Abstract
Calcium is an essential signaling molecule in developing B cells, thus altering calcium dynamics represents a potential target for toxicant effects. GW7845, a tyrosine analog and potent peroxisome proliferator-activated receptor γ agonist, induces rapid mitogen-activated protein kinase (MAPK)–dependent apoptosis in bone marrow B cells. Changes in calcium dynamics are capable of mediating rapid initiation of cell death; therefore, we investigated the contribution of calcium to GW7845-induced apoptosis. Treatment of a nontransformed murine pro/pre-B cell line (BU-11) with GW7845 (40μM) resulted in intracellular calcium release. Multiple features of GW7845-induced cell death were suppressed by the calcium chelator BAPTA, including MAPK activation, loss of mitochondrial membrane potential, cytochrome c release, caspase-3 activation, and DNA fragmentation. A likely mechanism for the calcium-mediated effects is activation of CaMKII, a calcium-dependent MAP4K. We observed that three CaMKII isoforms (β, γ, and δ) are expressed in lymphoid tissues and bone marrow B cells. Treatment with GW7845 increased CaMKII activity. All features of GW7845-induced cell death, except loss of mitochondrial membrane potential, were suppressed by CaMKII inhibitors (KN93 and AIP-II), suggesting the activation of multiple calcium-driven pathways. To determine if CaMKII activation is a common feature of early B cell death following perturbation of Ca2+ flux, we dissected tributyltin (TBT)-induced death signaling. High-dose TBT (1μM) is known to activate calcium-dependent death. TBT induced rapid apoptosis that was associated with intracellular calcium release, CaMKII activation and MAPK activation, and was inhibited by AIP-II. Thus, we show that early B cells are susceptible to calcium-triggered cell death through a CaMKII/MAPK-dependent pathway.
Keywords: bone marrow, B cells, CaMKII, apoptosis, MAPK
Peroxisome proliferator-activated receptor γ (PPARγ) agonists of diverse structures have been shown to cause apoptosis in a number of different cell types, including those of hematopoietic origin (Contractor et al., 2005; Konopleva et al., 2004; Padilla et al., 2000; Piva et al., 2005; Ray et al., 2004; Schlezinger et al., 2002, 2004), leading to the consideration of PPARγ as a target for treatment in hematologic malignancies (Tsao et al., 2010). However, these agonists also may initiate apoptosis through receptor-independent mechanisms (Berry et al., 2005; Chintharlapalli et al., 2005). We have shown that GW7845, a tyrosine analog PPARγ agonist, induces rapid apoptosis in several types of bone marrow B cells, primary pro-B cells, a pro/pre-B cell line (BU-11), and an immature B cell lymphoma line (WEHI231) (Schlezinger et al., 2006, 2007). While GW7845 was developed as a potent agonist for PPARγ, its ability to induce apoptosis in B cells results from a receptor-independent activation of a mitogen-activated protein kinase (MAPK) cascade (Schlezinger et al., 2006). The strong rapid death signal induced by GW7845 provided us with a tool to investigate potential receptor-independent pathways initiated by a PPARγ agonist, as well as to gain a broader understanding of death pathways in B cells.
Calcium is a key signaling molecule and changes in calcium dynamics are capable of rapid initiation of MAPK activation and cell death (Orrenius et al., 2003). Intracellular Ca2+ distribution is tightly controlled by active (ATP-dependent) membrane calcium pumps, and several types of perturbations in Ca2+ homeostasis will elicit cell death (Cerella et al., 2010). Ca2+ depletion in the endoplasmic reticulum (ER) leads to induction of apoptosis by the ER stress pathway and caspase-12 (Rao et al., 2004). Ca2+ release from the ER into the cytosol can initiate opening of the mitochondrial permeability transition pore leading to onset of necrosis (Orrenius et al., 2003; Szabadkai and Rizzuto, 2004). An increase in cytosolic Ca2+ and/or decrease in ER Ca2+ may lead to activation of pro-apoptotic BCL family members and initiate mitochondria-dependent apoptosis (Nutt et al., 2002; Pan et al., 2001). Furthermore, multiple pathways may be triggered at once, resulting in necrapoptosis (Lemasters et al., 2009). Ca2+-mediated pathways may be of particular significance to B lymphocytes, as it is an essential signaling molecule for B cell development and function (Scharenberg et al., 2007) and plays a key role in B-cell receptor–mediated apoptosis in developing B cells during the process of negative selection (Ruiz-Vela et al., 1999).
Calcium/calmodulin protein kinase II (CaMKII) is a conduit for translating cytosolic Ca2+ flux into physiological and pathological signals. CaMKII is a serine-threonine kinase that is activated by Ca2+/calmodulin binding, which results in a conformational change revealing the catalytic site (Hudmon and Schulman, 2002). CaMKII is a multifunctional signaling protein that regulates numerous physiological processes including ion channel function (Pitt, 2007), transcription factor activation (Wheeler et al., 2008), MAPK activation (Gardner et al., 2005; Takeda et al., 2004), and pathological processes such as necrosis and apoptosis induced by ischemia/reperfusion injury in cardiomyoctyes (Vila-Petroff et al., 2007), by cholesterol accumulation in macrophages (Timmins et al., 2009), and by toxicant exposure (Fladmark et al., 2002; Olofsson et al., 2008; Wang et al., 2009). Knowledge of the participation of CaMKII in B cell physiology and apoptotic pathways in B lymphocytes is limited (Valentine et al., 1995).
Here, we employed toxicants as putative Ca2+ flux inducers to investigate the role of CaMKII in the induction of apoptosis in bone marrow–derived developing B cells. In addition to GW7845, we examined the contribution of Ca2+ flux to B cell death induced by tributyltin (TBT). TBT is a known immunotoxicant (Vos et al., 1984), which alters calcium dynamics and induces apoptosis in both thymocytes and B cells (De Santiago and Aguilar-Santelises, 1999; Gennari et al., 2000; Raffray and Cohen, 1993; Stridh et al., 1999). Here, we show that both GW7845 and TBT initiate catastrophic cytoplasmic calcium release and CaMKII activation. All features of GW7845-activated cell death (MAPK activation, loss of mitochondrial membrane potential, cytochrome c release, caspase-3 activation, and DNA fragmentation) were suppressed by the calcium chelator BAPTA. CaMKII inhibitors (KN93 and AIP-II) suppressed both GW7845- and TBT-activated cell death. The results are consistent with the conclusion that toxicants that alter Ca2+ flux in B lymphocytes induce cell death through activation of CaMKII, leading to MAPK-stimulated mitochondrial permeabilization and apoptosis, in concert with necrosis.
MATERIALS AND METHODS
Materials.
GW7845 was the generous gift of Dr T. Willson (GlaxoSmithKline, Research Triangle Park, NC). BAPTA-AM and KN93 were from Biomol (Plymouth Meeting, PA). AIP-II-Antennapedia was from EMD Biosciences (San Diego, CA). Antibodies specific for cleaved caspase-3, p38 MAPK, phospho-p38 MAPK, ATF-2, and phospho-ATF-2 were from Cell Signaling Technology (Beverly, MA). The cytochrome c-specific antibody was from BD Biosciences Clontech (Palo Alto, CA). Plasmocin was from Invivogen (San Diego, CA). Fluo3-AM, Fluo-4-AM, and JC-1 were from Molecular Probes (Eugene, OR). Murine rIL-7 was from Research Diagnostics (Flanders, NJ). The JNK1-specific antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Propidium iodide, Protease Inhibitor Cocktail for Mammalian Cells, and the β-actin antibody were from Sigma-Aldrich (St Louis, MO). All other reagents were from Fisher Scientific (Pittsburgh, PA).
Cell cultures.
Stromal cell-dependent CD43+ BU-11 cells expressing rearranged cytoplasmic Ig heavy chains (pro/pre-B cells) (Yamaguchi et al., 1997) were cocultured on cloned BMS2 bone marrow–derived stromal cells (Pietrangeli et al., 1988) (kindly provided by Dr P. Kincade, Oklahoma Medical Research Foundation). Stocks of BU-11 cells were maintained on BMS2 cell monolayers in an equal mixture of Dulbecco's modified Eagle medium and RPMI 1640 medium with 5% fetal bovine serum (FBS) (Thermo Fisher Scientific, formerly Hyclone), plasmocin, L-glutamine and 2-mercaptoethanol. All cultures were maintained at 37°C in a humidified, 5% CO2 atmosphere. Cell cultures were determined to be mycoplasma negative by PCR (MycoAlert Mycoplasma Detection Kit; Cambrex, East Rutherford, NJ).
All animal studies were reviewed and approved by the Institutional Animal Care and Use Committee at Boston University. Spleen, thymus, and bone marrow were collected from wild-type C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME). Primary bone marrow pro-B cell cultures were prepared essentially as described (Tze et al., 2000). Bone marrow was flushed from the femurs of 4- to 8-week-old mice. Red blood cells were lysed by incubation in 0.17M NH4Cl, 10mM KHCO3, and 1mM EDTA at 37°C for 5 min. The remaining cells were cultured for 5–7 days in primary B cell medium (RPMI containing 10% FBS, penicillin/streptomycin, L-glutamine, 2-mercaptoethanol, and 16 ng/ml murine rIL-7). This procedure results in a B cell culture in which at least 95% of the cells express CD43, B220, and CD19.
For experiments, BU-11 cells were cultured (0.5–1 × 106 cells/ml medium) overnight without BMS2 cells in RPMI with 5% FBS and IL7 (16 ng/ml) and treated with Vh (ethanol:dimethyl sulfoxide [DMSO], 50:50, 0.1% final concentration) or GW7845 (40μM) and TBT (1 μM) for 5 min—4 h. Where applicable, cells were pretreated with Vh (DMSO, 0.1% final concentration), BAPTA (5–15μM), KN-93 (1–5μM), or AIP-II (4μM) for 30 min.
Flow cytometry.
For analysis of free intracellular calcium, cells were loaded with Fluo-3-AM (1μM), or in later experiments with the enhanced analog Fluo-4-AM, for 30 min. After a 15-min treatment, B cells were transferred to tubes without washing and analyzed immediately for green fluorescence (FL-1) on a Becton/Dickinson FACScan flow cytometer. For experiments with GW7845, batch fluorescence also was determined using a multifunctional plate reader (Synergy 2; Biotek Instruments, Winooski, VT).
For analysis of mitochondrial membrane potential (ΔΨm), 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1, 1.4μM) was added to cultures at the time of dosing. At the time of harvest, B cells were transferred to FACS tubes without washing and analyzed immediately for green (FL-1) and red (FL-2) fluorescence by flow cytometry. Only cells in the live gate were analyzed. Cells with low mitochondrial membrane potential () were determined to be those having an increased green fluorescence with or without a loss of red fluorescence.
Cell death assays.
To analyze caspase-3 activation, the Caspase-Glo 3/7 Assay Kit was used (Promega). To analyze LDH release, the CytoTox-Glo Assay Kit was used (Promega). Luminescence values in experimental wells were normalized by that measured in Naive cells to determine “Fold Change from Naïve” values.
For analysis of cellular DNA content, cells were harvested into cold PBS containing 5% FBS and 10μM azide. Cells were resuspended in 0.25 ml hypotonic buffer containing 50 μg/ml propidium iodide (PI), 1% sodium citrate, and 0.1% Triton X-100 and analyzed for red fluorescence with FL-2 in the log mode. The percentage of cells undergoing apoptosis was determined to be those having a weaker PI fluorescence than cells in the G0/G1 phase of the cell cycle.
Immunoblotting.
For analysis of caspase expression or kinase expression and phosphorylation, cytoplasmic extracts were prepared from tissues or cells as described previously (Schlezinger et al., 2006, 2007). For analysis of cytochrome c release, cytoplasmic fractions from digitonin-permeabilized cells were prepared as described previously (Schlezinger et al., 2006). Protein concentrations were determined by the Bradford method.
Proteins (5–60 μg) were resolved on 12% (ATF-2, p38 MAPK, and JNK) or 15% (cytochrome c) gels, transferred to a 0.2 μm nitrocellulose membrane, and incubated with primary antibody. Primary antibodies included anti-cleaved caspase-3 (catalog # 9661), anti-cytochrome (S2050), anti-JNK1 antibody (sc-474), anti-phospho-ATF-2 (Thr71) (5112), and anti-phospho-p38 MAPK (Thr180/Tyr182) (9215). The secondary antibodies were horseradish peroxidase-linked goat anti-rabbit and goat-anti mouse IgG (Bio-Rad, Hercules, CA). Immunoreactive bands were visualized with enhanced chemiluminescence. To control for equal protein loading, blots were re-probed with antibodies to total p38 MAPK (9212), total ATF-2 (9226), or β-actin (A5441) and analyzed as above. To quantify changes in protein expression, band densities were determined using the UVP Bioimaging System and the Labworks 4 program (UVP, Inc., Upland, CA). For kinases, the band density of the phospho-specific protein was divided by the band density of the total protein. For all other proteins, the band density of the specific protein was divided by the band density of β-actin. β-actin-normalized data are reported as “Relative Expression.” When appropriate, the phospho/total ratio or the protein/β-actin ratio for experimental samples then was divided by the ratio in the Naïve sample from that experiment, and the data are reported as “Fold Change from Naïve.”
Enzyme activity assays.
For JNK activation, B cells were harvested and washed once in cold PBS. Cytoplasmic extracts were prepared by pipetting cells in lysis buffer (20mM Tris-HCl [pH 7.5], 150mM NaCl, 1mM EDTA, 1mM ethylenebis(oxyethylenenitrilo)tetraaceticacid, 1% Triton, 10% glycerol, 10mM sodium fluoride, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM sodium orthovanadate, 1 μg/ml leupeptin, and 1mM phenylmethylsulfonyl fluoride). Lysates were cleared by centrifugation at 14,000 rpm. Protein concentrations were determined using the Bradford method. JNK1 was immunoprecipitated from 200 μg extract with an anti-JNK1 antibody (sc-474) and Protein A-Sepharose beads by nutation at 4°C for 1 h. Following multiple, successive washes with kinase buffer (40mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [HEPES] [pH 7.5], 40mM β-glycerophosphate, 40mM MgCl2, 1.5mM sodium orthovanadate, 1.5mM dithiothreitol) containing 3M urea, then kinase buffer alone, immune complexes were incubated with GST-c-jun and [γ-32P]ATP for 30 min at 30°C. GST-c-jun was then purified from the reaction mixture with glutathione-Sepharose beads, followed by resolution on a 12% acrylamide gel and autoradiography. To ensure equal pull down of JNK1, an identical sample was analyzed by immunblotting to determine the level of JNK1 expression. Relative JNK activity was quantified as described above. For CaMKII activation, the SignaTect Assay kit was used (Promega, Madison, WI). Cytoplasmic extracts were prepared and the protein concentrations determined by the Bradford method. To determine specific activity, experimental reactions were run for 6 min, along with a 3-min reaction with extracts from untreated cells to determine background activity. All counts per minute (CPM) values were normalized for protein content, and the protein-corrected CPM values determined in the background reaction were subtracted from all experimental reactions run concurrently. Corrected CPM values in experimental wells were normalized by that measured in Naïve cells to determine “Fold Change from Naïve” values.
RT-PCR for CaMKII isoform expression.
Total RNA was prepared from tissues and cells using RNA-Stat 60 (Tel-Test Inc., Friendswood, TX) and quantified. Equal amounts of total RNA were reverse transcribed using iScript (Bio-Rad, Hercules, CA). PCR specific for each isoform was performed as described previously (Lorenz et al., 2002). As a control, PCR for β-actin also was performed.
Statistics.
Statistical analyses were performed with StatView (SAS Institute, Cary, NC). Data are presented as means ± SE. One-factor ANOVAs were used, in combination with the Dunnett's or Tukey-Kramer multiple comparisons tests, to analyze the data and determine significant differences.
RESULTS
Calcium-Dependence of GW7845-Induced Apoptosis
GW7845 (40μM) activates a rapid increase in the percentage of cells with a sub-G0/G1 DNA content, indicative of apoptosis, in cultured pro/pre-B cells and primary B cells (Schlezinger et al., 2007). In addition, necrosis is evident following treatment with GW7845 at late time points (Supplementary fig. S1). A MAPK cascade is activated within minutes of exposure and contributes significantly to GW7845-activated B cell death (Schlezinger et al., 2006). One mechanism for the rapid activation of MAPKs is the accumulation of Ca2+ in the cytosol (Takeda et al., 2004). Therefore, we investigated the contribution of cytoplasmic Ca2+ to the activation of MAPKs and cell death in cultured pro/pre-B cells following treatment with GW7845.
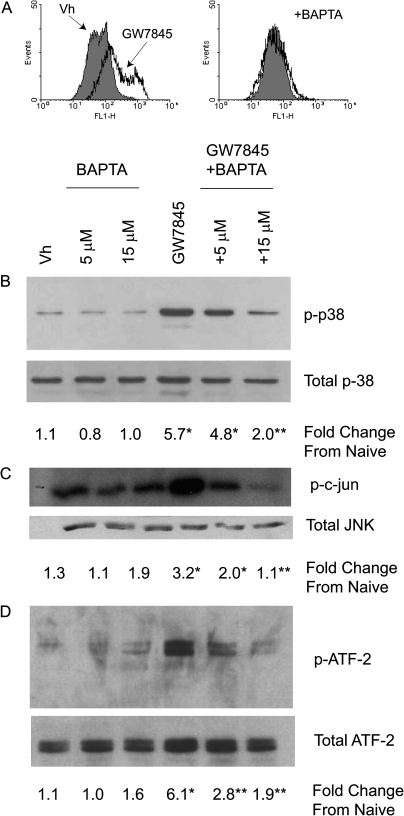
Cytoplasmic Ca2+ was determined in Fluo-3-loaded BU-11 cells, a nontransformed murine pro/pre-B cell line. BU-11 cells were treated with vehicle (DMSO:ethanol, 50:50, 0.1% final concentration) or GW7845 (40μM) and analyzed for Fluo-3 fluorescence. There was a distinct increase in Fluo-3 fluorescence, indicative of cytoplasmic Ca2+, from 33 ± 3 relative fluorescence units (RFUs) in Vh-treated cells to 86 ± 20 RFUs in GW7845-treated cells (p < 0.05, ANOVA, Dunnett's) (Fig. 1A). This increase was prevented by co-treatment with the calcium chelator BAPTA. In order to determine the contribution of Ca2+ to the activation of MAPKs, BU-11 cells were pretreated with BAPTA (5–15μM), treated with vehicle or GW7845, and then analyzed for MAPK phosphorylation and activation. A significant increase in the phosphorylation of p38 MAPK, indicative of activation, was evident following treatment with GW7845, and this was significantly decreased by co-treatment with BAPTA (Fig. 1B). Similarly, JNK was activated by treatment with GW7845, as indicated by an in vitro kinase assay, and this was significantly decreased by co-treatment with BAPTA (Fig. 1C). We have shown previously that ATF-2 is an endogenous target of both p38 MAPK and JNK following GW7845 treatment (Schlezinger et al., 2006). Here, we show that GW7845-stimulated ATF-2 phosphorylation also is significantly decreased by BAPTA co-treatment (Fig. 1D). From these experiments, it was concluded that GW7845 treatment results in a significant increase in cytoplasmic Ca2+ and calcium-dependent MAPK-activation.
FIG. 1.
GW7845 activates calcium-dependent MAPK activation in pro/pre-B cells. (A) Cytoplasmic Ca2+ was detected by loading BU-11 cells with Fluo3-AM (1μM) prior to treatment with Vh (DMSO, 0.1%) or GW7845 (± 15μM BAPTA) for 15 min, followed by flow cytometry. (B–D) For MAPK activation, BU-11 cells were pretreated with Vh (DMSO, 0.1%) or BAPTA (5–15μM) for 30 min and then treated with Vh (ethanol:DMSO, 50:50, 0.1%) or GW7845 (40μM) for 30 min. Cytoplasmic extracts were prepared and analyzed for p38 MAPK activation by detection of phosphorylated p38 MAPK by immunoblotting (B), for JNK activation by in vitro kinase assay (C), and for endogenous p38 MAPK/JNK activity by detection of phosphorylated ATF-2 by immunoblotting (D). Protein expression was quantified as described in the Materials and Methods and presented as “Fold Change from Naive.” Data are representative of or are presented as means ± SE from at least three independent experiments. *Statistically different from Vh-treated (p < 0.05, ANOVA, Tukey-Kramer). **Statistically different from GW-treated alone (p < 0.05, ANOVA, Tukey-Kramer).
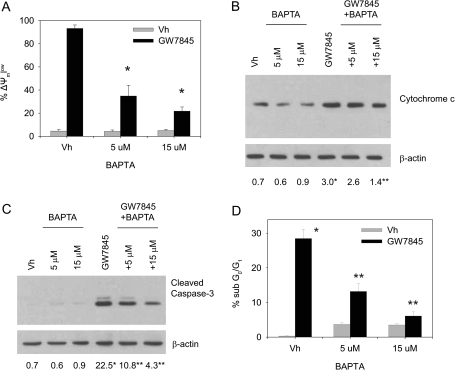
Given the critical role of MAPKs in GW7845-induced cell death (Schlezinger et al., 2006), we next examined the effect of BAPTA on features of GW7845-induced apoptosis. BU-11 cells were pretreated with vehicle (DMSO:ethanol, 50:50, 0.1% final volume) or BAPTA (5–15μM), treated with vehicle or GW7845 (40μM), and analyzed for changes in mitochondria, as well as for capase-3 activation and DNA fragmentation. GW7845 induced a nearly complete loss of mitochondrial membrane potential (Fig. 2A) and a significant release of cytochrome c (Fig. 2B), both of which were suppressed by co-treatment with BAPTA. Accordingly, the downstream events of GW7845-induced cell death, caspase-3 activation (Fig. 2C) and DNA fragmentation (Fig. 2D), were inhibited by co-treatment with BAPTA. The data are consistent with the conclusion that GW7845 activates a calcium-dependent death pathway via MAPK-dependent mitochondrial permeabilization.
FIG. 2.
Calcium chelation suppresses GW7845-induced mitochondrial changes and apoptosis. BU-11 cells were pretreated with Vh (DMSO, 0.1%) or BAPTA (5–15μM) for 30 min and then treated with Vh (ethanol:DMSO, 50:50, 0.1%) or GW7845 (40μM). (A) Mitochondrial membrane potential was analyzed after 30 min of treatment by JC-1 staining, followed by flow cytometry. (B) Cytochrome c release was analyzed after 45 min of treatment by immunoblotting of cytoplasmic extracts from digitonin-permeablized cells. Protein expression was quantified as described in the Materials and Methods and presented as “Fold Change from Naive.” (C) Formation of cleaved active caspase-3 (17 kDa) was analyzed after 30 min of treatment by immunoblotting of cytoplasmic extracts. (D) The percentage of cells with a sub-G0/G1 DNA content was analyzed after 2 h of treatment by PI staining, followed by flow cytometry. Data are presented as means ± SE from at least three independent experiments. *Statistically different from Vh-treated (p < 0.05, ANOVA, Tukey-Kramer). **Statistically different from GW-treated alone (p < 0.05, ANOVA, Tukey-Kramer).
Role of CaMKII in Ca2+ Flux-Induced Apoptosis
Given the requirement for Ca2+ in GW7845-induced MAPK activation, we sought to determine how Ca2+ triggers that activation. CaMKII is a calcium-dependent MAP4K that is known to activate MAPKs (Takeda et al., 2004) and has been associated with toxicant-induced cell death (Fladmark et al., 2002; Gardner et al., 2005; Olofsson et al., 2008). Therefore, we investigated the contribution of CaMKII to GW7845-induced cell death.
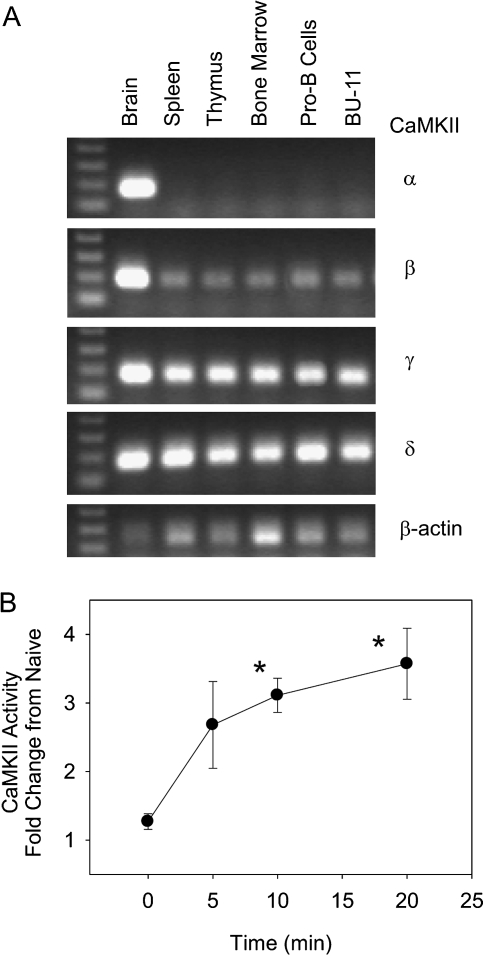
There is limited information on the distribution of CaMKII isoforms in the immune system; therefore, we began by determining the expression of CaMKII α, β, γ, and δ in brain, primary immune tissues, cultured primary B cells, and a B cell line by RT-PCR. As expected, all CaMKII isoforms were detected in the brain, and brain was the only tissue in which CaMKII α was detected (Fig. 3A). In contrast, β, γ, and δ all were detected in spleen, thymus, and bone marrow and in cultured primary pro-B cells and the pro/pre-B cell line BU-11. To determine if CaMKII was activated following initiation of the Ca2+ flux, BU-11 cells were treated with vehicle (DMSO:ethanol, 50:50, 0.1% final volume) or GW7845 (40μM). CaMKII activity increased within 5 min and reached significance at 10 min. These data demonstrate that B cells express multiple isoforms of CaMKII, and CaMKII can be activated rapidly by treatment with GW7845.
FIG. 3.
Multiple CaMKII isoforms are expressed in B cells, and CaMKII is activated following GW7845 exposure. (A) Total RNA was prepared from tissues and cells, reverse transcribed, and amplified using CaMKII isoform- and β-actin-specific primers. (B) BU-11 cells were treated with Vh (ethanol:DMSO, 50:50, 0.1%) or GW7845 (40μM) for 5–20 min. CaMKII activity was determined in cytoplasmic extracts using the SignaTect Assay (Promega). CPM values in experimental wells were normalized by that measured in untreated cells and are presented as “Fold Change from Naïve.” Data are presented as means ± SE from at least three independent experiments. *Statistically different from t = 0 (p < 0.05, ANOVA, Dunnett's).
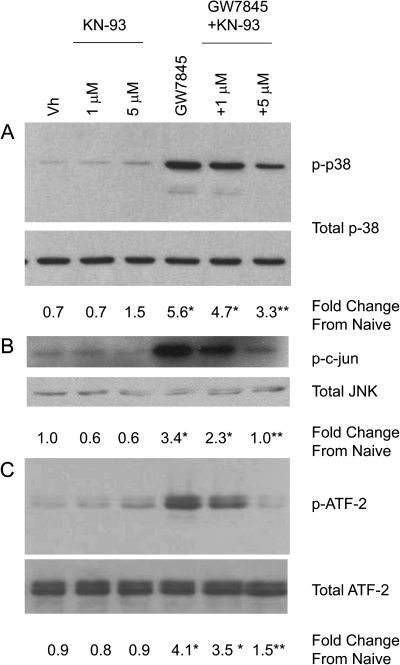
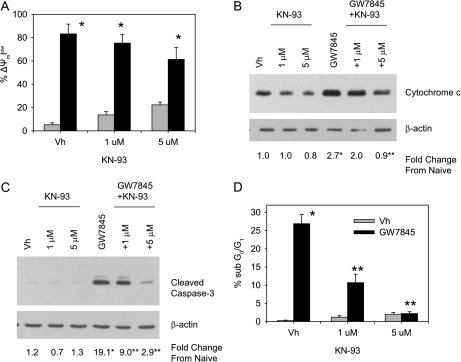
To test the contribution of CaMKII activation to Ca2+ flux-induced MAPK activation and apoptosis, BU-11 cells were pretreated with vehicle (DMSO, 0.1%) or KN93 (1–5μM), a CaMKII inhibitor (Sumi et al., 1991). BU-11 cells then were treated with vehicle or GW7845 and analyzed for MAPK activation, alterations to the mitochondria, and apoptosis. A significant increase in the phosphorylation of p38 MAPK was evident following treatment with GW7845, and this was significantly decreased by co-treatment with KN93 (Fig. 4A). Similarly, JNK was activated by treatment with GW7845, as indicated by an in vitro kinase assay, and this was significantly decreased by co-treatment with KN93 (Fig. 4B). GW7845-stimulated ATF-2 phosphorylation also was significantly decreased by KN93 co-treatment (Fig. 4C). Accordingly, multiple features of GW7845-induced death were significantly suppressed, including GW7845-induced cytochrome c release (Fig. 5B), caspase-3 activation (Fig. 5C), and DNA fragmentation (Fig. 5D). The one exception was that KN93 did not suppress GW7845-induced loss of mitochondrial membrane potential (Fig. 5A); however, this is consistent with the previous observations that GW7845 appears to induce multiple independent changes in mitochondria (Schlezinger et al., 2007). The suppression of multiple aspects of GW7845-induced cell death by KN93 supports the conclusion that GW7845-induced apoptosis results from activation of CaMKII. As with all inhibitors, caution must be taken interpreting the results; KN93 also causes a reduction in currents of L-type calcium channels (Gao et al., 2006). However, the suppression of GW7845-induced caspase-3 activation by the structurally distinct CaMKII peptide inhibitor AIP-II (Ishida et al., 1995) (see below) provides further support for the conclusion.
FIG. 4.
A CaMKII inhibitor, KN93, suppresses GW7845-induced MAPK activation. BU-11 cells were pretreated with Vh (DMSO, 0.1%) or KN93 (1–5μM) for 30 min and then treated with Vh (ethanol:DMSO, 50:50, 0.1%) or GW7845 (40μM) for 30 min. Cytoplasmic extracts were prepared and analyzed for p38 MAPK activation by detection of phosphorylated p38 MAPK by immunoblotting (A), for JNK activation by in vitro kinase assay (B), and for endogenous p38 MAPK/JNK activity by detection of phosphorylated ATF-2 by immunoblotting (C). Protein expression was quantified as described in the Materials and Methods and presented as “Fold Change from Naive.” Data are presented as means ± SE from at least three independent experiments. *Statistically different from Vh-treated (p < 0.05, ANOVA, Tukey-Kramer). **Statistically different from GW-treated alone (p < 0.05, ANOVA, Tukey-Kramer).
FIG. 5.
KN93 inhibits GW7845-activated apoptosis. BU-11 cells were pretreated with Vh (DMSO, 0.1%) or KN93 (1–5μM) for 30 min and then treated with Vh (ethanol:DMSO, 50:50, 0.1%) or GW7845 (40μM). (A) Mitochondrial membrane potential was analyzed after 30 min of treatment by JC-1 staining, followed by flow cytometry. (B) Cytochrome c release was analyzed after 45 min of treatment by immunoblotting of cytoplasmic extracts from digitonin-permeablized cells. Protein expression was quantified as described in the Materials and Methods and presented as “Fold Change from Naive.” (C) Formation of cleaved active caspase-3 (17 kDa) was analyzed after 30 min of treatment by immunoblotting of cytoplasmic extracts. (D) The percentage of cells with a sub-G0/G1 DNA content was analyzed after 2 h of treatment by PI staining, followed by flow cytometry. Data are presented as means ± SE from at least three independent experiments. *Statistically different from Vh-treated (p < 0.05, ANOVA, Tukey-Kramer). **Statistically different from GW-treated alone (p < 0.05, ANOVA, Tukey-Kramer).
CaMKII-Dependent Cell Death is Activated Following TBT-Induced Ca2+ Flux
TBT has long been known as an immunotoxicant. High dose acute TBT exposure induces thymic atrophy and suppresses immune responses to infection in rodent models (Vos et al., 1984), potentially via inducing thymocyte apoptosis (Raffray and Cohen, 1993; Stridh et al., 1999). While TBT generally is thought of as a thymocyte toxicant, B lymphocytes also are highly susceptible to TBT-induced apoptosis (De Santiago and Aguilar-Santelises, 1999). Micromolar concentrations of TBT influence calcium dynamics, and this is thought to be a mechanism through which TBT induces apoptosis in multiple cell types (Gennari et al., 2000; Grundler et al., 2001; Nakatsu et al., 2007). Here, we tested the hypothesis that high-dose TBT induces a calcium- and CaMKII-dependent apoptotic pathway in B cells.
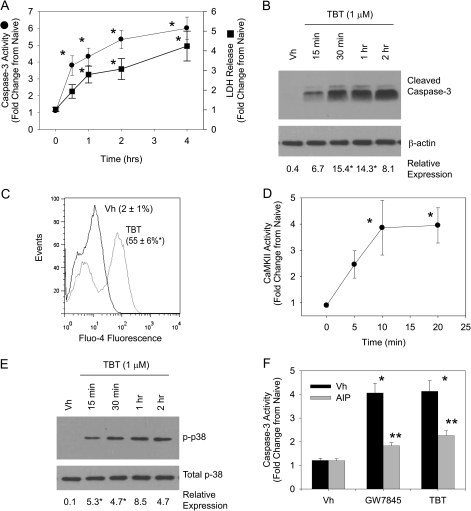
First, we characterized the timing of high-dose TBT–induced B cell death. BU-11 cells were treated with vehicle (DMSO, 0.1%) or TBT (1μM) and analyzed for caspase-3 activation and LDH release. TBT rapidly induced apoptosis, as evidenced by significant caspase-3 activation occurring within 2 h of treatment (Figs. 6A and 6B). Significant LDH release indicated that necrosis occurred concurrently with TBT-induced apoptosis (Fig. 6A). Next, we examined TBT-treated BU-11 cells for release of Ca2+ into the cytoplasm and activation of CaMKII. There was a significant increase in Fluo-4 fluorescence, indicative of cytoplasmic Ca2+, within 15 min of treatment with 1μM TBT (Fig. 6C) that was accompanied by a significant increase in CaMKII activity (Fig. 6D) and activation of p38 MAPK (Fig. 6E). Finally, we tested the contribution of CaMKII activation to TBT-induced apoptosis. BU-11 cells were pretreated with vehicle (DMSO, 0.1%) or AIP-II (4 μM), a CaMKII inhibitor (Ishida et al., 1995). BU-11 cells then were treated with vehicle, GW7845, as a positive control, or TBT (1μM) and analyzed for capsase-3 activity. AIP-II significantly suppressed both TBT- and GW7845-induced caspase activation. The data support the conclusion that CaMKII is a common conduit to apoptosis for agents that stimulate catastrophic release of Ca2+ into the cytoplasm of B cells.
FIG. 6.
The immunotoxicant TBT induces calcium-dependent cell death. BU-11 cells were treated with Vh (DMSO, 0.1%) or TBT (1μM) for 5 min 2 h. (A) Caspase-3 activity and LDH release were assessed using the Caspase3/7-Glo Assay and the CytoTox-Glo Assay (Promega). Luminescence values in experimental wells were normalized by that measured in untreated cells and are presented as “Fold Change from Naïve.” (B) Formation of cleaved active caspase-3 (17 kDa) was analyzed by immunoblotting of cytoplasmic extracts. Protein expression was quantified as described in the Materials and Methods and presented as “Relative Expression.” (C) Cytoplasmic Ca2+ was detected by loading BU-11 cells with Fluo4-AM (1μM) prior to treatment with Vh or TBT for 15 min, followed by flow cytometry. (D) CaMKII activity was determined in cytoplasmic extracts using the SignaTect Assay (Promega). CPM values in experimental wells were normalized by that measured in untreated cells and are presented as “Fold Change from Naïve.” (E) p38 MAPK activation was determined by detection of phosphorylated p38 MAPK in cytoplasmic extracts by immunoblotting. (F) BU-11 cells were pretreated with Vh (DMSO, 0.1%) or AIP-II (4μM) for 30 min and then treated with Vh (DMSO, 0.1%) or TBT (1 μM). Caspase-3 activity was assessed using the Caspase3/7-Glo Assay, as described in (A). Data are presented as means ± SE from at least three independent experiments. *Statistically different from Vh-treated (p < 0.05, ANOVA, Tukey-Kramer). ** Statistically different from GW-treated alone (p < 0.05, ANOVA, Tukey-Kramer).
DISCUSSION
Ca2+ is a second messenger that is an essential signaling molecule for B cell development and function (Scharenberg et al., 2007) and plays a key role in B-cell receptor–mediated apoptosis in developing B cells during the process of negative selection (Ruiz-Vela et al., 1999); therefore, we hypothesized that changes in calcium dynamics are a potential target for toxicant-induced effects. Given the growing recognition of the role that changes in Ca2+ dynamics play in multiple death pathways (Zhu et al., 2007), we investigated the contribution of Ca2+ to toxicant-induced apoptosis in a developing B cell model and the mechanism of signal transduction between increased cytosolic calcium and activation of the apoptotic machinery.
We have shown previously that GW7845 induces rapid, MAPK-dependent apoptosis in bone marrow B cells (Schlezinger et al., 2006, 2007). GW7845 activates an intrinsic apoptotic pathway dependent upon mitochondrial permeabilization and release of cytochrome c, but not loss of mitochondrial membrane potential (Schlezinger et al., 2007). Here, we show that GW7845 stimulates accumulation of Ca2+ in the cytosol and that a calcium chelator suppresses upstream signaling (p38 MAPK and JNK activation), alteration of the mitochondria (loss of mitochondrial membrane potential and cytochrome c release), downstream apoptotic signaling (caspase-3 activation), and a terminal apoptotic feature (DNA fragmentation). These results are consistent with the conclusion that changes in calcium flux are an apical event in GW7845-induced cell death. Thus, we sought to determine the mechanism by which Ca2+ leads to initiation of this MAPK-dependent death pathway.
A likely intermediary for the calcium-dependent effects is CaMKII. Of the four CaMKII isoforms, α and β are abundantly expressed in neurons, whereas γ and δ are more widely expressed (Tobimatsu and Fujisawa, 1989). However, a recent study has suggested that CaMKII, including the β isoform, not only is expressed in T lymphocytes but also contributes significantly to T cell physiology (Lin et al., 2005). In concurrence with these studies, we have found that CaMKIIβ, γ and δ, but not α, are expressed in lymphoid tissues (spleen, thymus, and bone marrow) and have extended this analysis to show that these isoforms are expressed in primary pro-B cells and a developing B lymphocyte model (Fig. 3). To date, CaMKIIδ is the one isoform that has specifically been associated with initiation of cell death (Zhu et al., 2007; Timmins et al., 2009). CaMKII targets at least two MAP3Ks, apoptosis signaling kinase 1 and TGF-β-activated kinase 1 (Takeda et al., 2004; Wang et al., 2009), which could initiate the MAPK cascade and result in MAPK-dependent cell death as seen following GW7845 treatment. Further investigations are required to determine the specific CaMKII isoform(s) that contribute to GW7845-induced B cell death, as well as the CaMKII target(s).
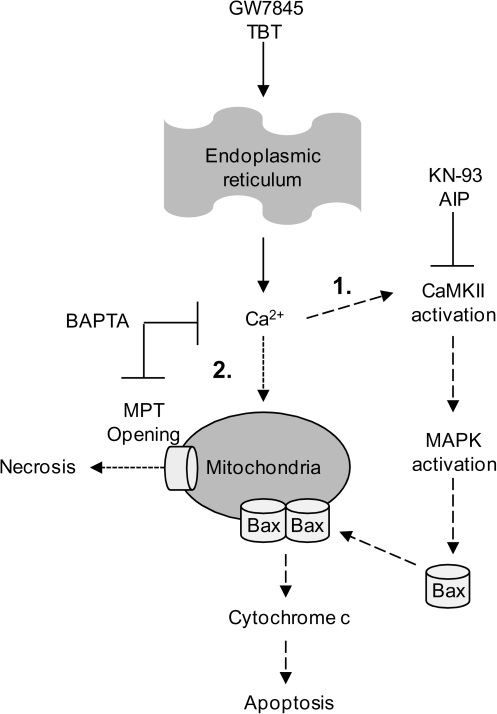
Evidently, GW7845 activates a calcium- and CaMKII-dependent apoptosis pathway. However, the facts that BAPTA, but not KN93, suppresses the loss of mitochondrial membrane potential (Fig. 2) and that GW7845 also stimulates LDH release indicative of necrosis suggest that more than one calcium-mediated mechanism is at work. These results are in line with our previous observation that cyclosporin A, an inhibitor of permeability transition pore opening, significantly reduces GW7845-induced mitochondrial membrane potential loss, but not cytochrome c release or apoptosis (Schlezinger et al., 2007). The data are consistent with the conclusion that calcium initiates activation of CaMKII leading to MAPK-dependent mitochondrial permeabilization (Fig. 7, pathway 1) and also has a direct effect on the mitochondria leading to opening of the permeability transition pore (Fig. 7, pathway 2). The mitochondria can act as a sink for Ca2+ released into the cytosol; however, when the sequestering capacity of the mitochondria is exceeded, opening of the permeability transition pore ensues leading to collapse of the mitochondrial membrane potential, loss of the ability to produce ATP and necrosis, all of which can occur concurrently with apoptosis (necrapoptosis) (Lemasters et al., 2009).
FIG. 7.
Hypothesized pathways of GW7845- and TBT-induced, calcium-mediated death.
In order to test whether activation of CaMKII is a common feature of Ca2+-mediated cell death in B lymphocytes, we examined its role in cell death induced by an immunotoxicant known to influence calcium dynamics, high-dose TBT. Micromolar concentrations of TBT activate calcium-dependent apoptosis in thymocytes (Gennari et al., 2000; Grundler et al., 2001). Here, we extended these studies by demonstrating that developing bone marrow B cells also are susceptible to high-dose TBT–activated apoptosis. As with thymocytes, this cell death is mediated by a calcium-activated pathway (Fig. 6). Yu et al. (2000) demonstrated p38 MAPK and JNK activation following TBT exposure, and data presented here show for the first time that CaMKII activation is the signal transduction mechanism leading from cytosolic Ca2+ accumulation to MAPK activation and apoptosis. B lymphocytes appear to be highly susceptible to TBT exposure, as concentrations as low as 100nM induce apoptosis in mature human B cells (De Santiago and Aguilar-Santelises, 1999). Interestingly, data suggest that distinct dose-dependent mechanisms lead to the activation of different apoptotic pathways by exposure to high (micromolar) and low (nanomolar) concentrations of TBT (Nakatsu et al., 2007). Indeed, in our own hands, low-dose TBT (100nM) activates a slower onset of apoptosis than high-dose TBT (1μM) (40% apoptosis occurring within 16 vs. 2 h [data not shown]), which we hypothesize results from a difference in the contribution of calcium to activation of the death pathways.
GW7845 and TBT share two prominent characteristics, the ability to activate PPARγ at low doses and to cause substantial changes in Ca2+ flux at high doses. Interestingly, exposure to thiazolidinediones, therapeutic PPARγ agonists, also results in receptor-independent alteration of Ca2+ flux and activation of CaMKII (Gardner et al., 2005). It is unknown at this time whether these compounds perturb Ca2+ homeostasis by similar or disparate mechanisms. Once calcium flux is initiated by these compounds, however, CaMKII activation is a common result. In concurrence with the growing recognition of the contribution that calcium plays in apoptotic pathways, our data show that the end result of CaMKII activation following substantial cytoplasmic calcium release in an early B cell model is the initiation of apoptosis. Given the minimal information available on the role of CaMKII in physiologic and pathological processes in B cells and the dearth of knowledge of how it regulates developing lymphocyte apoptosis, the present study on the contribution of CaMKII to apoptosis signaling and its activation by toxicants, including environmental chemicals, represents an important step forward in defining toxicant-induced CaMKII signaling leading to rapid death in developing B cells.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Superfund Research Program at the National Institutes of Health (P42 ES007381 to J.J.S.).
Supplementary Material
Acknowledgments
The authors would like to thank Mr Matthew Rarrick for his superb technical assistance and Dr David Sherr for his helpful comments on the manuscript.
References
- Berry EB, Keelan JA, Helliwell RJ, Gilmour RS, Mitchell MD. Nanomolar and micromolar effects of 15-deoxy-delta 12,14-prostaglandin J2 on amnion-derived WISH epithelial cells: differential roles of peroxisome proliferator-activated receptors gamma and delta and nuclear factor kappa B. Mol. Pharmacol. 2005;68:169–178. doi: 10.1124/mol.104.009449. [DOI] [PubMed] [Google Scholar]
- Cerella C, Diederich M, Ghibelli L. The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int. J. Cell Biol. 2010;2010:546163. doi: 10.1155/2010/546163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Konopleva M, Andreef M, Samudio I, Safe S. 2-Cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds inhibit growth of colon cancer cells through peroxisome proliferator-activated receptor gamma-dependent and -independent pathways. Mol. Pharmacol. 2005;68:119–128. doi: 10.1124/mol.105.011437. [DOI] [PubMed] [Google Scholar]
- Contractor R, Samudio IJ, Estrov Z, Harris D, McCubrey JA, Safe SH, Andreeff M, Konopleva M. A novel ring-substituted diindolylmethane,1,1-bis[3'-(5-methoxyindolyl)]-1-(p-t-butylphenyl) methane, inhibits extracellular signal-regulated kinase activation and induces apoptosis in acute myelogenous leukemia. Cancer Res. 2005;65:2890–2898. doi: 10.1158/0008-5472.CAN-04-3781. [DOI] [PubMed] [Google Scholar]
- De Santiago A, Aguilar-Santelises M. Organotin compounds decrease in vitro survival, proliferation and differentiation of normal human B lymphocytes. Hum. Exp. Toxicol. 1999;18:619–624. doi: 10.1191/096032799678839437. [DOI] [PubMed] [Google Scholar]
- Fladmark KE, Brustugun OT, Mellgren G, Krakstad C, Boe R, Vintermyr OK, Schulman H, Doskeland SO. Ca2+/calmodulin-dependent protein kinase II is required for microcystin-induced apoptosis. J. Biol. Chem. 2002;277:2804–2811. doi: 10.1074/jbc.M109049200. [DOI] [PubMed] [Google Scholar]
- Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem. Biophys. Res. Commun. 2006;345:1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- Gardner OS, Shiau CW, Chen CS, Graves LM. Peroxisome proliferator-activated receptor gamma-independent activation of p38 MAPK by thiazolidinediones involves calcium/calmodulin-dependent protein kinase II and protein kinase R: correlation with endoplasmic reticulum stress. J. Biol. Chem. 2005;280:10109–10118. doi: 10.1074/jbc.M410445200. [DOI] [PubMed] [Google Scholar]
- Gennari A, Viviani B, Galli CL, Marinovich M, Pieters R, Corsini E. Organotins induce apoptosis by disturbance of [Ca(2+)](i) and mitochondrial activity, causing oxidative stress and activation of caspases in rat thymocytes. Toxicol. Appl. Pharmacol. 2000;169:185–190. doi: 10.1006/taap.2000.9076. [DOI] [PubMed] [Google Scholar]
- Grundler W, Dirscherl P, Beisker W, Marx K, Stampfl A, Maier K, Zimmermann I, Nusse M. Early functional apoptotic responses of thymocytes induced by Tri-n-butyltin. Cytometry. 2001;44:45–56. [PubMed] [Google Scholar]
- Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem. Biophys. Res. Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Elstner E, McQueen TJ, Tsao T, Sudarikov A, Hu W, Schober WD, Wang RY, Chism D, Kornblau SM, et al. Peroxisome proliferator-activated receptor gamma and retinoid X receptor ligands are potent inducers of differentiation and apoptosis in leukemias. Mol. Cancer Ther. 2004;3:1249–1262. [PubMed] [Google Scholar]
- Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MY, Zal T, Ch'en IL, Gascoigne NR, Hedrick SM. A pivotal role for the multifunctional calcium/calmodulin-dependent protein kinase II in T cells: from activation to unresponsiveness. J. Immunol. 2005;174:5583–5592. doi: 10.4049/jimmunol.174.9.5583. [DOI] [PubMed] [Google Scholar]
- Lorenz JM, Riddervold MH, Beckett EA, Baker SA, Perrino BA. Differential autophosphorylation of CaM kinase II from phasic and tonic smooth muscle tissues. Am. J. Physiol. Cell Physiol. 2002;283:C1399–C1413. doi: 10.1152/ajpcell.00020.2002. [DOI] [PubMed] [Google Scholar]
- Nakatsu Y, Kotake Y, Ohta S. Concentration dependence of the mechanisms of tributyltin-induced apoptosis. Toxicol. Sci. 2007;97:438–447. doi: 10.1093/toxsci/kfm039. [DOI] [PubMed] [Google Scholar]
- Nutt LK, Chandra J, Pataer A, Fang B, Roth JA, Swisher SG, O'Neil RG, McConkey DJ. Bax-mediated Ca2+ mobilization promotes cytochrome c release during apoptosis. J. Biol. Chem. 2002;277:20301–20308. doi: 10.1074/jbc.M201604200. [DOI] [PubMed] [Google Scholar]
- Olofsson MH, Havelka AM, Brnjic S, Shoshan MC, Linder S. Charting calcium-regulated apoptosis pathways using chemical biology: role of calmodulin kinase II. BMC Chem. Biol. 2008;8:2. doi: 10.1186/1472-6769-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Padilla J, Kaur K, Cao HJ, Smith TJ, Phipps RP. Peroxisome proliferator activator receptor-γ agonists and 15-deoxy-Δ12,14-PGJ2 induce apoptosis in normal and malignant B-lineage cells. J. Immunol. 2000;165:6941–6948. doi: 10.4049/jimmunol.165.12.6941. [DOI] [PubMed] [Google Scholar]
- Pan Z, Bhat MB, Nieminen AL, Ma J. Synergistic movements of Ca(2+) and Bax in cells undergoing apoptosis. J. Biol. Chem. 2001;276:32257–32263. doi: 10.1074/jbc.M100178200. [DOI] [PubMed] [Google Scholar]
- Pietrangeli C, Hayashi S-I, Kincade P. Stromal cell lines which support lymphocyte growth: characterization, sensitivity to radiation and responsiveness to growth factors. Eur. J. Immunol. 1988;18:863–872. doi: 10.1002/eji.1830180606. [DOI] [PubMed] [Google Scholar]
- Pitt GS. Calmodulin and CaMKII as molecular switches for cardiac ion channels. Cardiovasc. Res. 2007;73:641–647. doi: 10.1016/j.cardiores.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Piva R, Gianferretti P, Ciucci A, Taulli R, Belardo G, Santoro MG. 15-Deoxy-delta 12,14-prostaglandin J2 induces apoptosis in human malignant B cells: an effect associated with inhibition of NF-kappa B activity and down-regulation of antiapoptotic proteins. Blood. 2005;105:1750–1758. doi: 10.1182/blood-2004-04-1360. [DOI] [PubMed] [Google Scholar]
- Raffray M, Cohen GM. Thymocyte apoptosis as a mechanism for tributyltin-induced thymic atrophy in vivo. Arch. Toxicol. 1993;67:231–236. doi: 10.1007/BF01974341. [DOI] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Ray DM, Bernstein SH, Phipps RP. Human multiple myeloma cells express peroxisome proliferator-activated receptor gamma and undergo apoptosis upon exposure to PPARgamma ligands. Clin. Immunol. 2004;113:203–213. doi: 10.1016/j.clim.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Ruiz-Vela A, Gonzalez de Buitrago G, Martinez AC. Implication of calpain in caspase activation during B cell clonal deletion. EMBO J. 1999;18:4988–4998. doi: 10.1093/emboj/18.18.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharenberg AM, Humphries LA, Rawlings DJ. Calcium signalling and cell-fate choice in B cells. Nat. Rev. Immunol. 2007;7:778–789. doi: 10.1038/nri2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlezinger JJ, Emberley JK, Bissonnette SL, Sherr DH. An L-tyrosine derivative and PPARγ agonist, GW7845, activates a multifaceted caspase cascade in bone marrow B cells. Toxicol. Sci. 2007;98:125–136. doi: 10.1093/toxsci/kfm071. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Emberley JK, Sherr DH. Activation of multiple mitogen-activated protein kinases in pro/pre-B cells by GW7845, a peroxisome proliferator-activated receptor gamma agonist, and their contribution to GW7845-induced apoptosis. Toxicol. Sci. 2006;92:433–444. doi: 10.1093/toxsci/kfl003. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Howard GJ, Hurst CH, Emberley JK, Waxman DJ, Webster T, Sherr DH. Environmental and endogenous peroxisome proliferator-activated receptor gamma agonists induce bone marrow B cell growth arrest and apoptosis: interactions between mono(2-ethylhexyl)phthalate, 9-cis-retinoic acid, and 15-deoxy-delta12,14-prostaglandin J2. J. Immunol. 2004;173:3165–3177. doi: 10.4049/jimmunol.173.5.3165. [DOI] [PubMed] [Google Scholar]
- Schlezinger JJ, Jensen BA, Mann KK, Ryu H-Y, Sherr DH. Peroxisome proliferator-activated receptor γ-mediated NF-κB activation and apoptosis in pre-B cells. J. Immunol. 2002;169:6831–6841. doi: 10.4049/jimmunol.169.12.6831. [DOI] [PubMed] [Google Scholar]
- Stridh H, Gigliotti D, Orrenius S, Cotgreave I. The role of calcium in pre- and postmitochondrial events in tributyltin-induced T-cell apoptosis. Biochem. Biophys. Res. Commun. 1999;266:460–465. doi: 10.1006/bbrc.1999.1821. [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem. Biophys. Res. Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Rizzuto R. Participation of endoplasmic reticulum and mitochondrial calcium handling in apoptosis: more than just neighborhood? FEBS Lett. 2004;567:111–115. doi: 10.1016/j.febslet.2004.04.059. [DOI] [PubMed] [Google Scholar]
- Takeda K, Matsuzawa A, Nishitoh H, Tobiume K, Kishida S, Ninomiya-Tsuji J, Matsumoto K, Ichijo H. Involvement of ASK1 in Ca2+-induced p38 MAP kinase activation. EMBO Rep. 2004;5:161–166. doi: 10.1038/sj.embor.7400072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J. Clin. Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J. Biol. Chem. 1989;264:17907–17912. [PubMed] [Google Scholar]
- Tsao T, Kornblau S, Safe S, Watt JC, Ruvolo V, Chen W, Qiu Y, Coombes KR, Ju Z, Abdelrahim M, et al. Role of peroxisome proliferator-activated receptor-gamma and its coactivator DRIP205 in cellular responses to CDDO (RTA-401) in acute myelogenous leukemia. Cancer Res. 2010;70:4949–4960. doi: 10.1158/0008-5472.CAN-09-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tze LE, Baness EA, Hippen KL, Behrens TW. Ig light chain receptor editing in anergic B cells. J. Immunol. 2000;165:6796–6802. doi: 10.4049/jimmunol.165.12.6796. [DOI] [PubMed] [Google Scholar]
- Valentine MA, Czernik AJ, Rachie N, Hidaka H, Fisher CL, Cambier JC, Bomsztyk K. Anti-immunoglobulin M activates nuclear calcium/calmodulin-dependent protein kinase II in human B lymphocytes. J. Exp. Med. 1995;182:1943–1949. doi: 10.1084/jem.182.6.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundina-Weilenmann C, Mattiazzi A. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc. Res. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Vos JG, de Klerk A, Krajnc EI, Kruizinga W, van Ommen B, Rozing J. Toxicity of bis(tri-n-butyltin)oxide in the rat. II. Suppression of thymus-dependent immune responses and of parameters of nonspecific resistance after short-term exposure. Toxicol. Appl. Pharmacol. 1984;75:387–408. doi: 10.1016/0041-008x(84)90177-7. [DOI] [PubMed] [Google Scholar]
- Wang C, Chen T, Zhang N, Yang M, Li B, Lu X, Cao X, Ling C. Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha kinase-NFkappaB. J. Biol. Chem. 2009;284:3804–3813. doi: 10.1074/jbc.M807191200. [DOI] [PubMed] [Google Scholar]
- Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J. Cell Biol. 2008;183:849–863. doi: 10.1083/jcb.200805048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Matulka RA, Schneider AM, Toselli P, Trombino AF, Yang S, Hafer LJ, Mann KK, Tao X-J, Tilly JL, et al. Induction of preB cell apoptosis by 7,12-dimethylbenz[a]anthracene in long-term primary murine bone marrow cultures. Toxicol. Appl. Pharmacol. 1997;147:190–203. doi: 10.1006/taap.1997.8263. [DOI] [PubMed] [Google Scholar]
- Yu ZP, Matsuoka M, Wispriyono B, Iryo Y, Igisu H. Activation of mitogen-activated protein kinases by tributyltin in CCRF-CEM cells: role of intracellular Ca(2+) Toxicol. Appl. Pharmacol. 2000;168:200–207. doi: 10.1006/taap.2000.9033. [DOI] [PubMed] [Google Scholar]
- Zhu W, Woo AY, Yang D, Cheng H, Crow MT, Xiao RP. Activation of CaMKIIdeltaC is a common intermediate of diverse death stimuli-induced heart muscle cell apoptosis. J. Biol. Chem. 2007;282:10833–10839. doi: 10.1074/jbc.M611507200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.