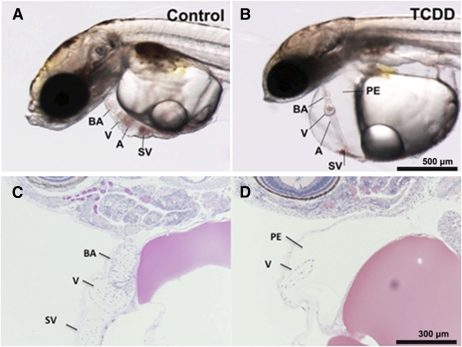
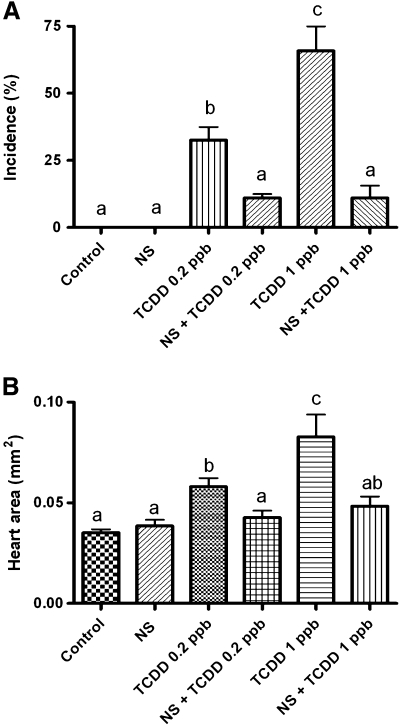
Medaka embryos exposed to dioxin between 0.05 and 1 ppb showed increased incidence and severity of well-described developmental phenotypes in fish embryos. These included PE, yolk sac edema, decreased vascular flow extending to stasis, hemorrhage, and disruption in heart morphogenesis including improper heart looping. Signs of heart failure were evident at 5 dpe with loss of ventricular contractions and circulation. Hearts failed to loop correctly and appeared as an elongated linear tube stretching across the pericardial cavity. Pericardial edema was extreme and heart area was drastically diminished (Fig. 1).
FIG. 1.
Cardiovascular toxicity in medaka embryos. Orientation of images is such that head is to the left, dorsal wall is at the top, and ventral wall is toward bottom of figure with tail to the right. (A) Dechorionated medaka embryo after 8 days development in 0.1% DMSO shows normal structure. Heart ventricle (V) and atrium (A) are adjacent indicating proper looping. Bulbus arteriosus (BA) is cranial to ventricle and sinus venosus (SV) is caudoventral to the heart structures. (B) 1.0 ppb TCDD (1-h exposure)-treated medaka embryo demonstrates significant heart dysmorphogenesis. Elongated heart failed to loop correctly and appears as a long linear tube stretching across the enlarged pericardial cavity. Pericardial edema is extreme and heart is visibly altered. (C and D) GMA sections of medaka hearts stained with H&E. (C) Control ventricle (V) and BA are readily visible. In this orientation, the atrium is out of the plane of section. Myocardium and endocardium appear as a thin wall at this magnification. (D) In this TCDD-treated heart significant PE is observed. Part of the elongated ventricle is in the plane of section.
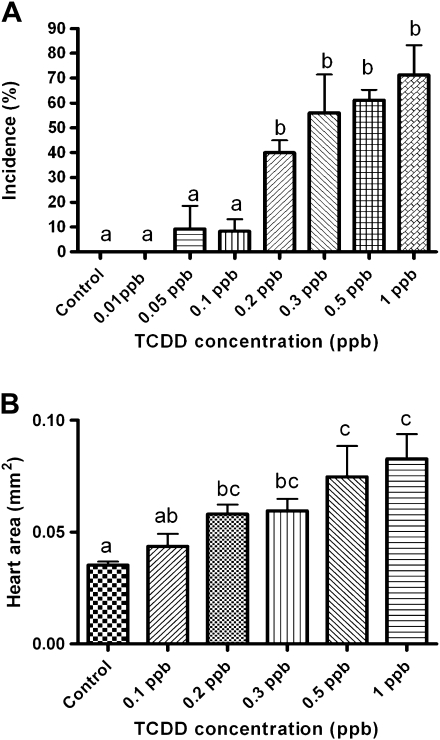
Incidence and severity of cardiovascular phenotypes including PE were dose dependent (Figs. 2A and 2B). PE was first observed 5 dpe at a dose of >0.5 ppb. An increase in size of the pericardial sac was observed with a continued increase in pericardial volume at each subsequent day of analysis. Significant change from control in PE incidence and severity was observed 6 dpe at concentration ≥ 0.2 ppb. All subsequent analyses were conducted at this time point. Severity and incidence of PE was determined by measuring total pericardial area in lateral recumbency using NIH Image J software. Incidence of PE was scored positive when the pericardial area of the individual was larger than the than the average (+/−) SE of the pericardial area within the nontreated control group.
FIG. 2.
Incidence and severity of PE in medaka embryos. (A) Incidence and (B) severity of PE phenotype at 6 dpe to 0.01–1 ppb TCDD (1-h exposure). Severity and incidence of PE was determined as described in the “Materials and Methods” section. Significant differences between treatment was determined using one-way ANOVA, p value < 0.05, followed by Newman-Keuls Multiple Comparison test within the Prism4 software package (GraphPad Software, Inc.). All experiments consisted of three replicate experimental wells containing 10 embryos per well and each experiment replicated at least three times.
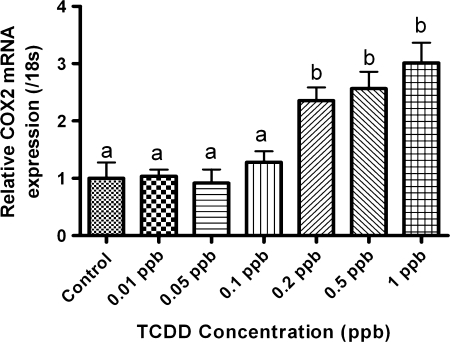
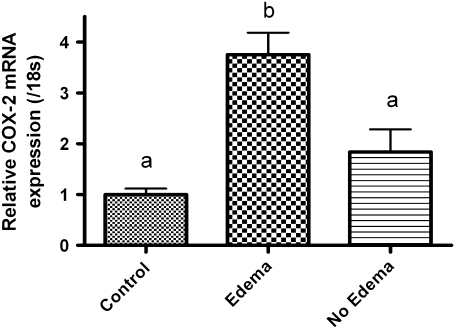
To examine the premise that inflammatory regulators mediate PE, we determined whether expression of COX-2 was altered following TCDD treatment. Medaka embryos (4 hpf) were exposed to TCDD between 0.01 and 1 ppb, for 1 h, then washed in embryo rearing media, and incubated for a total of 6 days. Three sets of 10 embryos each were pooled, and COX-2 expression was determined by quantitative PCR (qPCR). Results demonstrate that TCDD exposure produced a concentration-dependent rise in COX-2 gene expression when compared with control (Fig. 3). Significant induction of COX-2 mRNA was observed at >0.2 ppb TCDD consistent with the concentration required for onset of PE. COX-2 expression increased concomitant with TCDD concentration with a maximum of threefold at 1 ppb. Changes in COX-2 expression were significant at p≥ 0.05 for concentrations 0.2, 0.5, and 1 ppb TCDD. To further investigate the relation between PE and COX-2 gene expression, a second cohort of embryos were exposed to 1 ppb TCDD as described above, incubated for 6 days, and then separated into two populations. The first population was comprised of individual TCDD-exposed embryos exhibiting PE. The second population consisted of individual TCDD-exposed embryos not exhibiting PE. qPCR was then conducted with RNAs isolated from control embryos and the two isolated populations. As demonstrated in Figure 4, COX-2 expression increased 3.75-fold over control in the cohort exhibiting PE. By comparison, the nonedematous cohort exhibited a slight, nonsignificant increase in COX-2 expression compared with control. This increase is likely due to a latent onset of edema in this cohort as all TCDD embryos exposed to 1 ppb TCDD, exhibit edema after grow out to 10 dpe.
FIG. 3.
Quantitative, real-time PCR data for COX-2 expression in medaka embryos 6 dpe to 0.01–1 ppb TCDD. Data were normalized to medaka 18s and are represented as the mean mRNA level ± SEM. A significant change in COX-2 was determined using one-way ANOVA, p value < 0.05, followed by Newman-Keuls Multiple Comparison test within the Prism4 software package (GraphPad Software Inc.). Data were logarithmically transformed as needed to improve equality of variances. All experiments consisted of three replicate experimental wells containing 10 embryos per well and each experiment replicated at least three times. All qPCR was conducted in triplicate.
FIG. 4.
Quantitative, real-time PCR data for COX-2 expression in medaka embryos 6 dpe to 1 ppb TCDD or DMSO vehicle. TCDD-treated embryos were separated into two populations “Edema” or “No-Edema” based upon presence of pericardial swelling as described in “Materials and Methods” section. Relative COX-2 mRNA transcript levels were normalized to medaka 18s and are represented as the mean mRNA level ± SEM. A significant change in COX-2 was determined using one-way ANOVA, p value < 0.05, followed by Newman-Keuls Multiple Comparison test within the Prism4 software package (GraphPad Software, Inc.). Data were logarithmically transformed as needed to improve equality of variances. All experiments consisted of three replicate experimental wells containing 10 embryos per well and each experiment replicated at least three times. All qPCR was conducted in triplicate.
To determine whether pharmacological or genetic inhibition COX-2 rescues TCDD-induced PE, studies were conducted using NS398 (Caymen chemical Co., # 70590), a selective pharmacological inhibitor of COX-2. 0.2 and 1 ppb TCDD-treated embryos were continuously incubated with 20μM NS398 up to 6 dpe at which time embryos were scored for PE incidence and severity. As demonstrated in Figure 5, treatment with NS398 after a 1-h exposure to TCDD, significantly inhibited both PE incidence (Fig. 5A) and severity (Fig. 5B) at low and at high TCDD concentrations.
FIG. 5.
Incidence and severity of PE phenotype at 6 dpe to 0.2 or 1.0 ppb TCDD with and without coexposure to 20μM NS398. (A) Incidence and (B) severity. Medaka embryos were treated to TCDD and NS398 as described in “Materials and Methods” section. Severity and incidence of PE was determined as described in “Materials and Methods” section. Significant differences between treatments were determined using one-way ANOVA, p value < 0.05, followed by Newman-Keuls Multiple Comparison test within the Prism4 software package (GraphPad Software, Inc.). All experiments consisted of three replicate experimental wells containing 10 embryos per well and each experiment replicated at least three times.
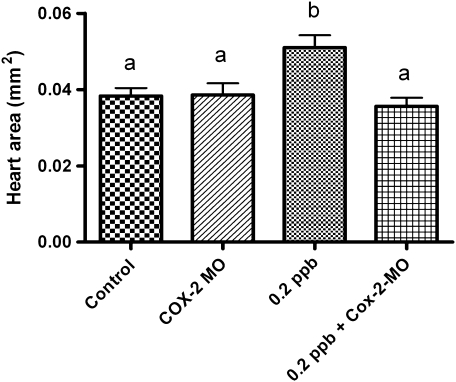
To further test the role of COX-2 expression in TCDD-induced PE phenotype, we modified COX-2 gene induction using MO gene knockdown approach. Our earlier finding that NS398 reduced COX-2 gene expression allowed us to test the possibility that by blocking COX-2 induction, we would protect against onset and severity of PE. A translation initiation MO was designed to medaka COX-2 and injected into 1-2 cell-stage embryos. Embryos were then exposed to 0.2-ppb TCDD for 1 h, grown out for a 6-day incubation period, and assessed for incidence and severity of PE (Fig. 6). Under these conditions, injections of COX-2 morpholinos consistently inhibited onset and severity of PE.
FIG. 6.
Effect of medaka COX-2 translation initiation morpholino on (A). Pericardial edema severity. Medaka embryos were microinjected with morpholinos at the one- to two-cell–stage followed by exposure to 0.2 ppb TCDD as described in “Materials and Methods” section. Severity of PE was determined by measuring total pericardial area using NIH Image J software. Significant differences between treatments were determined using one-way ANOVA, p value < 0.05, followed by Newman-Keuls Multiple Comparison test within the Prism4 software package (GraphPad Software, Inc.). All experiments consisted of three replicate experimental wells containing 10 embryos per well and each experiment replicated at least three times. All qPCR was conducted in triplicate.
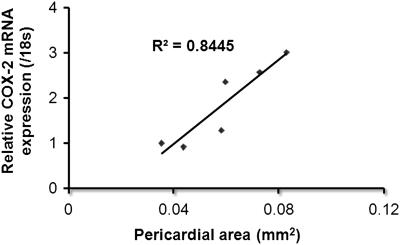
Correlation analysis was conducted to demonstrate the relationship between COX-2 gene expression and severity of pericardial phenotype. Medaka embryos were exposed to 0.05–1 ppb TCDD for 1 h and grown out for 6 days. Embryos were subsequently assessed for severity of PE and COX-2 expression using qPCR. Figure 7 illustrates a direct correlation between the two determinants with an R2 value of 0.844.
FIG. 7.
Correlation analysis for relative COX-2 mRNA expression and severity of PE. Medaka embryos were treated to TCDD at a concentration of 0.05, 0.1, 0.2, 0.3, 0.5, or 1 ppb for 1 h. Severity of PE was determined by measuring total pericardial area using NIH Image J software. Following assessment of severity, embryos were harvested for mRNA and subjected to qPCR for medaka COX-2 transcript. qPCR data were normalized to medaka 18s and are represented as the mean mRNA level ± SEM. Correlation analysis was conducted in Prism4 software package (GraphPad Software, Inc.) by plotting relative medaka COX-2 expression on the y-axis and pericardial area on the x-axis. All experiments consisted of three replicate experimental wells containing 10 embryos per well and each experiment replicated at least three times. All qPCR was conducted in triplicate.
AA-Induced Pericardial Edema and COX-2 Expression
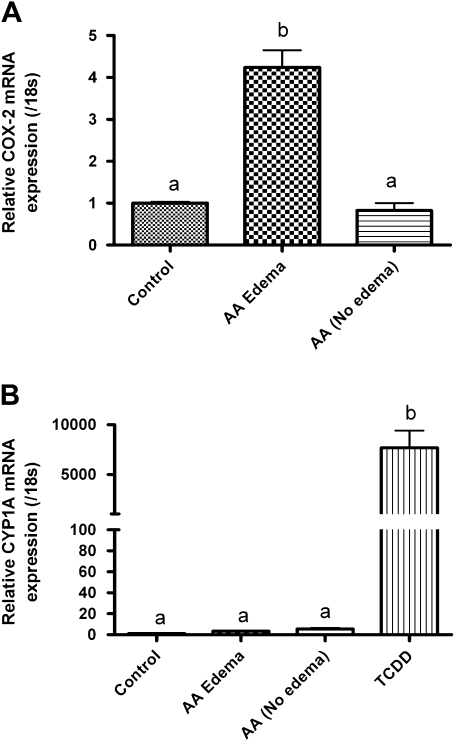
Given that upregulation in cyclooxygenase (COX) expression likely results in an increased production of cellular AA, we examine whether AA itself could recapitulate some of the developmental phenotypes observed with TCDD exposure. After conducting a range finding study (data not presented), we found that a continuous 6-day exposure between 100 and 450μM AA was sufficient to initiate PE. AA exposures resulted in a similar array of developmental cardiovascular phenotypes as observed with TCDD including PE, yolk sac edema, decreased and static blood flow, hemorrhage, and elongation of the heart tube with improper looping. We additionally observed formation of a truncated tail and shortening of the interocular distance with AA treatment (data not shown). As with TCDD, we then partitioned 200μM AA-treated embryos into two cohorts following a 6-day continuous treatment, i.e., those individuals exhibiting PE and those individuals that did not develop PE by day 6. COX-2 gene expression was determined for the control vehicle-treated group and the two AA-exposed populations. As illustrated in Figure 8A, COX-2 gene expression was upregulated in the presence of AA. When compared with control, AA treatment resulted in a 4.2-fold induction of COX-2 gene expression in the cohort of embryos exhibiting PE. Conversely, those individual embryos exposed to AA but lacking the PE phenotype had no appreciable increase in COX-2 gene expression compared with both control and the PE group. To test the possibility that AA itself functioned through the classical AhR/ARNT/DRE mechanism, we examined whether CYP1A expression was induced in AA-treated PE and non-PE embryo cohorts. As demonstrated in Figure 8B, there was no significant induction of CYP1A expression in any of the AA-treated cohorts, suggesting that PE induction and COX-2 expression can be induced independently of AhR/ARNT/DRE.
FIG. 8.
Quantitative, real-time PCR data of COX-2 and Cyp1A expression in medaka embryos following a constant 6-day exposure to 200μM AA or DMSO vehicle. AA treated embryos were separated into two populations “Edema” or “No-Edema” based upon presence of pericardial swelling as described in the “Materials and Methods” section. (A) Relative COX-2 expression and (B) relative Cyp1A expression. mRNA transcript levels were normalized to medaka 18s and are represented as the mean mRNA level ± SEM. A significant change in transcript was determined using one-way ANOVA, p value < 0.05, followed by Newman-Keuls Multiple Comparison test within the Prism4 software package (GraphPad Software, Inc.). Data were logarithmically transformed as needed to improve equality of variances. All experiments consisted of three replicate experimental wells containing 10 embryos per well, and each experiment was replicated at least three times. All qPCR was conducted in triplicate.
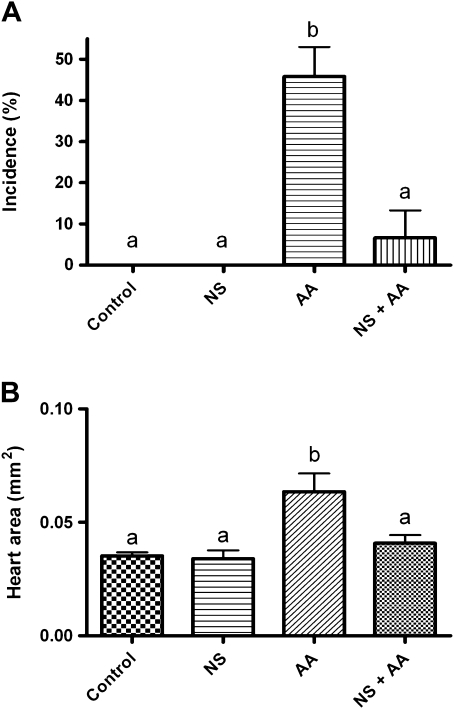
Lastly, we determined whether the COX inhibitor NS398 would additionally inhibit AA-induced PE phenotype as described earlier for TCDD. Here medaka embryos were coexposed to 200μM AA in the presence of 20μM NS398 continuously for a 6-day incubation period. As demonstrated in Figure 9, control vehicle–exposed embryos and embryos exposed to NS398 only exhibited no PE. Embryos treated with 200μM AA resulted in a significant increase in PE compared with control, whereas addition of NS398 significantly rescued the AA-induced PE incidence and severity (Figs. 9A and 9B).
FIG. 9.
Incidence and severity of PE phenotype following a constant 6-day exposure to 200μM AA with and without coexposures to 20μM NS398. (A) Incidence and (B) severity. Medaka embryos were treated to AA and NS398 as described in the “Materials and Methods” section. Severity and incidence of PE was determined as described in the “Materials and Methods” section. Significant differences between treatments were determined using one-way ANOVA, p value < 0.05, followed by Newman-Keuls Multiple Comparison test within the Prism4 software package (GraphPad Software, Inc.). All experiments consisted of three replicate experimental wells containing 10 embryos per well and each experiment was replicated at least three times.
DISCUSSION
Dioxin and other AhR ligands are well known to result in multiple, defined developmental phenotypes including PE and circulatory failure in small aquarium fish models. In this study, we demonstrate that AhR activation by TCDD results in stimulation of inflammation and eicosanoid pathways. We have focused on PE as a prototypic, apical, and sensitive end point of AhR-induced developmental cardiovascular toxicity. We additionally take advantage of the extended developmental timing of medaka embryos where we relate onset and progression of cardiovascular events to critical stages in heart development in this species. Our observed characteristics of PE include typical swelling of the pericardial sac and elongation of the heart tube. PE first appears at stage 30 and arises after bilateral heart development and heart tube formation have occurred. This is consistent with initiation of medaka heart chamber formation and looping. This aspect of cardiovascular development occurs prior to concentric heart chamber growth beginning at stage 34 (Taneda et al., 2010).
Few investigations have demonstrated a linkage between AhR-induced cardiovascular phenotypes and inflammatory mediators. Here we questioned whether production of PE is associated with modifications in embryonic inflammatory response. We targeted COX-2 given its well-established role in the production of AA, a precursor of pro- and anti-inflammatory prostanoids capable of impacting heart morphogenesis and vascular permeability. Assessment of the medaka genome in the ensembl database (http://uswest.ensembl.org/Oryzias_latipes/Info/Index) suggests that medaka contain a single-copy gene for both COX-1 and COX-2. We thus first established that medaka embryos express basal levels of COX-2 as early as stage 28 (data not shown). This was necessary given that aspects of cellular immunity are under development in embryos. For example, Lam et al. (2004) stated that morphologic and functional maturity of the immune system in medaka occurs 4–6 weeks after fertilization. On the other hand, Grabher et al. (2007) showed definitive properties of differentiated macrophages cells as early as stage 18 (26 hpf) in the rostral-most lateral mesoderm surrounding the cardiac field. These cells actively patrolled the entire embryo and their migratory path was not random but followed well-defined routes and respected tissue barriers.
Our results demonstrate that TCDD-induced PE correlates with expression of the inflammatory mediator COX-2. Previous in vitro studies demonstrated that TCDD induces a rapid mobilization of intracellular Ca+2 and induction of COX-2 in mouse hepatoma cells (Puga et al., 1997). Similarly, PCBs are well known to elicit inflammatory responses in several cell types including vascular endothelial cells (Arzuaga et al., 2007), neutrophils (Bezdecny et al.2005, 2007), rat fibroblasts (Kim et al., 2005), and human mast cells (Kwon et al., 2002). These findings suggest a linkage between exposure to chlorinated organics, cardiovascular disease, and atherosclerosis (Kopf and Walker, 2009; Vogel et al., 2004). Such linkage may be due to activation of inflammatory-mediated responses including activation and/or release of proinflammatory mediators (COX-2), IL-1β, IL-6, and tumor necrosis factor-α. Several recent mammalian studies propose that exposure to TCDD triggers a rapid increase in intracellular calcium concentration, activation of calcium-dependent cytosolic PLA2 and subsequent induction of COX-2 and AA release (Dong and Matsumura, 2008, 2009). This response occurs in multiple cell types including foam cells, macrophages, adipocytes, epithelial cells, and others (Dong and Matsumura, 2008; Li and Matsumura, 2008; Sciullo et al., 2009; Vogel et al., 2004). In fact, non-coplanar PCBs and o,p′-dichlorodiphenyltrichloroethane, which are not AhR ligands, elicit strong inflammatory responses that are mediated by the induction of COX activity (Bezdecny et al., 2007, 2005; Han et al., 2008; Kim et al., 2005; Kwon et al., 2002).
Within both mouse and human COX-2 promoters regions, DREs have been identified (Sirois and Richards, 1993) along with cis elements for CCAAT enhancer binding protein, cAMP response element-binding protein, and activator protein 1 (Yang and Bleich, 2004). Using ChiP analysis, Degner et al. (2009) demonstrated that TCDD induced the association of human AhR to a DRE within the human COX-2 promoter. TCDD treatment additionally recruited histone acetyl transferase p300 and acetylated histone H4 (AcH4), suggesting that regulation of COX-2 occurs through a classical genomic (nuclear) mechanism. Conversely, additional reports suggest a putative nonclassical/nonnuclear mechanism of AhR-driven COX-2 gene expression (Dong et al., 2010; Wong et al., 2010). Using a combination of small interfering RNA against ARNT, DRE-decoy treatments, DRE mutations, and AhRnls mice, where AhR does not translocate to the nucleus, an alternate mechanism of COX-2 induction is proposed (Dong and Matsumura, 2009; Dong et al., 2010).
To establish some insight into the mechanism of COX-2 induction in medaka embryos, we sought to inhibit COX-2–mediated onset and progression of the PE phenotype. Using both pharmacological (NS398) and genetic (morpholino) approaches, we were able to significantly reduce COX-2 expression and subsequent incidence and severity of PE. Although we have not definitively established a mechanism of COX-2 induction in medaka, we demonstrate that both TCDD and AA upregulate COX-2 gene expression. COX-2 induction is additionally observed in zebrafish following exposure to aristocholic acid (Huang et al., 2007) and tetradecanoylphorbol acetate (Ishikawa and Herschman, 2007) where PE is observed. The fact that PE is induced by non-AhR agonists suggests that some cardiovascular phenotypes can be initiated independently of AhR/ARNT/DRE signaling pathway. What remains to be resolved is whether TCDD functions to induce medaka COX-2 through the nuclear actions of AhR/ARNT/DRE, through a nonnuclear mechanisms as described by Matsumura (2009), or through an alternative mechanism such as complexation of AhR with alternate dimerization partners.
COX isoforms have complex roles in normal cardiovascular function and disruption in COX-1/COX-2 activity is associated with several cardiovascular pathologies (Carney and Chen et al., 2006; Chang et al., 2006; Streicher and Wang, 2008; Suzuki et al., 2006; Zidar et al., 2007, 2008). Furthermore, it has been recently observed that chronic use of COX-2 inhibitors increases the risk of heart disease (Streicher and Wang, 2008). The exact role of COX-2 in cardiac pathogenesis remains equivocal, but the role of COX 2 in promoting PG synthesis and subsequent inflammation response is well established (Vane et al., 1998). COX-2–mediated production and homeostatic regulation of PG signaling is essential for proper cardiovascular development. A recent study in zebrafish describes the role of prostanoid signaling in atrioventricular morphogenesis (Scherz et al., 2008). Valve formation in zebrafish occurs through multiple phases and each is sensitive to subtle morphological changes after modification in COX-2–dependent signaling. Embryos treated with selective COX-2 inhibitors NS398 and CAY10404 exhibited improper endocardial thickening and deficits in valve function resulting in retrograde blood flow. COX-2 inhibition alters myocardial cell shape in the posterior dorsal outer curvature of the ventricle resulting in myocardial bending (Scherz et al., 2008). Effects of COX-2 inhibition were traced to PGF2 and TXA2 where an effective balance between these PGs is essential for proper myocardial cell shape and valve formation. Comparatively, zebrafish embryos exposed to TCDD exhibit heart morphologies similar to those observed with COX-2 inhibition including blood regurgitation from dysmorphogenic atrioventricular and ventriculobulbar valves (Mehta et al., 2008). These effects are consistent with disruption of cellular patterns associated with normal valve development and, at later stages of development, interference with endothelial cell positioning essential for formation of functional valves (Auman and Yelon, 2004; Auman et al., 2007).
It is well known that COX-2 is important for supplying PGs to maintain tissue homeostasis and that both COX-1 and COX-2 are likely involved in the development of inflammatory diseases (Loftin et al., 2002). We speculate that proper COX-2 expression is essential for regulating appropriate balance in prostanoid signaling during heart development and that disruption in this pathway significantly disrupts cardiovascular morphogenesis (He et al., 2010). This fact is illustrated by our ability to recapitulate cardiovascular toxicities by exposing embryos to AA. Myocardial phospholipids are the primary source of AA in heart and AA is liberated through the hydrolytic actions of cardiac PLA2 (Jenkins et al., 2009). AA serves as a precursor to eicosanoid production through the actions of COX and lipoxygenases. Eicosanoids regulate a diverse array of cellular functions that promote adaptive changes in the myocardium under normal physiologic conditions. Myocardium, endocardium, and vascular smooth muscle rely on paracrine action of eicosanoids to regulate contractile and hemodynamic function. Chronic stress and perturbation of the heart, however, can result in aberrant eicosanoid signaling and deleterious, maladaptive cardiovascular function promoting heart failure (Jenkins et al., 2009). In fish models, this fact is illustrated by Teraoka et al. (2009, where inhibition of COX-2 and addition of a competitive antagonist to the thromboxane receptor rescued TCDD-induced circulatory failure, and, through induction of COX-2–mediated PE and ventricular hypertrophy following exposure to aristocholic acid (Huang et al., 2007).
In our studies, the phenomenon of COX-2–triggered cardiovascular toxicities offers the potential to serve as a tool to elucidate inflammatory pathways involved in AhR-mediated heart dysmorphogenesis. Here we propose a model in which a potent AhR ligand mediates induction of embryonic COX-2. This action likely results in subsequent modification of prostanoid production affecting an essential balance in signaling necessary for appropriate heart morphogenesis in these fish models of human disease.
FUNDING
National Cancer Institute (R21CA105084-01A1 to S.W.K.); North Carolina Agricultural Research Grant (02225 to S.W.K.); and National Science Foundation of China and the program for New Century Excellent Talent in University (30360090 and NCET-04-0262 to W.D.).
Acknowledgments
We wish to thank Erin Kollitz, Erin Yost, Arnaud Van Wettere, and Gwijun Kwon for medaka care, culture, and maintenance. We also thank James Bonner, Gerald LeBlanc, and David Hinton for critical review of an early draft of the manuscript.
References
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84:368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Peterson RE, Heideman W. Blocking expression of AHR2 and ARNT1 in zebrafish larvae protects against cardiac toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 2006;94:175–182. doi: 10.1093/toxsci/kfl093. [DOI] [PubMed] [Google Scholar]
- Aragon AC, Kopf PG, Campen MJ, Huwe JK, Walker MK. In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure: effects on fetal and adult cardiac gene expression and adult cardiac and renal morphology. Toxicol. Sci. 2008;101:321–330. doi: 10.1093/toxsci/kfm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzuaga X, Reiterer G, Majkova Z, Kilgore MW, Toborek M, Hennig B. PPARalpha ligands reduce PCB-induced endothelial activation: possible interactions in inflammation and atherosclerosis. Cardiovasc. Toxicol. 2007;7:264–272. doi: 10.1007/s12012-007-9005-8. [DOI] [PubMed] [Google Scholar]
- Auman HJ, Coleman H, Riley HE, Olale F, Tsai HJ, Yelon D. Functional modulation of cardiac form through regionally confined cell shape changes. PLoS Biol. 2007;5:e53. doi: 10.1371/journal.pbio.0050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auman HJ, Yelon D. Vertebrate organogenesis: getting the heart into shape. Curr. Biol. 2004;14:R152–R153. [PubMed] [Google Scholar]
- Bezdecny SA, Karmaus P, Roth RA, Ganey PE. 2,2',4,4'-Tetrachlorobiphenyl upregulates cyclooxygenase-2 in HL-60 cells via p38 mitogen-activated protein kinase and NF-kappaB. Toxicol. Appl. Pharmacol. 2007;221:285–294. doi: 10.1016/j.taap.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdecny SA, Roth RA, Ganey PE. Effects of 2,2',4,4'-tetrachlorobiphenyl on granulocytic HL-60 cell function and expression of cyclooxygenase-2. Toxicol. Sci. 2005;84:328–334. doi: 10.1093/toxsci/kfi093. [DOI] [PubMed] [Google Scholar]
- Carney MW, Erwin K, Hardman R, Yuen B, Volz DC, Hinton DE, Kullman SW. Differential developmental toxicity of naphthoic acid isomers in medaka (Oryzias latipes) embryos. Mar. Pollut. Bull. 2008;57:255–266. doi: 10.1016/j.marpolbul.2008.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney SA, Chen J, Burns CG, Xiong KM, Peterson RE, Heideman W. Aryl hydrocarbon receptor activation produces heart-specific transcriptional and toxic responses in developing zebrafish. Mol. Pharmacol. 2006a;70:549–561. doi: 10.1124/mol.106.025304. [DOI] [PubMed] [Google Scholar]
- Carney SA, Prasch AL, Heideman W, Peterson RE. Understanding dioxin developmental toxicity using the zebrafish model. Birth. Defects Res. A. Clin. Mol. Teratol. 2006b;76:7–18. doi: 10.1002/bdra.20216. [DOI] [PubMed] [Google Scholar]
- Chang H, Hanawa H, Liu H, Yoshida T, Hayashi M, Watanabe R, Abe S, Toba K, Yoshida K, Elnaggar R, et al. Hydrodynamic-based delivery of an interleukin-22-Ig fusion gene ameliorates experimental autoimmune myocarditis in rats. J. Immunol. 2006;177:3635–3643. doi: 10.4049/jimmunol.177.6.3635. [DOI] [PubMed] [Google Scholar]
- Chen CM, Cooper KR. Developmental toxicity and EROD induction in the Japanese medaka (Oryzias latipes) treated with dioxin congeners. Bull. Environ. Contam. Toxicol. 1999;63:423–429. doi: 10.1007/s001289900997. [DOI] [PubMed] [Google Scholar]
- Degner SC, Papoutsis AJ, Selmin O, Romagnolo DF. Targeting of aryl hydrocarbon receptor-mediated activation of cyclooxygenase-2 expression by the indole-3-carbinol metabolite 3,3'-diindolylmethane in breast cancer cells. J. Nutr. 2009;139:26–32. doi: 10.3945/jn.108.099259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Matsumura F. Roles of cytosolic phospholipase A2 and Src kinase in the early action of 2,3,7,8-tetrachlorodibenzo-p-dioxin through a nongenomic pathway in MCF10A cells. Mol. Pharmacol. 2008;74:255–263. doi: 10.1124/mol.107.044669. [DOI] [PubMed] [Google Scholar]
- Dong B, Matsumura F. The conversion of rapid TCCD nongenomic signals to persistent inflammatory effects via select protein kinases in MCF10A cells. Mol. Endocrinol. 2009;23:549–558. doi: 10.1210/me.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B, Nishimura N, Vogel CF, Tohyama C, Matsumura F. TCDD-induced cyclooxygenase-2 expression is mediated by the nongenomic pathway in mouse MMDD1 macula densa cells and kidneys. Biochem. Pharmacol. 2010;79:487–497. doi: 10.1016/j.bcp.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabher C, Cliffe A, Miura K, Hayflick J, Pepperkok R, Rorth P, Wittbrodt J. Birth and life of tissue macrophages and their migration in embryogenesis and inflammation in medaka. J. Leukoc. Biol. 2007;81:263–271. doi: 10.1189/jlb.0806526. [DOI] [PubMed] [Google Scholar]
- Grimes AC, Erwin KN, Stadt HA, Hunter GL, Gefroh HA, Tsai HJ, Kirby ML. PCB126 exposure disrupts zebrafish ventricular and branchial but not early neural crest development. Toxicol. Sci. 2008;106:193–205. doi: 10.1093/toxsci/kfn154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han EH, Kim JY, Kim HK, Hwang YP, Jeong HG. o, p'-DDT induces cyclooxygenase-2 gene expression in murine macrophages: role of AP-1 and CRE promoter elements and PI3-kinase/Akt/MAPK signaling pathways. Toxicol. Appl. Pharmacol. 2008;233:333–342. doi: 10.1016/j.taap.2008.09.003. [DOI] [PubMed] [Google Scholar]
- He Q, Harding P, LaPointe MC. PKA, Rap1, ERK1/2, and p90RSK mediate PGE2 and EP4 signaling in neonatal ventricular myocytes. Am. J. Physiol. Heart. Circ. Physiol. 2010;298:H136–H143. doi: 10.1152/ajpheart.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Jr, Martin LJ, Tabangin ME, Mazwi ML, Cripe LH, Benson DW. Hypoplastic left heart syndrome is heritable. J. Am. Coll. Cardiol. 2007;50:1590–1595. doi: 10.1016/j.jacc.2007.07.021. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Spitsbergen JM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters cardiovascular and craniofacial development and function in sac fry of rainbow trout (Oncorhynchus mykiss) Toxicol. Sci. 1999;47:40–51. doi: 10.1093/toxsci/47.1.40. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chen PC, Huang CW, Yu J. Aristolochic acid induces heart failure in zebrafish embryos that is mediated by inflammation. Toxicol. Sci. 2007;100:486–494. doi: 10.1093/toxsci/kfm235. [DOI] [PubMed] [Google Scholar]
- Ishikawa TO, Herschman HR. Two inducible, functional cyclooxygenase-2 genes are present in the rainbow trout genome. J. Cell. Biochem. 2007;102:1486–1492. doi: 10.1002/jcb.21368. [DOI] [PubMed] [Google Scholar]
- Ivnitski I, Elmaoued R, Walker MK. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary development is preceded by a decrease in myocyte proliferation and an increase in cardiac apoptosis. Teratology. 2001;64:201–212. doi: 10.1002/tera.1065. [DOI] [PubMed] [Google Scholar]
- Iwamatsu T. Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 2004;121:605–618. doi: 10.1016/j.mod.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Jenkins CM, Cedars A, Gross RW. Eicosanoid signalling pathways in the heart. Cardiovasc. Res. 2009;82:240–249. doi: 10.1093/cvr/cvn346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim YH, Shin KJ, Oh YS, Lee CS, Kang KO, Ryu SH, Suh PG. 2,2',4,6,6'-Pentachlorobiphenyl-induced apoptosis is limited by cyclooxygenase-2 induction. Toxicol. Sci. 2005;83:397–404. doi: 10.1093/toxsci/kfi026. [DOI] [PubMed] [Google Scholar]
- Kopf PG, Walker MK. Overview of developmental heart defects by dioxins, PCBs, and pesticides. J. Environ. Sci. Health. C. Environ. Carcinog. Ecotoxicol. Rev. 2009;27:276–285. doi: 10.1080/10590500903310195. [DOI] [PubMed] [Google Scholar]
- Kwon O, Lee E, Moon TC, Jung H, Lin CX, Nam KS, Baek SH, Min HK, Chang HW. Expression of cyclooxygenase-2 and pro-inflammatory cytokines induced by 2,2',4,4',5,5'-hexachlorobiphenyl (PCB 153) in human mast cells requires NF-kappa B activation. Biol. Pharm. Bull. 2002;25:1165–1168. doi: 10.1248/bpb.25.1165. [DOI] [PubMed] [Google Scholar]
- Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004;28:9–28. doi: 10.1016/s0145-305x(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Li W, Matsumura F. Significance of the nongenomic, inflammatory pathway in mediating the toxic action of TCDD to induce rapid and long-term cellular responses in 3T3-L1 adipocytes. Biochemistry. 2008;47:13997–14008. doi: 10.1021/bi801913w. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Loftin CD, Tiano HF, Langenbach R. Phenotypes of the COX-deficient mice indicate physiological and pathophysiological roles for COX-1 and COX-2. Prostaglandins Other Lipid. Mediat. 2002;68–69:177–185. doi: 10.1016/s0090-6980(02)00028-x. [DOI] [PubMed] [Google Scholar]
- Matsumura F. The significance of the nongenomic pathway in mediating inflammatory signaling of the dioxin-activated Ah receptor to cause toxic effects. Biochem. Pharmacol. 2009;77:608–626. doi: 10.1016/j.bcp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Mehta V, Peterson RE, Heideman W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin exposure prevents cardiac valve formation in developing zebrafish. Toxicol. Sci. 2008;104:303–311. doi: 10.1093/toxsci/kfn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Matsumura F, Vogel CF, Nishimura H, Yonemoto J, Yoshioka W, Tohyama C. Critical role of cyclooxygenase-2 activation in pathogenesis of hydronephrosis caused by lactational exposure of mice to dioxin. Toxicol. Appl. Pharmacol. 2008;231:374–383. doi: 10.1016/j.taap.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Tanguay RL, Mehta V, Heideman W, Peterson RE. Identification of zebrafish ARNT1 homologs: 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the developing zebrafish requires ARNT1. Mol. Pharmacol. 2006;69:776–787. doi: 10.1124/mol.105.016873. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Teraoka H, Carney SA, Dong W, Hiraga T, Stegeman JJ, Heideman W, Peterson RE. Aryl hydrocarbon receptor 2 mediates 2,3,7,8-tetrachlorodibenzo-p-dioxin developmental toxicity in zebrafish. Toxicol. Sci. 2003;76:138–150. doi: 10.1093/toxsci/kfg202. [DOI] [PubMed] [Google Scholar]
- Puga A, Hoffer A, Zhou S, Bohm JM, Leikauf GD, Shertzer HG. Sustained increase in intracellular free calcium and activation of cyclooxygenase-2 expression in mouse hepatoma cells treated with dioxin. Biochem. Pharmacol. 1997;54:1287–1296. doi: 10.1016/s0006-2952(97)00417-6. [DOI] [PubMed] [Google Scholar]
- Scherz PJ, Huisken J, Sahai-Hernandez P, Stainier DY. High-speed imaging of developing heart valves reveals interplay of morphogenesis and function. Development. 2008;135:1179–1187. doi: 10.1242/dev.010694. [DOI] [PubMed] [Google Scholar]
- Sciullo EM, Dong B, Vogel CF, Matsumura F. Characterization of the pattern of the nongenomic signaling pathway through which TCDD-induces early inflammatory responses in U937 human macrophages. Chemosphere. 2009;74:1531–1537. doi: 10.1016/j.chemosphere.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmera D, Hu N, Weiss KM, Keller BB, Denslow S, Thompson RP. Cellular changes in experimental left heart hypoplasia. Anat. Rec. 2002;267:137–145. doi: 10.1002/ar.10098. [DOI] [PubMed] [Google Scholar]
- Sirois J, Richards JS. Transcriptional regulation of the rat prostaglandin endoperoxide synthase 2 gene in granulosa cells. Evidence for the role of a cis-acting C/EBP beta promoterelement. J. Biol. Chem. 1993;268:21931–21938. [PubMed] [Google Scholar]
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- Streicher JM, Wang Y. The role of COX-2 in heart pathology. Cardiovasc. Hematol. Agents Med. Chem. 2008;6:69–79. doi: 10.2174/187152508783329948. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Ogawa M, Futamatsu H, Kosuge H, Tanaka H, Isobe M. A cyclooxygenase-2 inhibitor alters Th1/Th2 cytokine balance and suppresses autoimmune myocarditis in rats. J. Mol. Cell. Cardiol. 2006;40:688–695. doi: 10.1016/j.yjmcc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Taneda Y, Konno S, Makino S, Morioka M, Fukuda K, Imai Y, Kudo A, Kawakami A. Epigenetic control of cardiomyocyte production in response to a stress during the medaka heart development. Dev. Biol. 2010;340:30–40. doi: 10.1016/j.ydbio.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Kubota A, Dong W, Kawai Y, Yamazaki K, Mori C, Harada Y, Peterson RE, Hiraga T. Role of the cyclooxygenase 2-thromboxane pathway in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced decrease in mesencephalic vein blood flow in the zebrafish embryo. Toxicol. Appl. Pharmacol. 2009;234:33–40. doi: 10.1016/j.taap.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Kubota A, Kawai Y, Hiraga T. Interdisciplinary Studies on Environmental Chemistry--Biological Responses to Chemical Pollutants. 2008. Prostanoid signaling mediates circulation failure caused by TCDD in developing zebrafish. (Y. Murakami, K. Nakayama, S. -I. Kitamura, H. Iwata and S. Tanabe, Eds.), pp. 61–80. TERRAPUB, Tokyo, Japan. [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Matsumura F. Activation of inflammatory mediators and potential role of ah-receptor ligands in foam cell formation. Cardiovasc. Toxicol. 2004;4:363–373. doi: 10.1385/ct:4:4:363. [DOI] [PubMed] [Google Scholar]
- Volz DC, Bencic DC, Hinton DE, Law JM, Kullman SW. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces organ-specific differential gene expression in Japanese medaka (Oryzias latipes) Toxicol. Sci. 2005;85:572–584. doi: 10.1093/toxsci/kfi109. [DOI] [PubMed] [Google Scholar]
- Walker MK, Catron TF. Characterization of cardiotoxicity induced by 2,3,7, 8-tetrachlorodibenzo-p-dioxin and related chemicals during early chick embryo development. Toxicol. Appl. Pharmacol. 2000;167:210–221. doi: 10.1006/taap.2000.8992. [DOI] [PubMed] [Google Scholar]
- Wong PS, Vogel CF, Kokosinski K, Matsumura F. Aryl hydrocarbon receptor activation in NCI-H441 cells and C57BL/6 mice: possible mechanisms for lung dysfunction. Am. J. Respir. Cell. Mol. Biol. 2010;42:210–217. doi: 10.1165/rcmb.2008-0228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Bleich D. Transcriptional regulation of cyclooxygenase-2 gene in pancreatic beta-cells. J. Biol. Chem. 2004;279:35403–35411. doi: 10.1074/jbc.M404055200. [DOI] [PubMed] [Google Scholar]
- Zidar N, Dolenc-Strazar Z, Jeruc J, Jerse M, Balazic J, Gartner U, Jermol U, Zupanc T, Stajer D. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the normal human heart and in myocardial infarction. Cardiovasc. Pathol. 2007;16:300–304. doi: 10.1016/j.carpath.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Zidar N, Odar K, Glavac D, Jerse M, Zupanc T, Stajer D. Cyclooxygenase in normal human tissues—is COX-1 really a constitutive isoform, and COX-2 an inducible isoform? J. Cell. Mol. Med. 2008;13:3753–3763. doi: 10.1111/j.1582-4934.2008.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]